Abstract
Background
The aim of this study was to evaluate the safety and efficacy of low-dose everolimus treatment in patients with tuberous sclerosis complex (TSC)-associated angiomyolipoma (AML) with renal dysfunction or low body weight.
Methods
We investigated a total of 50 adult patients underwent everolimus treatment for AML associated with TSC. For patients with renal dysfunction (serum creatinine level ≥ 1.5 mg/dl) or low body weight (body weight < 35 kg), 5 mg of everolimus was administered daily (low-dose group). For patients without renal dysfunction or low body weight, 10 mg of everolimus was administered daily (conventional-dose group). The treatment effects and adverse events were compared between the two groups.
Results
There were 20 patients in the low-dose group, and 30 in the conventional-dose group. The average reduction rate of the AML volume in the low-dose group was 52%, whereas it was 60% in the conventional-dose group. No significant differences were found in the average reduction rate between the groups (P = 0.24). The average blood everolimus trough levels were 7.7 ± 3.1 ng/mL in the low-dose group and 12.2 ± 5.7 ng/mL in the conventional-dose group. The level was significantly higher in the conventional-dose group than in the low-dose group (P = 0.004). The incidences of stomatitis and irregular menstruation were significantly lower in the low-dose group than in the conventional-dose group (P = 0.009, P = 0.045, respectively).
Conclusions
The present study demonstrates that low-dose everolimus treatment is safe and effective for TSC-associated AML. This treatment was well tolerated and adverse events were mild in all cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberous sclerosis complex (TSC) is a systemic disease with an autosomal dominant mode of inheritance, in which hamartomas form throughout the body, leading to the development of various organ disorders [1]. TSC is most often caused by mutations of the TSC1 gene (which encodes hamartin), or TSC2 gene (which encodes tuberin) located in chromosomes 9 and 16, respectively [2, 3]. AML is the most frequent benign renal tumor and is observed in 50–80% of TSC patients [4]. TSC-associated AML differs from sporadic AML, in that it frequently develops at multiple locations or bilaterally [5]. Most AML cases are asymptomatic and their renal function is normal [6]. TSC-associated AML is rarely observed in infancy, and it generally develops between the second and third decades of life [7]. When the tumor size is ≥ 4 cm, the growing speed is fast, and the incidence of hemorrhaging and hematuria increases [5].
Approximately 30% of TSC patients die due to the aggravation of renal lesions [8]. For this reason, it is of the utmost importance that these lesions be treated and managed appropriately. The International Tuberous Sclerosis Complex Consensus Conference (ITSCCC) held in 2012 recommended mammalian target of rapamycin (mTOR) inhibitors as the first-line treatment for AML when lesions were enlarged to ≥ 3 cm, even when asymptomatic [9]. Transcatheter arterial embolization (TAE) and partial nephrectomy are recommended as second-line treatments [9]. The standard dose for everolimus for adult TSC-associated AML is 10 mg daily. The dose may be increased or decreased depending on the patient's condition and the trough level. However, there are quite a few patients who receive low-dose everolimus treatment due to renal dysfunction or low body weight. There have been few studies investigating the effects of low-dose everolimus in the treatment of TSC-associated AML. In this retrospective study, we investigated the effects and adverse events of low-dose everolimus in patients with TSC-associated AML.
Methods
Patients and study design
TSC-associated AML was defined according to the diagnostic criteria of the ITSCCC after discussions with an internist and a dermatologist. From January 2014 through December 2018, a total of 50 adult patients met the diagnostic criteria and underwent everolimus treatment for TSC-associated AML. This treatment was performed for AML ≥ 4 cm in size. The reduction ratio of AML was calculated according to the volume of AML measured on multi-slice helical CT scans for three-dimensional imaging. The volume of the AML was compared to the baseline volume, which was measured before the initiation of treatment. During treatment with everolimus, abdominal CT scans were obtained at 3-month intervals. Adverse events were defined according to the Common Terminology Criteria for Adverse Events v5.0–JCOG (National Cancer Institute, Bethesda, MD, USA). The present study received approval from the institutional review board of JR Tokyo General Hospital (No. R01-22). All patients or their families provided their written informed consent before entering the study.
