Abstract
Objective
To investigate factors influencing the volume response of everolimus and sirolimus in tuberous sclerosis complex (TSC) associated-angiomyolipomas (AML).
Methods
A retrospective analysis of 30 cases of TSC-AML treated by mTOR inhibitors (everolimus 18 cases, and sirolimus 12 cases) between April 2014 and November 2017 at our center was carried out. Epidemiological data, therapeutic response and influence factors were reviewed and analyzed. Age, sex, associated with SEGA and/or LAM or not, plasma rapamycin concentration, AML volume at baseline, and mean CT value of AML in the maximum cross-section at baseline were analyzed as potential influencing factors.
Results
Eighteen patients with 32 lesions in everolimus group and 12 patients with 15 lesions in sirolimus group were included. There was no statistically significant difference of baseline characteristics except for involved side (P = 0.008) between two groups. The mean volume of AML was 1000 ± 1276 cm3 at baseline and 633 ± 1121 cm3 at 6 months after treatment (P < 0.001) in everolimus group, and 1984 ± 2861 cm3 at baseline and 1733 ± 2533 cm3 at 6 months after treatment (P = 0.001) in sirolimus group, respectively. The mean volume reduction of the AML in everolimus and sirolimus groups were 55.56% ± 23.79% and 30.5% ± 22.8% (P = 0.001). Stepwise multiple linear regression analysis revealed that factors influencing the short-term volume response of everolimus and sirolimus for TSC-associated AML were AML volume at baseline (P < 0.001 and 0.038, respectively) and mean CT value at baseline (P < 0.001 and 0.020, respectively). The rates of ≥ 50% volume reduction in high CT value group was much higher than that in low CT value group (90.5% vs. 18.2%, P < 0.001).
Conclusions
Everolimus at 10 mg daily might be more effective than sirolimus at 2 mg daily in treatment of patients with TSC-AML. AML volume and mean CT value at baseline were factors influencing the short-term volume response of everolimus or sirolimus for TSC-AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder characterized by hamartomas in organs including the brain, kidney, lung, skin, and heart [1]. The birth incidence of TSC has been estimated to be approximately 1 in 6000 [2]. The most common kidney manifestation of TSC is angiomyolipomas (AML), which occurs in 70–90% TSC patients [3]. mTOR inhibitors have shown promising efficacy in patients with TSC-associated AML [4]. Everolimus has demonstrated that it can effect a significant reduction in renal angiomyolipoma volume compared with placebo in the phase 3 EXIST-2 trial [5]. Sirolimus, another mTOR inhibitor, has also been demonstrated to be effective in TSC-AML in previous clinical trials [6,7,8,9,10,11]. However, considering individual difference, different patients may have various therapeutic responses to mTOR inhibitors. There are approximately 50% of patients who could not get a ≥ 50% reduction from baseline in the sum of volumes of target angiomyolipomas in EXIST-2 trial [5].
To our knowledge, there have been few previous reports regarding investigating the factors influencing the efficacy of mTOR inhibitors for TSC-AML. The objective of the present study was to evaluate the volume response of everolimus and sirolimus for TSC-AML and investigate the possible predictive factors affecting its efficacy. We could also compare the therapeutic efficacy between everolimus and sirolimus at the currently conventional doses for TSC-AML in this study.
Materials and methods
Participants
We retrospectively analyzed the clinical data of TSC-AML patients who received everolimus or sirolimus for TSC-AML in Peking Union Medical College Hospital from April 2014 to November 2017. The diagnosis of TSC was made based on the clinical diagnostic criteria of the 2012 international tuberous sclerosis complex consensus conference [3]. TSC patients who aged over 18 years old with at least one renal AML ≥ 2 cm in diameter were candidates for treatment. The exclusion criteria for sirolimus treatment included recent AML bleeding, history of surgery/embolism within 6 months, current or planned pregnancy, lactation, and renal/hepatic/metabolic abnormalities. Informed consent was obtained from all the patients before treatment. However, only the patients who received everolimus or sirolimus treatment for TSC-AML for the first time were included in this study. At last, 30 patients (18 cases for everolimus, and 12 for sirolimus) were included for analysis. Our study was approved by the Ethics Committee of Peking Union Medical College Hospital.
