Abstract
Background
Six-month adjuvant chemotherapy with S-1 is standard care for resected pancreatic cancer in Japan; however, the optimal duration has not been established. We aimed to evaluate the impact of duration of adjuvant chemotherapy with S-1.
Methods
We performed a multicenter, randomized, open-label, phase II study. Patients with histologically proven invasive pancreatic ductal carcinoma, pathological stage I–III, and no local residual or microscopic residual tumor were eligible. Patients were randomized 1:1 to receive 6- or 12-month adjuvant chemotherapy with S-1. The primary endpoint was 2-year overall survival (OS). Secondary endpoints were disease-free survival (DFS) and feasibility.
Results
A total of 170 patients were randomized (85 per group); the full analysis set was 82 in both groups. Completion rates were 64.7% (6-month group) and 44.0% (12-month group). Two-year OS was 71.5% (6-month group) and 65.4% (12-month group) (hazard ratio (HR): 1.143; 80% confidence interval CI 0.841–1.553; P = 0.5758). Two-year DFS was 46.4% (6-month group) and 44.9% (12-month group) (HR: 1.069; 95% CI 0.727–1.572; P = 0.6448). In patients who completed the regimen, 2-year DFS was 56.5% (6-month group) and 75.0% (12-month group) (HR: 0.586; 95% CI 0.310–1.105; P = 0.0944). Frequent (≥ 5%) grade ≥ 3 adverse events comprised anorexia (10.5% in the 6-month group) and diarrhea (5.3% vs. 5.1%; 6- vs. 12-month group, respectively).
Conclusions
In patients with resected pancreatic cancer, 12-month adjuvant chemotherapy with S-1 was not superior to 6-month therapy regarding OS and DFS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite recent improvements in preoperative evaluation, surgical techniques, postoperative management, and neoadjuvant/adjuvant chemotherapy, pancreatic cancer remains one of the most lethal malignancies [1, 2]. In the early 2000s, surgical resection was the only curative treatment for pancreatic cancer, which was reported resectable in 15–20% of cases [3]. However, even in resectable cases, 95% of pancreatic cancers recurred within 2 years after surgery [4], with a median survival of 11.0–16.9 months and a 5-year survival rate of only 8.2% [5, 6]. Improved outcomes with surgical resection were limited, and there was an urgent need to develop better multimodal treatment.
The European Study Group for Pancreatic Cancer (ESPAC) 1 trial demonstrated that adjuvant chemotherapy with fluorouracil plus folic acid provided a significant survival benefit in patients with resected pancreatic cancer, whereas no additional effect was observed with radiation therapy [6]. The Charite Onkologie (CONKO) 001 trial showed that adjuvant chemotherapy with gemcitabine (GEM) delayed recurrence and improved survival compared with surgery alone [7]. The PRODIGE 24-ACCORD/CCTG PA6 trial performed by the Unicancer and Canadian Cancer Trials Group (CCTG) showed that in patients with resected pancreatic cancer, adjuvant chemotherapy with modified fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), although more toxic than GEM, was significantly more effective regarding survival [8].
The Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC) 01 trial randomized 385 pancreatic cancer patients who underwent radical resection to GEM or S-1 as adjuvant chemotherapy for 6 months. The final analysis of follow-up data revealed a hazard ratio (HR) for mortality of 0.57 (95% confidence interval CI 044–0.72), with a 5-year overall survival (OS) of 24.4% (18.6–30.8 T) in the GEM group and 44.1% (16.9–51.1%) in the S-1 group [9]. In accordance with these results, adjuvant chemotherapy with S-1 has become the standard treatment for resected pancreatic cancer in Japan. The duration of administration is considered 6 months; however, the optimal duration has not been established. The Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC) showed that adjuvant chemotherapy with S-1 significantly prolonged survival compared with surgery alone for curatively-resected gastric cancer [10]. Accordingly, 12 months of S-1 was established as the standard dosing period for resected gastric cancer in Japan and other Asian countries. The Japan Clinical Oncology Group (JCOG) 1104 trial investigated the non-inferiority of 6- vs. 12-month therapy of S-1 regarding recurrence-free survival. However, the predictive probability for showing non-inferiority at the final analysis was 2.9%; therefore, the trial was stopped [11].
