Abstract
Background
Granulocyte colony-stimulating factor (G-CSF) is an essential supportive agent for chemotherapy-induced severe myelosuppression. We proposed two clinical questions (CQ): CQ #1, “Does primary prophylaxis with G-CSF benefit chemotherapy for non-round cell soft tissue sarcoma (NRC-STS)?” and CQ #2, “Does G-CSF-based intensified chemotherapy improve NRC-STS treatment outcomes?” for the Clinical Practice Guidelines for the Use of G-CSF 2022 of the Japan Society of Clinical Oncology.
Methods
A literature search was performed on the primary prophylactic use of G-CSF for NRC-STSs. Two reviewers assessed the extracted papers and analyzed overall survival, incidence of febrile neutropenia, infection-related mortality, quality of life, and pain.
Results
Eighty-one and 154 articles were extracted from the literature search for CQs #1 and #2, respectively. After the first and second screening, one and two articles were included in the final evaluation, respectively. Only some studies have addressed these two clinical questions through a literature review.
Conclusion
The clinical questions were converted to future research questions because of insufficient available data. The statements were proposed: “The benefit of primary G-CSF prophylaxis is not clear in NRC-STS” and “The benefit of intensified chemotherapy with primary G-CSF prophylaxis is not clear in NRC-STSs.” G-CSF is often administered as primary prophylaxis when chemotherapy with severe myelosuppression is administered. However, its effectiveness and safety are yet to be scientifically proven.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas (STSs) are malignant tumors that occur throughout the body and account for approximately 1% of all malignant tumors. STSs comprise non-round cell soft tissue sarcomas (NRC-STSs) such as undifferentiated pleomorphic sarcomas, myxofibrosarcomas, liposarcomas, and leiomyosarcomas, and round cell STSs, including rhabdomyosarcomas, extra-skeletal Ewing sarcomas, and CIC-rearranged sarcomas [1]. Surgery with negative margins is the primary treatment for localized and resectable NRC-STSs [2]. In advanced and unresectable cases, chemotherapy and radiotherapy are considered. Anthracyclines and alkylating agents are commonly used for perioperative settings and the first-line treatment for advanced NRC-STSs, with a high risk of myelosuppression [3].
Granulocyte colony-stimulating factor (G-CSF) is a supportive care drug that prevents chemotherapy-induced febrile neutropenia (FN), and appropriate use increases chemotherapy’s benefits [4]. G-CSF may prevent associated death, improve quality of life (QOL), and prolong survival. However, G-CSF has undesirable side effects, including musculoskeletal pain, further hospital visits, and increased drug costs.
The ASCO Guidelines, ESMO Guidelines 2010, and NCCN Guidelines have no specific descriptions of NRC-STS and state that G-CSF primary prophylaxis is recommended when the incidence of FN is ≥ 20% [5,6,7]. Adriamycin and ifosfamide (AI) and mesna, adriamycin, ifosfamide, and dacarbazine (MAID) are listed in the ESMO Guidelines 2010 and the NCCN Guidelines as regimens with a risk of FN ≥ 20% among chemotherapy regimens for NRC-STSs.
Because G-CSF is an essential medical treatment that may influence patient survival and QOL, guidelines are required to ensure appropriate use. The Japan Society of Clinical Oncology Working Group for Revising Clinical Practice Guidelines for the Use of G-CSF was established to develop guidelines to support decision-making by patients and healthcare providers through evidence-based evaluation of the balance of benefits and harms.
In this systematic review, we assess the usefulness of G-CSF as a primary prophylaxis for adult patients with NRC-STS.