Exclusion criteria
Patients with any of the following conditions were excluded from the present study: (1) poor respiratory condition due to lung lymphangioleiomyomatosis; (2) uncontrollable epileptic seizures (despite treatment with antiepileptic agents); (3) swallowing disorders; and (4) pregnancy. Patients who were unable to periodically visit the hospital were also excluded from the study.
Everolimus treatment
For patients with renal dysfunction (serum creatinine level ≥ 1.5 mg/dl) or low body weight (body weight < 35 kg), 5 mg of everolimus was administered daily (low-dose group). For patients without renal dysfunction or low body weight, 10 mg of everolimus was administered daily (conventional-dose group). We compared the treatment effects and adverse events of the low-dose and conventional-dose groups. The blood everolimus trough levels were measured in both groups. Examinations were performed every month after the start of treatment. During these examinations blood testing, urinalysis, and chest radiography were performed and adverse events were assessed. KL-6 was measured to evaluate interstitial pulmonary disease. When an adverse event of Grade ≥ 3 was noted, the treatment was temporarily suspended. Everolimus could be resumed following the resolution of the adverse event.
Statistical analyses
Wilcoxon’s signed-rank test was used to compare the serial tumor volume measurements and adverse events due to everolimus. P < 0.05 was considered to be significant.
Results
Patient characteristics
We analyzed 50 adult patients with TSC-associated AML who underwent everolimus treatment (Table 1). There were 20 patients in the low-dose group and 30 in the conventional-dose group. All of them met the diagnostic criteria for TSC. One patient in the low-dose group showed a performance status of 2 due to mental retardation and muscle weakness. In the low-dose group, there were 17 patients with renal dysfunction (serum creatinine level ≥ 1.5 mg/dl) and 3 with low body weight (body weight < 35 kg). Three patients (15%) in the low-dose group and four (13%) in the conventional-dose group had AML ≥ 10 cm in diameter.
Treatment efficacy
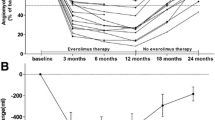
The median everolimus treatment periods were 17 months (range 10–33 months) in the low-dose group and 19 months (range 12–37 months) in the conventional-dose group. The average relative dose intensities of everolimus were 41.2% in the low-dose group and 80.1% in the conventional-dose group (P < 0.001). The volume reduction rate was calculated according to the AML volume after the start of treatment (the baseline AML volume was defined as 100%). Figure 1 shows the largest rate of reduction from baseline in both groups. The average reduction rate of AML volume in the low-dose group was 52%, whereas it was 60% in the conventional-dose group. No significant differences were found in the average reduction rate between the groups (P = 0.24). The average blood everolimus trough levels were 7.7 ± 3.1 ng/mL in the low-dose group and 12.2 ± 5.7 ng/mL in the conventional-dose group. The level was significantly higher in the conventional-dose group than in the low-dose group (P = 0.004).
Best percentage change in the AML volume from baseline in the low-dose group and conventional-dose group. The average reduction rate was 52% in the low-dose group and 60% in the conventional-dose group. No significant difference was found in the average reduction rate between the groups (P = 0.24). The dotted lines show the average reduction rates for each group
Adverse events
Adverse events related to everolimus treatment in the two groups are shown in Table 2. Stomatitis and irregular menstruation were frequently observed in both groups. The incidence of these events was significantly lower in the low-dose group than in the conventional-dose group (P = 0.009 and P = 0.045, respectively). The incidences of other events did not differ markedly between the two groups. Adverse events of Grade ≥ 3 were observed in 3 patients (10%) in the conventional-dose group, all of whom developed stomatitis, which was improved by symptomatic treatment, and resumed everolimus; in contrast, such events were noted in none of the low-dose group.
Abnormal everolimus treatment-related laboratory test values are shown in Table 3. For non-hematologic toxicities, the rate of elevation of the serum KL-6 level was significantly higher in the conventional-dose group than in the low-dose group (P = 0.041). Patients with elevated serum KL-6 levels were followed up carefully, since such patients are at a high risk of developing interstitial lung disease (ILD). There was only one case of ILD in each group (Table 2). The incidence of other laboratory abnormalities did not differ markedly between the two groups. There were no laboratory abnormalities of Grade ≥ 3 in either group. Everolimus treatment has been continued in both groups, and the renal function has not been aggravated in any of them as of yet.