The aim of this study was to evaluate the effect of the duration of adjuvant chemotherapy with S-1 for patients with resected pancreatic cancer. This was a multicenter, randomized, clinical trial to evaluate and compare the efficacy and safety of 6- vs. 12-month S-1 therapy and to select the more promising regimen, setting 6 months as the standard duration.
Patients and methods
Study design
This was a randomized, open-label, multicenter, phase II trial of 6- vs. 12-month adjuvant chemotherapy with S-1 in patients with resected pancreatic cancer; the postoperative adjuvant chemotherapy S-1 (PACS-1) trial.
Participants
The eligibility criteria for participants were histologically proven pancreatic ductal carcinoma in accordance with the General Rules for the Study of Pancreatic Cancer [12]; pathological stage I–III according to the TNM Classification of Malignant Tumors [13]; no local residual tumor (R0) or microscopic residual tumor (R1); and no cancer cells in intraoperative peritoneal lavage fluid cytology. Participants also had to satisfy the following criteria: age ≥ 20 years; Eastern Cooperative Oncology Group performance status [14]: 0 or 1; no history of chemotherapy or radiotherapy within the past 3 years; enrollment within 10 weeks after surgery; and adequate bone marrow, liver, and kidney function within 14 days before registration (leucocytes: 3000–12,000/mm3; platelets: ≥ 100,000/mm3; hemoglobin: ≥ 8.0 g/dL; total bilirubin: ≤ 2.0 mg/dL; aspartate aminotransferase and alanine aminotransferase: ≤ 100 IU/L; and serum creatinine: ≤ 1.2 mg/dL).
Patients were excluded if they had the following: history of chemotherapy for locally advanced pancreatic cancer; previously treated with S-1; serious drug allergy; recurrence confirmed before registration; severe pleural effusion or ascites; pulmonary fibrosis or interstitial pneumonia; or inadequately-controlled diarrhea. The following were additional exclusion criteria: active infectious disease; blood transfusion within 2 weeks before registration; complicating psychiatric disorder; active multiple primary cancers; pregnancy; men who were willing to conceive a child; receiving flucytosine, phenytoin, or warfarin potassium; or judged unsuitable for inclusion.
Randomization
Patients were randomly assigned (1:1) to receive either 6- or 12-month adjuvant chemotherapy with S-1 at the data center by a modified minimization method, balancing the adjustment factors of residual tumor status (R0 or R1), nodal status (N0 or N1), and study site. The investigators were not masked to the patients’ allocated treatment. Patients were aware of their group assignment.
Procedure
Patients received S-1 orally, as follows, on the basis of body surface area (BSA): BSA < 1.25 m2: 40 mg; BSA 1.25 m2 to < 1.5 m2: 50 mg; and BSA ≥ 1.5 m2: 60 mg. S1 was administered twice daily for 4 weeks followed by a 2-week rest (one cycle). After the second course, S-1 was administrated for 2 weeks followed by a 1-week rest, depending on the onset of toxicity or other factors. This regimen was repeated for 6 or 12 months.
To start each cycle of S-1, patients had to satisfy the following criteria: leucocytes: ≥ 3000/mm3; neutrophils: ≥ 1500/mm3; platelets: ≥ 100,000/mm3; hemoglobin: ≥ 6.5 g/dL; no pyrexia of ≥ 38 °C; serum creatinine: ≤ 1.5 mg/dL; aspartate aminotransferase: ≤ 150 IU/L; alanine aminotransferase: ≤ 150 IU/L; and no other non-hematological adverse events (AE) of grade ≥ 2. In addition to the criteria to rest and restart S-1 in each cycle, if any of the following AEs were observed, further administration of S-1 in the ongoing cycle was suspended: leucocytes: ≤ 1000/mm3; platelets: ≤ 50,000/mm3; pyrexia ≥ 38 °C; serum creatinine ≥ upper limit of the facility criteria; and non-hematological AEs grade ≥ 2. Once administration of S-1 was suspended, the daily dose of S-1 for the next cycle was reduced, as follows: from 120 to 100 mg; 100 mg to 80 mg; or 80 mg to 50 mg daily, on the basis of BSA.