Patients and methods
Data search and screening
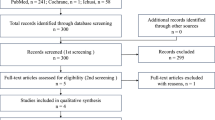
A systematic review, adhering to the Medical Information Network Distribution Service (Minds) Handbook for Clinical Practice Guideline Development 2014 [8] and Minds Clinical Practice Guideline Development Guide 2017 [9], was conducted utilizing PubMed, Ichushi web (Japan Medical Abstracts Society database), and the Cochran Library databases. In April 2020, we searched for studies employing Mesh terms and keywords, including “sarcoma/soft tissue neoplasms” AND “G-CSF,” with publication dates from 1 January 1990 to 31 December 2019, in English and Japanese (Supplementary Tables S1, S2). Relevant literature from other databases was incorporated based on the judgment of a systematic review team. According to titles and abstracts, an initial screening was independently conducted by two reviewers (K.T. and M.I.) to identify ineligible reports (Fig. 1a, b). In the second screening, articles selected in the first screening were carefully assessed by reading the entire article according to the inclusion and exclusion criteria. The reasons for exclusion were recorded, and duplicates were removed. Discrepancies were resolved by consensus among the co-authors. Articles that met the selection criteria were further scrutinized for quality, as outlined below.
Selection criteria
The inclusion criteria were as follows: (1) randomized controlled trial (RCT), non-RCT, and cohort or case–control trial design; (2) adult population diagnosed with NRC-STS; and (3) studies that included patients in the treatment group who received standard therapy. We excluded guidelines, reviews, letters, abstracts without full articles, laboratory studies, systematic reviews, meta-analyses, and gray literature.
PICO setting
Our study addressed these Clinical Questions (CQs): #1: “Does primary prophylaxis with G-CSF benefit chemotherapy for non-round cell soft tissue sarcoma (NRC-STS)?” #2: “Does G-CSF-based intensified chemotherapy improve NRC-STS treatment outcomes?”.
Regarding CQ #1, PICO consisted of the following: (P) patients, which included patients with NRC-STS receiving chemotherapy; (I) intervention, which included use of G-CSF as primary prophylaxis; (C) comparison, which did not use G-CFS as primary prophylaxis; (O) outcomes, which included (1) OS, (2) incidence of FN, (3) mortality rate for infection, (4) QOL, and (5) pain. Concerning CQ #2, PICO comprised (P) patients, which included patients with NRC-STS who received chemotherapy; (I) intervention, which included use of intensified chemotherapy based on the premise of G-CSF as primary prophylaxis; (C) comparison, which included conventional and non-intensified chemotherapy; (O) outcomes, which included (1) OS, (2) incidence of FN, (3) mortality rate for infection, (4) QOL, and (5) pain.
Reviewing
After the second screening, a systematic review team member independently reassessed the articles and extracted data using standardized data abstraction forms. The evidence indicated by individual studies on critical outcomes was assessed based on study design and quality. The authors reviewed the outcomes under the Population, Intervention, Comparator, and Outcome (PICO) frameworks for the benefits and harms of prophylactic G-CSF. Literature quality and evidence body were assessed by the reviewers. Conflicts and questions were resolved by the team leader (M.E.).
Results
For CQ #1, 53 articles in English and 28 in Japanese were identified using PubMed and Ichushi, respectively. After the first screening, seven of the 81 papers were extracted by title and abstract. Subsequently, a full-text evaluation was performed for eligibility, and only one paper, which described an RCT, was selected for the second screening. That RCT included 48 patients who underwent MAID chemotherapy (Table 1) [10]. The patients were randomly allocated to the prophylactic group, in which they received G-CSF as primary prophylaxis, or the non-prophylactic group, in which they did not receive G-CSF. The OS was not reported in that paper. The occurrence rates of FN in the prophylactic and non-prophylactic groups were 23% and 58%, respectively (p = 0.02), during the first cycle. No adverse deaths related to the infection were observed. Regarding pain, bone pain was observed in 23% of the prophylactic group and 3% of the non-prophylactic group, respectively (p = 0.06), during the first treatment cycle. There were no comments on the QOL. We found it challenging to evaluate CQ #1 directly based on the results of this systematic review. Therefore, we replaced CQ with FQ and proposed that “the effectiveness of primary prophylaxis with G-CSF is not clear for the treatment of NRC-STS.”