Treatment and follow-up
We obtained baseline data and there were no contraindications in all the patients before treatment. Pretreatment, abdominal CT/MRI, head CT/MRI, and chest CT examinations were obtained in all patients. Everolimus (10 mg/day) or sirolimus (2 mg/day) was orally administrated for at least 6 months. Plasma rapamycin concentration was monitored. We examined therapeutic response of TSC-AMLs at 6 months after treatment, including the volume of AML (cm3) and the mean CT value of AML maximum cross-section (hereinafter referred to as mean CT value, Hounsfield Units, HU). One patient in everolimus group died due to bleeding at 16 weeks, and all the other 29 patients received follow-up at 6 months. For evaluating AML lesions, 17 and 10 patients in everolimus group and sirolimus group got abdominal CT, respectively, and the other three patients got abdominal MRI. During imaging analysis, if there existed multiple bilateral AML lesions, the largest one on each side was analyzed. However, lesions with maximum diameter < 2 cm were excluded. The comparisons of volume and CT value were conducted for each lesion, respectively.
Statistical analysis
All statistical analyses were performed using SPSS19.0 software (SPSS Inc., USA). Data were expressed as means ± standard deviation (mean ± SD) or n (%) as appropriate. Statistical significance was determined by paired samples t test or Wilcoxon test for changes between pre- and post-treatment. Chi square test was used for comparison of dichotomic variables between two groups, and t test or Mann–Whitney test was used to determine differentiation state of continuous variables between two groups. Stepwise multiple linear regression analysis was used to assess the factors influencing the volume response of everolimus or sirolimus for TSC-AML. A P value of less than 0.05 was considered statistically significant.
Results
In total, 18 patients with 32 lesions were included in everolimus group, and 12 patients with 15 lesions in sirolimus group. The characteristics of patients at baseline were shown in Table 1. Only involved side had statistically significant difference between the two groups (P = 0.008). However, another five patients in sirolimus group had AML lesions with maximum diameter < 2 cm on the other side. There was no statistically significant difference between the two groups when they were included (P = 0.678).
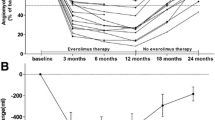
In sirolimus group, the mean volume of AMLs at 6 months after treatment reduced to 1733 ± 2533 cm3, and statistical analysis showed a statistically significant difference between the pre- and post-treatment AML volumes (P = 0.014). In everolimus group, the mean volume reduced to 633 ± 1121 cm3 at 6 months after treatment (P < 0.001) (Fig. 1). The mean volume reduction of the AML was 55.56% ± 23.79% in everolimus group and 30.52% ± 22.80% in sirolimus group (P = 0.001). 20 lesions (62.5%) in everolimus group and 3three lesions (20.0%) had a > 50% reduction in the AML volumes relative to the baseline (P = 0.016). The mean CT value was − 6.7 ± 45.7 at baseline and − 34.6 ± 48.7 Hu at 6 months after treatment (P = 0.002) in everolimus group, and − 11.2 ± 52.9 Hu at baseline and − 24.8 ± 58.0 Hu at 6 months after treatment (P < 0.001) in sirolimus group, respectively. One patient in everolimus group died due to bleeding at 16 weeks, and no other bleeding event was discovered in imaging during follow-up. No new gross or microscopic hematuria occurred during 6 months’ treatment, and the microscopic hematuria changed to 11.8% and 33.3% in everolimus and sirolimus groups at 6 months. Two patients in everolimus group experienced dosage reduction (to 5 mg/day) due to serious mucositis oral for 2 weeks and 4 weeks, respectively, and one patient in everolimus group stopped treatment for 1 week due to pneumonia. No withdrawl or dosage reduction occurred in sirolimus group.
We used stepwise multiple linear regression to explore the factors influencing volume response of everolimus or sirolimus for TSC-AML. For each group, the possible independent variables included age, sex, associating with SEGA and/or LAM or not, AML volume at baseline, mean CT value of AML in the maximum cross-section at baseline, and plasma rapamycin concentration. Statistical analysis showed that only AML volume at baseline and mean CT value of AML in the maximum cross-section at baseline were main influence factors (Table 2). Age, sex, with SEGA and/or LAM or not, and plasma rapamycin concentration of rapamycin did not influence everolimus or sirolimus volume response in TSC-AML. To further analyze the effect of CT value for response, we pooled all the lesions in the two groups together and grouped them anew by median of CT value (− 7.0 Hu) as high CT value group (≥ − 7 Hu) and low CT value group (< − 7 Hu). The rates of ≥ 50% volume reduction in high and low CT value groups were 90.5% and 18.2% (P < 0.001) respectively.
Discussion
TSC is a genetic disease that involves multiple organs, including brain, kidneys, heart, lungs, and skin [1]. The incidence is about 1/6000, but it may be underestimated for undiagnosed cases with mild or no symptoms [2]. Two genes have been identified to be responsible for TSC: TSC1 and TSC2. TSC1 locates on chromosome 9q34 and encodes hamartin, and TSC2 locates on chromosome 16p13.3 and encodes tuberin [12, 13]. Mutations of TSC1 and TSC2 can lead to defective production of hamartin and tuberin, respectively. However, mutations of TSC2 will usually result in more severe manifestations [14, 15]. TSC1 and TSC2 are both tumor suppressor genes, whose mutations will result in uncontrolled proliferation of benign tumors at several sites [16]. However, TSC1 or TSC2 mutations in 10 ~ 25% TSC patients cannot be shown by conventional genetic testing [3].