During the protocol treatment, we evaluated tumor markers every 6 weeks (one cycle), and we repeated abdominal computed tomography (CT) or magnetic resonance imaging every 3 months. After the protocol treatment period, tumor markers were evaluated every 3 months, and abdominal computed tomography or magnetic resonance imaging was performed every 3 months during the first 2 years after enrollment, and every 6 months thereafter.
Outcomes
The primary endpoint was 2-year OS. OS was defined as the time interval from randomization to death from any cause. The secondary endpoints were disease-free survival (DFS) and the incidence of AEs. DFS was the time from registration to date of recurrence or death from any cause. We assessed AEs using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) [15].
Statistical analysis
In the JASPAC 01 trial [9], the 2-year OS for the 6-month S-1 therapy group was 70%. Based on this data, we assumed that 2-year OS for the 6-month group would be 70%. In the ACTS-GC trial [10], a study of 2-year OS for patients who took S-1 for less than 6 months and between 6 and 12 months and those who were able to take S-1 for 12 months showed an additional benefit of about 10%. Based on these results, assuming a 10% improvement in survival in the 12-month group compared to 70% survival in the 6-month group, the 2-year OS for 12-month group would be 80%. Assuming that the survival function follows an exponential distribution, we expected that the HR for mortality in the 12-month group compared with that in the 6-month group would be 0.62. For the log-rank test with two-sided significance of 20% and power of 70%, the number of events required was 59. Assuming an enrollment period of 3 years, follow-up period of 2 years, and no dropouts during the observation period, approximately 78 cases per group were required. Considering dropouts after enrollment, the target enrollment number was set at 85 cases per group, for a total of 170 cases. Patient characteristics were summarized with descriptive statistics and contingency tables. We estimated OS and DFS using the Kaplan–Meier method, and differences were compared using the log-rank test. HRs and Cis were obtained using a Cox proportional hazards model. All statistical analyses were performed using JMP 16.0 software (SAS Institute Inc., Cary, NC, USA).
Results
Patients’ characteristics
Between 11 February 2014 and 10 August 2016, 170 patients were enrolled; 85 patients were randomly assigned to the 6-month group and 85 to the 12-month group. After randomization, four patients were found ineligible, and two patients did not receive adjuvant chemotherapy. As a result, 82 patients in both the 6- and 12-month groups comprised the per-protocol population (Fig. 1). Table 1 shows the baseline characteristics of the per-protocol population. The patients’ demographics and tumor characteristics were well-balanced between the groups.
Completion rate
Fifty-three (64.7%) patients in the 6-month group and 36 (44.0%) in the 12-month group completed the S-1 chemotherapy regimen (Table 2). Disease relapse and AEs were the most common primary and secondary reasons for treatment discontinuation in both groups.
Survival
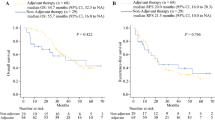
Two-year OS rates were 71.5% (6-month group) and 65.4% (12-month group) (HR: 1.143; 80% CI 0.841–1.553; P = 0.5758) (Fig. 2A). Recurrence was observed in 46 (56.1%) patients in the 6-month group and 51 (62.2%) in the 12-month group. Two-year DFS rates were 46.4% (6-month group) and 44.9% (12-month group) (HR: 1.069; 95% CI 0.727–1.572; P = 0.6448) (Fig. 2B). There were no significant differences in 2-year OS and DFS between the groups.