For CQ #2, 126 articles in English and 28 in Japanese were listed on PubMed and Ichushi, respectively. After the first screening, the titles and abstracts of 14 of 154 papers were extracted. Subsequently, a full-text evaluation was performed for eligibility, and only two papers were selected for the second screening. The reports included one RCT and one non-RCT, including patients with sarcoma who underwent AI therapy. In the RCT, 60 patients were randomly allocated to arm A, in which they received 60 mg/m2 of adriamycin and 5 g/m2 of ifosfamide every 3 weeks with prophylactic G-CSF, or arm B, in which they received 60 mg/m2 of adriamycin and 9 g/m2 of ifosfamide every 4 weeks without prophylactic G-CSF (Table 1) [4]. On the other hand, in the non-RCT, 33 patients were treated in two consecutive protocols. In the first protocol, the patients received 75 mg/m2 adriamycin and 10 g/m2 ifosfamide without prophylactic G-CSF (Table 1) [11]. The patients received 90 mg/m2 adriamycin and 10 g/m2 ifosfamide with prophylactic G-CSF in the second protocol. The OS was not reported in either trial. The occurrence of FN was only reported in the RCT, and all patients in both arms had FN. One patient in arm B died of grade 4 myelotoxicity in the RCT, whereas one patient with prophylactic G-CSF died of cardiotoxicity in the non-RCT. There were no comments regarding bone pain and QOL. Finally, we considered it difficult to answer CQ #2 directly based on the results of this systematic review. Therefore, we changed CQ to FQ and stated that “the effectiveness of intensified chemotherapy based on primary prophylaxis with G-CSF for treating NRC-STS is unclear.”
There is no specific description of the NRC-STS in published guidelines such as those of the ASCO, ESMO, and NCCN. They all recommend that primary prophylaxis be used for patients who receive chemotherapy whose FN occurrence rate exceeds 20% [5,6,7]. As for the regimen whose FN occurrence rate exceeds 20%, AI and MAID regimens are listed in the ESMO 2010 guidelines, and anthracycline-based regimens, including adriamycin alone and AI, are in the NCCN Guidelines. There are no reports on the usefulness of intensified chemotherapy with primary prophylaxis with G-CSF for treating NRC-STS.
Discussion
Surgery is the first-choice treatment for patients with resectable STSs. In Japan’s bone and soft tissue tumor registry, surgery was performed in over 80% of STSs [12]. The goal of treatment is to remove the tumor itself from the body and reduce the relapse rate [2]. Chemotherapy is usually applied to localized high-risk or advanced NRC-STSs. Several reports have described the usefulness of anthracycline-based chemotherapy [13,14,15]. Myelosuppression is a common side effect of anthracycline-based chemotherapy, and G-CSF is used to prevent severe myelosuppression. However, there have been no clear guidelines for the primary prophylaxis use of G-CSF for NRC-STSs. In the present study, we attempted to replace the conventional recommendation for G-CSF use for regimens with an incidence of FN of ≥ 20% with NRC-STS-specific recommendations. To fulfill this objective, we searched for evidence of primary prophylaxis use of G-CSF for NRC-STSs. However, the results showed that few reports existed, and evidence was poor for NRC-STSs.
As for CQ #1, an RCT investigated the prophylactic use of G-CSF for NRC-STS patients receiving MAID [10]. In that report, the occurrence rate of FN in the prophylactic group was higher than in the non-prophylactic group (p = 0.02). However, the MAID regimen is not commonly used nowadays because of severe adverse events, including myelosuppression. Instead of MAID, the AI regimen is most frequently used in preoperative settings. AI has been reported to have an FN incidence rate of 46% in clinical trials of patients with advanced disease [13] and 18–36% in clinical trials of perioperative patients [16, 17]. Although the primary prophylactic use of G-CSF is not recommended from this analysis, G-CSF is widely administered in clinical practice as a primary prophylaxis during AI regimens, considering the above FN incidence rates.