Renal lesions in TSC patients mainly include AML and multiple renal cysts. Approximately 80% of TSC patients develop AML, which is a significant cause for death. The vascular component of AML can cause bleeding, leading to dialysis or even renal transplantation [17]. The risk of spontaneous bleeding of AML is related to its volume, and approximately 25 ~ 50% of AML patients with diameter > 3 ~ 4 cm will experience hemorrhage [18, 19]. 40% of AML patients have renal function decline, but it is unclear what all the mechanisms causing impaired kidney function are [20]. Following the recommendations of the 2012 international tuberous sclerosis complex consensus conference [21], watchful waiting is acceptable for smaller AML and mTOR inhibitors can be chosen for asymptomatic progressive AMLs with diameter > 3 cm. The preferred treatment method for bleeding caused by aneurysm rupture of AML is selective renal artery embolization, and nephron sparing resection is second choice because it can lead to nephron loss.
To our knowledge, there has been no previous studies comparing the efficacy of sirolimus and everolimus for TSC-AML. Rapamycin and its analogs including temsirolimus and everolimus are widely used in treatment of tumors and prevention of solid organ transplant rejection. Until now, only everolimus has been approved for the treatment of TSC-SEGA and TSC-AML. However, it has been proven that sirolimus could benefit TSC patients in several clinical trials and prospective cohort studies [6,7,8,9,10,11]. In spite of this, everolimus at a dose of 10 mg daily might be more effective than sirolimus at a dose of 2 mg daily for TSC-AML at 6 months in our study. Comparative pharmacokinetics suggest that everolimus is more readily absorbed and exhibits greater oral bioavailability compared with sirolimus due to selective intestinal cell efflux for which sirolimus alone is a substrate [22]. The recommended plasma concentration for everolimus and sirolimus in previous studies is 5–15 ng/ml [6] and 4–8 ng/ml [11]. On the other hand, everolimus is 2.7-fold lower than sirolimus in hepatic metabolism [22]. It should be noted that tumors would progress after withdrawal of mTOR inhibitors in the above studies. Therefore, continuing benefit may need extending therapy of mTOR inhibitors. Currently, there has been no an exact recommendation for duration of treatment.
We analyzed the reasons regarding the difference of therapeutic response of volume to mTOR inhibitors for TSC-AML, and we found that AML volume and mean CT value at baseline, not age, sex, associating with or without SEGA/LAM, or plasma sirolimus concentration, were the influencing factors for treatment efficacy when considering volumetric change. Our patients with larger AML lesions had poor response. In addition, we found that the mean CT value at baseline also affected volume response to mTOR inhibitors. AML with higher mean CT value responded better to the therapy, indicating that components of the tumor might be an influence factor. AML are soft tissue perivascular epithelioid cell tumors composed of stromal elements including blood vessels, smooth muscle and fat which will lead to lower CT value. Several studies [5, 8, 23] demonstrated that plasma VEGF-D level was correlated with TSC-AML volume, and it could be used for monitoring the efficacy of mTOR inhibitors. Malinowska et al. reported that the variation trend of VEGF-D concentration was consistent with change of AML volume and concluded that serum VEGF-D may be useful for monitoring response to treatment and kidney angiomyolipoma size in patients with TSC [24]. Brugarolas et al. found that TSC2 could regulate VEGF through mTOR-dependent and independent pathways. In addition, they also found that inactivation of TSC2 gene could result in HIF-1α accumulation, and upregulate expression of some HIF-responsive genes including VEGF [25]. Therefore, better response to treatment with mTOR inhibitors in patients whose AMLs with more vascular components may be obtained due to the reduction of VEGF level by mTOR inhibitors. This may contribute to explain why the vascular components influence the therapy efficacy.
There are several limitations in the present study. This is a retrospective study with small samples, thus studies with larger number of patients are still needed to explore the influence of imaging characteristics on TSC-AML patients’ volume response to sirolimus and everolimus. In addition, the relationship between long-term efficacy of mTOR inhibitors and imaging features remains to be confirmed and this may be helpful for guiding mTOR inhibitors therapy. Furthermore, the optimal duration of treatment has not been defined. More efforts should be paid to investigate the predictive risk of AML progression in the absence of imaging characteristics.