Overall- and disease-free survival. We calculated p-values using the log-rank test. Numbers at the bottom indicate the numbers of patients at risk. A Overall survival 2 years after randomization was 71.5% in the 6-month group and 65.4% in the 12-month group. B Disease-free survival 2 years after randomization was 46.4% in the 6-month group and 44.9% in the 12-month group
For patients who completed the regimen, 2-year OS rates were 90.5% (6-month group) and 94.4% (12-month group) (HR: 0.596; 95% CI 0.229–1.551; P = 0.3836) (Fig. 3A). Recurrence was observed in 30/53 (56.6%) patients in the 6-month group and 14/36 (38.9%) in the 12-month group. Two-year DFS rates were 56.5% (6-month group) and 75.0% (12-month group) (HR: 0.586; 95% CI 0.310–1.105; P = 0.0944) (Fig. 3B). In patients who completed the regimen, the 12-month group tended to have a better DFS compared with the 6-month group, but this trend was not seen when compared to the 6-month group excluding 10 patients who had a recurrence between 6 and 12 months after the initiation of S-1 (2-year DFS: 69.7% vs. 75.0%; 6- vs. 12-month group, respectively; HR: 0.649; 95% CI 0.251–1.357; P = 0.2479) (Fig. 3C).
Overall- and disease-free survival in the patients who completed the regimen. We calculated p-values using the log-rank test. Numbers at the bottom indicate the numbers of patients at risk. A Overall survival 2 years after randomization was 90.5% in the 6-month group and 94.4% in the 12-month group. B Disease-free survival 2 years after randomization was 56.5% in the 6-month group and 75.0% in the 12-month group. In patients who completed the regimen, the 12-month group tended to have better disease-free survival compared with the 6-month group. C Disease-free survival 2 years after randomization excluding 10 patients in the 6-month group who had a recurrence between 6 and 12 months after the initiation of S-1 was 69.7% in the 6-month group and 75.0% in the 12-month group
Adverse events
Owing to insufficient data for six patients in the 6-month group and 4 in the 12-month group, AEs were compared for 76 patients and 78 patients, respectively. The most frequent (≥ 5%) grade ≥ 3 AEs were anorexia (10.5% in the 6-month group) and diarrhea (5.3% vs. 5.1%; 6- vs. 12-month group, respectively). Anorexia was observed at a significantly higher rate in the 6-month group (Table 3) vs. the 12-month group. However, for patients who completed the regimen, there was no significant difference in the AE rate between the groups (Table 4).
Discussion
This was a randomized, open-label, multicenter, phase II trial of 6- vs. 12-month adjuvant chemotherapy with S-1 in patients with curatively-resected pancreatic cancer; the (PACS-1) trial. In contrast to our expectations following the ACTS-GC and JCOG 1104 trials for patients with resected gastric cancer [10, 11], 12-month adjuvant chemotherapy with S-1 was not superior to 6-month therapy regarding OS and DFS in patients with resected pancreatic cancer. These results are similar to those of a recently reported nationwide survey by the Japan Pancreas Society based on real-world date [16]. One of the reasons may be the lower completion rate in the 12-month group. The JASPAC 01 trial revealed that 72% of the patients completed adjuvant chemotherapy with S-1. In the present study, the completion rate was 64.7% in the 6-month group, which was not significantly different compared with the findings in the JASPAC 01 trial; however, the rate was very low in the 12-month group (44.0%). The primary and secondary reasons for failure to complete adjuvant chemotherapy were recurrence and AEs. The recurrence rate was 66% in the JASPAC 01 trial compared with 56.1% in the 6-month group and 62.2% in the 12-month group in this study, which were not significantly different from the rate in the JASPAC 01 trial. For an unknown reason, the 6-month group had a significantly higher rate of grade ≥ 3 anorexia compared with the 12-month group. The anorexia rate in the JASPAC group was 8%, which was not significantly different from the rate of 10.5% in the 6-month group in this study [9]. When the comparison was limited to patients who completed adjuvant chemotherapy, there was no difference in OS; however, DFS tended to be better in the 12-month group compared with that in the 6-month group, suggesting that the lower completion rate in the 12-month group had a significant impact in this study. Of the 46 patients who did not complete the 12-month regimen, 28 discontinued within 6 months and 18 between 6 and 12 months. The reasons for discontinuation in these 18 patients were recurrence in 6 (33.3%), AEs in 8 (44.4%), and other in 4 (22.2%). These results suggest that more appropriate management of AEs may improve completion rates.