The ASCO, ESMO 2010 Guidelines, and NCCN Guidelines do not address the benefit of prophylactic G-CSF as a prerequisite for intensified chemotherapy in adults with NRC-STS [5,6,7]. The reports selected for CQ #2 included one RCT and one non-RCT with an AI regimen. The RCT reported only the occurrence rate of FN, in which all patients in both arms had FN. There is minimal data regarding OS, mortality rate from infection, QOL, and pain. It was challenging to answer CQ #2 directly with little evidence. More evidence is needed to demonstrate the effectiveness of G-CSF, which is an issue for further studies.
This systematic review has some limitations. First, only a few reports have addressed CQs. Second, more detailed information on QOL, pain, and other symptoms must be provided to assess the benefits and risks of prophylactic use of G-CSF. Third, the NRC-STS includes various histological subtypes, and the conclusion should be accepted with caution because the response to chemotherapy, even the results of G-CSF, may differ based on the difference in subtypes. In a previous report, tailored therapy based on five histological subtypes was found to be inferior to adriamycin-based treatment. However, this did not lead to the conclusion that histology-driven chemotherapy is inferior [18]. Future evidence should include sufficient data for each regimen and histological subtype.
In conclusion, we investigated the efficacy of primary prophylaxis with G-CSF for NRC-STS and found it challenging to directly evaluate the outcomes of CQ #1 and #2; these questions were converted to an FQ. The statements were “The benefit of primary G-CSF prophylaxis is not clear in NRC-STS” and “The benefit of intensified chemotherapy with primary G-CSF prophylaxis is not clear in NRC-STS.” In the future, it would be beneficial for decision-making if well-established studies on prophylactic G-CSF usage were performed to answer these CQs. G-CSF is widely administered as a primary prophylaxis for the AI regimen in clinical settings owing to its high FN incidence rates. However, its effectiveness and safety are yet to be scientifically proven.
Data availability
Data associated with this systematic review can be accessed from the corresponding author upon reasonable request.
References
Kawai A, Araki N, Ae K et al (2022) Japanese Orthopaedic Association (JOA) clinical practice guidelines on the management of soft tissue tumors 2020 – secondary publication. J Orthop Sci 27(3):533–550
Endo M, Lin PP (2018) Surgical margins in the management of extremity soft tissue sarcoma. Chin Clin Oncol 7(4):37
Nakayama R, Mori T, Okita Y et al (2020) A multidisciplinary approach to soft-tissue sarcoma of the extremities. Expert Rev Anticancer Ther 20(10):893–900
Erkisi M, Erkurt E, Ozbarlas S et al (1996) The use of recombinant human granulocyte colony-stimulating factor in combination with single or fractionated doses of ifosfamide and doxorubicin in patients with advanced soft tissue sarcoma. J Chemother 8(3):224–228
Crawford J, Caserta C, Roila F et al (2010) Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol 21(Suppl 5):v248–v251
NCCN (2022) NCCN Clinical Practice Guidelines in Oncology Hematopoietic Growth Factors Version 1.2022
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33(28):3199–3212
Morizane T, Yoshida M, Kojimahara N (2014) Medical Information Network Distribution Service (Minds) Handbook for clinical practice guideline development 2014 (in Japanese). Igaku Shoin, Tokyo
Minds Manual Developing Committee (2017) Minds manual for clinical practice guideline development 2017 (in Japanese). Japan Council for Quality Health Care, Tokyo
Bui BN, Chevallier B, Chevreau C et al (1995) Efficacy of lenograstim on hematologic tolerance to MAID chemotherapy in patients with advanced soft tissue sarcoma and consequences on treatment dose-intensity. J Clin Oncol 13(10):2629–2636
Patel SR, Vadhan-Raj S, Burgess MA et al (1998) Results of two consecutive trials of dose-intensive chemotherapy with doxorubicin and ifosfamide in patients with sarcomas. Am J Clin Oncol 21(3):317–321