Discussion
Recently, there have been an increasing number of reports on low-dose molecular-targeted drug therapies for renal cell carcinoma and breast cancer [10, 11]. Murata et al. reported the effect of low-dose axitinib administration for metastatic renal cell carcinoma [10]. Chang et al. showed that low-dose everolimus treatment was effective for neonatal cardiac rhabdomyoma [12]. The advantages of low-dose treatment are sustained drug effects, minimization of adverse events with treatment, and a reduction in medical costs [13].
The effectiveness of everolimus for TSC-associated AML has been demonstrated in the EXIST-2 and extension studies [14, 15]. Everolimus treatment for TSC-associated AML rarely achieves complete remission [14, 15]. The drug can shrink AML, but cannot cure it. Therefore, the patient must continue to take it for a long time. Several prospective studies have shown that the serum creatinine levels are increased in a significant proportion of patients receiving everolimus [16, 17]. In some TSC patients, the renal function decreases after TAE or renal surgery. In addition, some patients become underweight due to severe developmental disorders. The administration of a conventional-dose of everolimus to these patients may further reduce their renal function. For these reasons, we retrospectively investigated the effects and adverse events of low-dose everolimus treatment for TSC-associated AML.
The average reduction rates of the AML volume were equivalent between the low-dose and conventional-dose groups. The average blood everolimus trough level in the conventional-dose group was significantly higher than in the low-dose group. However, the recommended trough level of everolimus for TSC-associated AML is 5 to 15 ng/mL, and the average trough level of the low-dose group was within this range.
In the EXIST-2 extension study, the main adverse events of everolimus treatment were nasopharyngitis (43%), stomatitis (43%), and headache (30%). Adverse events of Grade ≥ 3 developed in 14% (16 of 112) of the cases. In our study, the incidences of stomatitis and irregular menstruation were relatively high in the conventional group (93% and 73%, respectively). Stomatitis in patients treated with everolimus, a frequently reported adverse effect, is often severe, and it may significantly reduce the patient's quality of life [18]. It is often necessary to reduce the dose of everolimus or to suspend administration altogether. Therefore, stomatitis with everolimus treatment is a major problem hampering continuous treatment. In addition, several mTOR inhibitors have been shown to reduce serum testosterone levels in men and serum progesterone levels in women, and irregular menstruation subsequently occurs in women [19−21]. Since many patients with TSC-associated AML are young, it is necessary to take measures to prevent hypogonadism. The incidences of stomatitis and irregular menstruation were significantly lower in the low-dose group than in the conventional-dose group. These findings suggest that low-dose everolimus treatment for TSC-associated AML may minimize adverse events associated with treatment.
The present study has some limitations. First, this was a retrospective study, and everolimus was administered at the discretion of the treating urologist rather than via a prospective protocol. Second, the serum creatinine level is the most widely used factor for assessing the renal function. Generally, the estimated glomerular filtration rate (eGFR) more precisely reflects the renal function than serum creatinine. However, the eGFR is also affected by age, body weight, and muscle mass [22]. Therefore, we defined renal dysfunction as a serum creatinine level ≥ 1.5 mg/dl. Third, this study did not include children. This is because the recommended dose of everolimus for pediatric TSC-associated AML is 3.0 mg/m2 daily, but the dose differs among pediatric cases. Further studies on the effects and adverse events of low-dose everolimus treatment for pediatric TSC-associated AML are necessary.
Conclusions
The present study demonstrates that low-dose everolimus treatment is safe and effective for TSC-associated AML. This treatment was well-tolerated and adverse events were mild in all cases. Based on our results, low-dose everolimus can be considered a treatment option for patients with TSC-associated AML, especially those who have renal dysfunction or low body weight. Further studies should be performed to broaden our knowledge on the safety and efficacy of low-dose everolimus for TSC-associated AML.