The ESPAC-3 trial demonstrated that completion of adjuvant chemotherapy with GEM or fluorouracil plus folic acid for pancreatic cancer was an independent survival factor, and OS favored patients who completed vs. did not complete the full course of treatment [17]. Additionally, patients who completed the planned adjuvant chemotherapy with S-1 for pancreatic cancer had longer survival times compared with those who discontinued treatment [18]. Furthermore, Kobayashi et al. reported the following: (1) total dose intensity was important for the long-term prognosis after radical resection for pancreatic cancer, with completion of adjuvant chemotherapy with S-1; (2) long OS was achieved only when a high total dose intensity of at least 60% was maintained; and (3) there was no significant difference between patients who received < 60% of the total dose vs. the no adjuvant chemotherapy group [1].
Because AEs are a major reason why postoperative adjuvant chemotherapy is not completed, determining feasibility of S-1 treatment is essential. A feasibility study of S-1 as adjuvant chemotherapy using a 2-week administration regimen followed by a 1-week rest period was previously performed in locally advanced squamous cell carcinoma of the head and neck to increase compliance with S-1 therapy. In the study, treatment with S-1 was continued for 6 months as a 2-week administration/1-week rest (2-week/1-week) regimen or a 4-week administration/2-week rest (4-week/2-week) regimen. The treatment completion rate was 40.0% and 29.4%, and the frequency of grade ≥ 3 AEs was 8.0% and 17.6% for the 2-week/1-week and 4-week/2-week regimens, respectively [19]. These results suggested that the lower AE rate with the 2-week/1-week regimen exerted a significant positive effect on the completion rate of S-1 treatment.
Numerous studies have evaluated the relationship between the administration interval of fluoropyrimidine anticancer agents, including S-1, and their antitumor effects and AEs [20,21,22,23,24]. Arai et al. performed an alternate-day treatment schedule with S-1 in 36 gastric cancer patients [20]. The authors administered S-1 every other day in accordance with the theory that normal cells can be rescued by interrupting the administration of fluoropyrimidine anticancer agents every 24 h, taking advantage of the difference in the cell cycle between normal cells and tumor cells. The authors reported that the patients had sufficient blood fluoropyrimidine concentrations and fewer side effects and adequate clinical response [20].
In conclusion, our findings showed that 12-month adjuvant chemotherapy with S-1 was not superior to 6-month therapy regarding OS and DFS for patients with resected pancreatic cancer. Preoperative treatment of resectable pancreatic cancer was not common at the time this study was conducted. The usefulness of preoperative neoadjuvant chemotherapy for resectable pancreatic cancer has been reported, and postoperative adjuvant chemotherapy is now included in the regimen, with S-1 in particular being used frequently in Japan [25]. Against this background, we believe that this study to examine the duration of indication for postoperative adjuvant chemotherapy with S-1 will be useful for the clinical application of S-1.