Conclusions
Our findings suggest that everolimus at a dose of 10 mg daily might be more effective than sirolimus at a dose of 2 mg daily in the treatment of patients with TSC-AML. AML volume and mean CT value may be the factors influencing the short-term efficacy of mTOR inhibitors for TSC-associated AML when considering volumetric change.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AML:
-
Angiomyolipomas
- LAM:
-
Lymphangioleiomyomatosis
- SEGA:
-
Subependymal giant cell astrocytoma
- TSC:
-
Tuberous sclerosis complex
References
Sasongko TH, Ismail NF, Zabidi-Hussin Z (2016) Rapamycin and rapalogs for tuberous sclerosis complex. Cochrane Database Syst Rev 7:CD011272
Osborne JP, Fryer A, Webb D (1991) Epidemiology of tuberous sclerosis. Ann NY Acad Sci 615:125–127
Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group (2013) Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol 49(4):243–254
Budde K, Gaedeke J (2012) Tuberous sclerosis complex-associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis 59(2):276–283
Bissler JJ, Kingswood JC, Radzikowska E et al (2013) Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381(9869):817–824
Bissler JJ, McCormack FX, Young LR et al (2008) Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358(2):140–151
McCormack FX, Inoue Y, Moss J et al (2011) Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 364(17):1595–1606
Dabora SL, Franz DN, Ashwal S et al. (2011) Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF-D levels decrease. PLoS ONE 6(9):e23379
Davies DM, de Vries PJ, Johnson SR et al (2011) Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res 17(12):4071–4081
Koenig MK, Hebert AA, Roberson J et al (2012) Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R&D 12(3):121–126
Cabrera-López C, Martí T (2012) Assessing the effectiveness of rapamycin on angiomyolipoma in tuberous sclerosis: a two years trial. Orphanet J Rare Dis 7:87
van Slegtenhorst M, de Hoogt R, Hermans C et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277(5327):805–808
European Chromosome 16 Tuberous Sclerosis Consortium (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75(7):1305–1315
Dabora SL, Jozwiak S, Franz DN et al (2001) Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 68(1):64–80
Cai Y, Li H, Zhang Y (2017) Assessment if tuberous sclerosis complex associated with renal lesions by targeted next-generation sequencing in mainland China. Urology 101:170.e1–170.e7
Gao X, Pan D (2001) TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev 15(11):1383–1392
Kozłowska J, Okoń K (2008) Renal tumors in postmortem material. Pol J Pathol 59(1):21–25
Dixon BP, Hulbert JC, Bissler JJ (2011) Tuberous sclerosis complex renal disease. Nephron Exp Nephrol 118(1):e15–e20
Aydin H, Magi-Galluzzi C, Lane BR et al (2009) Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol 33(2):289–297
Curatolo P, Bjørnvold M, Dill PE et al (2016) The role of mTOR inhibitors in the treatment of patients with tuberous sclerosis complex: evidence-based and expert opinions. Drugs 76(5):551–565
Krueger DA, Northrup H, International Tuberous Sclerosis Complex Consensus Group (2013) Tuberous sclerosis complex surveillance and management: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol 49(4):255–265
MacKeigan JP, Krueger DA (2015) Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro Oncol 17(12):1550–1559
Budde K, Zonnenberg BA, Frost M et al (2016) Pharmacokinetics and pharmacodynamics of everolimus in patients with renal angiomyolipoma and tuberous sclerosis complex or lymphangioleiomyomatosis. Br J Clin Pharmacol 81(5):958–970
Malinowska IA, Lee N, Kumar V et al (2013) Similar trends in serum VEGF-D levels and kidney angiomyolipoma responses with longer duration sirolimus treatment in adults with tuberous sclerosis. PLoS ONE 8(2):e56199
Brugarolas JB, Vazquez F, Reddy A et al (2003) TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4(2):147–158
Acknowledgements
We thanked Dr. Xu Wang in Department of Urology, Peking Union Medical College Hospital for participating in manuscript revision work.
Funding
The present study was supported by National Natural Science Foundation of China (No. 81670611).
Author information
Authors and Affiliations
Contributions
YZ and YC conceived and designed the study. HL helped to evaluate the feasibility of the study, and guided the implementation. WW and HG performed the data collection. WW conducted data analysis and produced the manuscript. BS and HS helped to provide the image data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The study was approved by the Ethics Committee of Peking Union Medical College Hospital (Beijing, China).
Informed consent
Consent for publication was obtained from all the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, W., Guo, H., Shi, B. et al. CT characteristics predict the response to everolimus or sirolimus of renal angiomyolipomas in patients with tuberous sclerosis complex. Int Urol Nephrol 51, 671–676 (2019). https://doi.org/10.1007/s11255-019-02093-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02093-6