Ogura K, Higashi T, Kawai A (2017) Statistics of soft-tissue sarcoma in Japan: report from the Bone and Soft Tissue Tumor Registry in Japan. J Orthop Sci 22(4):755–764
Judson I, Verweij J, Gelderblom H et al (2014) Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 15(4):415–423
Pervaiz N, Colterjohn N, Farrokhyar F et al (2008) A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113(3):573–581
Sarcoma Meta-analysis Collaboration (1997) Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 350(9092):1647–1654
Tanaka K, Machida R, Kawai A et al (2022) Perioperative Adriamycin plus ifosfamide vs. gemcitabine plus docetaxel for high-risk soft tissue sarcomas: randomised, phase II/III study JCOG1306. Br J Cancer 127(8):1487–1496
Tanaka K, Mizusawa J, Fukuda H et al (2015) Perioperative chemotherapy with ifosfamide and doxorubicin for high-grade soft tissue sarcomas in the extremities (JCOG0304). Jpn J Clin Oncol 45(6):555–561
Gronchi A, Palmerini E, Quagliuolo V et al (2020) Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J Clin Oncol 38(19):2178–2186
Acknowledgements
We extend our gratitude to Natsuki Fukuda for her dedicated work at the Japan Society of Clinical Oncology’s administrative office, to Masahiro Yoshida for his methodological advice in establishing this guideline, and to the literature search team members, Natsuki Narita and Yuko Mitsuoka for their support. We also thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of this study. T.H. and M.E. wrote the manuscript draft, and all authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kenji Tsuchihashi received honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, Taiho Pharmaceutical, and Novartis Pharma. Yukinori Ozaki received honoraria from Daiichi-Sankyo, Pfizer, Chugai, Lilly, and Kyowa Kirin. Eiki Ichihara received honoraria from Eli Lilly and research funding from MSD, Ono Pharmaceutical, Jansen Pharma, and Takeda Pharmaceutical. Yuji Miura received honoraria from Ono Pharmaceutical, MSD, Takeda Pharmaceuticals, Eisai, Bristol Myers Squibb, and research funding from Ono Pharmaceutical and MSD. Shingo Yano received research funding from Otsuka Pharmaceutical. Dai Maruyama received honoraria from Ono Pharmaceutical, Janssen Pharma, Nippon Shinyaku, Eisai, Mundipharma, Kyowa Kirin, Chugai Pharmaceutical, Zenyaku, MSD, SymBio, Sanofi, AbbVie, Takeda Pharmaceutical, AstraZeneca, Bristol Myers Squibb, and Genmab, and research funding from Amgen, Astellas Biopharma, Novartis, Kyowa Kirin, Ono Pharmaceutical, Chugai Pharmaceutical, Janssen Pharma, Takeda Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Astellas, Bristol Myers Squibb, AbbVie, Eisai, MSD, Taiho Pharmaceutical, AstraZeneca, Eli Lilly, and Genmab. Tetsuhiro Yoshinami received honoraria from Kyowa Kirin, Pfizer, Chugai Pharmaceutical, Eli Lilly, MSD, AstraZeneca, and Eizai. Takashi Motohashi received honoraria from AstraZeneca, Chugai Pharmaceutical, and Myriad Genetics. Eishi Baba received honoraria from Chugai Pharmaceutical and Daiichi-Sankyo, and research funding from Taiho Pharmaceutical and Chugai Pharmaceutical. Toshio Kubo received honoraria from Chugai Pharmaceutical. Takahiro Kimura received honoraria from Sanofi. Shinji Nakao received honoraria from Kyowa Kirin. Atsushi Sato received honoraria from Chugai Pharmaceutical, and Taiho Pharmaceutical, and research funding from Chugai Pharmaceutical, and Taiho Pharmaceutical. Toshimi Takano received honoraria from Daiichi-Sankyo, Chugai Pharmaceutical, and Eli Lilly. The other authors certify that no conflicts of interest exist in relation to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hirose, T., Ito, M., Tsuchihashi, K. et al. Primary prophylaxis with G-CSF for patients with non-round cell soft tissue sarcomas: a systematic review for the Clinical Practice Guidelines for the Use of G-CSF 2022 of the Japan Society of Clinical Oncology. Int J Clin Oncol 29, 1067–1073 (2024). https://doi.org/10.1007/s10147-024-02569-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02569-1