References
Kobayashi K, Einama T, Takihata Y et al (2022) Therapeutic efficacy of dose-reduced adjuvant chemotherapy with S-1 in patients with pancreatic cancer: a retrospective study. BMC Cancer 22:1028
Itoh S, Tsujita E, Fukuzawa K et al (2021) Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: a multi-institutional retrospective study. Pancreatology 21:1356–1363
Sultana A, Smith CT, Cunningham D et al (2007) Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. J Clin Oncol 25:2607–2615
Sperti C, Pasquali C, Piccoli A et al (1997) Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg 21:195–200
Klinkenbijl JH, Jeekel J, Sahmoud T et al (1999) Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230:776–782
Neoptolemos JP, Stocken DD, Friess H et al (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210
Oettle H, Post S, Neuhaus P et al (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277
Conroy T, Hammel P, Hebbar M et al (2018) FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395–2406
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388:248–257
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Yoshikawa T, Terashima M, Mizusawa J et al (2019) Four courses versus eight courses of adjuvant S-1 for patients with stage II gastric cancer (JCOG1104 [OPAS-1]): an open-label, phase 3, non-inferiority, randomized trial. Lancet Gastroenterol Hepatol 4:208–216
Japanese Pancreas Society (2009) General rules for the study of pancreatic cancer, 6th edn. Kanehara, Tokyo
Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, Hoboken
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Common Terminology Criteria for Adverse Events v4.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
Tomimaru Y, Eguchi H, Inoue Y et al (2023) Impact of S-1 adjuvant chemotherapy longer than 6 months on survival in patients with resected pancreatic cancer: a nationwide survey by the Japan Pancreas Society based on real-world data. Cancer 129:728–739
Valle JW, Palmer D, Jackson R et al (2014) Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 32:504–512
Yabusaki N, Fujii T, Yamada S et al (2016) The significance of relative dose intensity in adjuvant chemotherapy of pancreatic ductal adenocarcinoma including the analysis of clinicopathological factors influencing relative dose intensity. Medicine (Baltimore) 95:e4282
Tsukuda M, Kida A, Fujii M et al (2005) Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93:884–889
Arai W, Hosoya Y, Hyodo M et al (2004) Alternate-day oral therapy with TS-1 for advanced gastric cancer. Int J Clin Oncol 9:143–148
Rino Y, Takanashi Y, Yukawa N et al (2006) A phase I study of bi-weekly combination therapy with S-1 and docetaxel for advanced or recurrent gastric cancer. Anticancer Res 26:1455–1462
Okumura N, Soh J, Suzuki H et al (2021) Randomized phase II study of daily and alternate-day administration of S-1 for adjuvant chemotherapy in completely-resected stage I non-small cell lung cancer: results of the Setouchi Lung Cancer Group Study 1301. BMC Cancer 21:506
Moriwaki T, Sakai Y, Ishida H et al (2019) Phase II study of S-1 on alternate days plus bevacizumab in patients aged ≥ 75 years with metastatic colorectal cancer (J-SAVER). Int J Clin Oncol 24:1214–1222
Ojima T, Nakamura M, Nakamori M et al (2019) Triplet chemotherapy with docetaxel, cisplatin and S-1 for unresectable advanced squamous cell carcinoma of the esophagus: phase I/II trial results. Oncotarget 10:847–855
Motoi F, Kosuge T, Ueno H et al (2019) Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 49:190–194
Acknowledgements
We thank Eisuke Adachi (Kyushu Central Hospital) and Yasuharu Ikeda (Fukuoka City Hospital) for the data and safety monitoring. We thank Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HK: conceptualisation, data curation, methodology, formal analysis, investigation, resources, visualisation, writing – original draft, writing—review & editing. SI: conceptualisation, data curation, methodology, formal analysis, investigation, resources, visualization, project administration, writing—original draft, writing—review & editing. MS: conceptualisation, data curation, methodology, formal analysis, validation, writing—original draft, writing—review & editing. HH, HT, KF, MN, KA, Y-iY, KS, HU, YM, TU, TU, TM, HB: investigation, resources, writing—review & editing. TY: conceptualisation, methodology, project administration, supervision, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest regarding this article.
Ethical approval
The study protocol was approved by Kyushu University Hospital Institutional Review Board (Approval number: 25066). This trial was completed in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies of the Ministry of Health, Labour, and Welfare of Japan.
Informed consent
Informed consent was obtained from all patients for inclusion in the study.
Registration number
This study was registered at University hospital Medical Information Network (UMIN) Clinical Trials Registry as UMIN000012634 (https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014772).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kayashima, H., Itoh, S., Shimokawa, M. et al. Effect of duration of adjuvant chemotherapy with S-1 (6 versus 12 months) for resected pancreatic cancer: the multicenter clinical randomized phase II postoperative adjuvant chemotherapy S-1 (PACS-1) trial. Int J Clin Oncol 28, 1520–1529 (2023). https://doi.org/10.1007/s10147-023-02399-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02399-7