Abbreviations
- TSC:
-
Tuberous sclerosis complex
- AML:
-
Angiomyolipoma
- ITSCCC:
-
International Tuberous Sclerosis Complex Consensus Conference
- mTOR:
-
Mammalian target of rapamycin
- TAE:
-
Transcatheter arterial embolization
- ILD:
-
Interstitial lung disease
- eGFR:
-
Estimated glomerular filtration rate
References
Curatolo P, Bombardieri R, Jozwiak S (2008) Tuberous sclerosis. Lancet 372:657–658
van Slegtenhorst M, de Hoogt R, Hermans C et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277:805–808
The European Chromosome 16 Tuberous Sclerosis Consortium (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75:1305–1315
Castagnetti M, Vezzu B, Laverda A et al (2007) Urological counseling and follow up in pediatric tuberous sclerosis complex. J Urol 178:2155–2159
Kaneda MW, Tanaka M, Hamasaki T, Katayama I (2013) Trends in the prevalence of tuberous sclerosis complex manifestations: an epidemiological study of 166 Japanese patients. PLoS ONE 8:e63910
Rouviere O, Nivet H, Grenier N et al (2013) Kidney damage due to tuberous sclerosis complex: management recommendations. Diagn Interv Imaging 94:225–237
Flum AS, Hamoui N, Said MA et al (2016) Update on the diagnosis and management of renal angiomyolipoma. J Urol 195:834–846
Rakowski SK, Winterkorn EB, Paul E et al (2006) Renal manifestations of tuberous sclerosis complex: incidence, prognosis, and predictive factors. Kidney Int 70:1777–1782
Krueger DA, Northrup H (2013) International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: recommendation of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediat Neurol 48:255–265
Murata M, Ikeda Y, Hasegawa G et al (2019) Low-dose axitinib rechallenge with positive outcomes in a patient with metastatic renal cell carcinoma refractory to interferon a, sunitinib, and nivolumab therapies: a case report. J Med Case Rep 13:98
Wong AL, Sunder R, Wang TT et al (2016) Phase lb/ll randomized, open-label study of doxorubicin and cyclophosphamide with or without low-dose, short-course subitinib in the pre-operative treatment of breast cancer. Oncotarget 27:64089–64099
Chang JS, Chiou PY, Yao SH, Chou IC, Lin CY (2017) Regression of neonatal cardiac rhabdmyoma in two months through low-dose everolimus therapy: a report of three cases. Pediar Cardiol 38:1478–1484
Wei CC, Tsai JD, Sheu JN et al (2019) Continuous Low-dose everolimus shrinkage tuberous sclerosis complex-associated renal angiomyolipoma: a 48-month follow-up study. J investing Med 67:686–690
Bissler JJ, Kingswood JC, Radzikowska E et al (2013) Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet 381:817–824
Bissler JJ, Kingswood JC, Radzikowska E et al (2016) Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized, controlled trial. Nephrol Dial Transpl 31:111–119
Motzer RJ, Escudier B, Oudard S et al (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116:4256–4265
Yao JC, Shah MH, Ito T et al (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Eng J Med 364:514–523
Jones VE, McIntyre KJ, Paul D et al (2019) Evaluation of miracle mouthwash plus hydrocortisone versus prednisolone mouth rinses as prophylaxis for everolimus-associated stomatitis: a randomized phase II study. Oncologist 24:1153–1158
Huyghe E, Zairi A, Nohra J et al (2007) Gonadal impact of target of rapamycin inhibitors (sirolimus and everolimus) in male patients: an overview. Transpl Int 20:305–311
Fritsche L, Budde K, Dragun D et al (2004) Testosterone concentrations and sirolimus in male renal transplant patients. Am J Transplant 4:130–131
Braun M, Young J, Reiner CS et al (2012) Low-dose oral sirolimus and the risk of menstrual-cycle disturbances and ovarian cysts: analysis of the randomized controlled SUISSE ADPKD trial. PLoS ONE 7:e45868
Salgado JV, Neves FA, Bastos MG et al (2010) Monitoring renal function: measured and estimated glomerular filtration rates – a review. Braz J Med Biol Res 43:528–536
Acknowledgements
We thank the patients and their families for participating.
Author information
Authors and Affiliations
Contributions
TH participated in the design of the study and drafted the manuscript. KE participated in data acquisition. MT participated in the design of the study and helped to write the paper. All authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hatano, T., Endo, K. & Tamari, M. Efficacy and safety of low-dose everolimus treatment for renal angiomyolipoma associated with tuberous sclerosis complex. Int J Clin Oncol 26, 163–168 (2021). https://doi.org/10.1007/s10147-020-01792-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01792-w