Abstract
Background
Taxane/platinum (TP)-based combination chemotherapy is standard for the treatment of metastatic or recurrent cervical cancer. The aim of this study was to investigate the efficacy of postoperative TP therapy in early stage cervical cancer.
Methods
A retrospective review of patients with FIGO IB–IIB stage cervical cancer who were treated with radical hysterectomy and displayed surgical-pathological risk factors was performed. 122 patients were identified between 2003 and 2012. Survival was analyzed by Kaplan–Meier method and compared by the log-rank test. The Cox proportional hazards model was used to investigate predictors of survival.
Results
The median follow-up period was 82.4 months. The postoperative adjuvant therapy was TP in 82 (67.2%) patients, other chemotherapies in 10 (8.2%), radiotherapy (RT) in 25 (20.5%), and no further therapy (NFT) in 5 (4.1%). Survival was analyzed using 4 subgroups according to the postoperative adjuvant therapy. The estimated 5-year overall survival was 95.1% in the TP group, 90.0% in the other chemotherapy group, 78.9% in the RT group, and 100% in the NFT group. No significant difference of survival was observed in the subgroups. However, when analyzing only patients who displayed high-risk factors, non-TP adjuvant therapy (including RT and other chemotherapies) was independently associated with shorter survival on multivariate analysis. In the TP group, multivariate analysis revealed that a positive surgical margin was a significant predictor of shorter survival.
Conclusions
Postoperative TP is effective in patients with surgically treated early stage cervical cancer. In these populations, a positive surgical margin could be associated with poor prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uterine cervical cancer is the fourth most frequent cancer in women with an estimated 530,000 new cases worldwide in 2012 [1]. Cervical cancer is most commonly diagnosed at early stage, with the highest incidence rates being in younger women [2, 3]. The majority of early stage cervical cancer patients who undergo surgical treatment with radical hysterectomy receive adjuvant therapy based on surgical–pathological risk factors [4].
Risk factors for recurrence after radical hysterectomy have been evaluated in many studies [5,6,7,8,9,10,11,12,13,14,15,16,17]. Positive lymph nodes, positive surgical margins, and parametrial invasion are classified as high-risk factors [5], while a large tumor size, lymphovascular space involvement (LVSI), and deep cervical stromal invasion are categorized as intermediate-risk factors [8]. Pelvic external beam radiotherapy (RT) with or without concurrent cisplatin-containing chemotherapy has been a standard adjuvant treatment for patients with these risk factors since the 2000s [4, 18,19,20]. Although the improvement in survival for early stage cervical cancer has been confirmed, severe complications, including lower-limb lymphedema, bowel obstruction, radiation cystitis, and urinary disturbance, have also been reported [5, 8, 21,22,23]. RT-induced complications are not easy to treat and related to a poor quality of life. Moreover, for young patients who received ovary-sparing surgery, postoperative RT leads to early menopause. RT often causes a fibrosis of the vagina [24], making it difficult for patients to retain a sexual function after treatment. One possible solution for these problems would be to use chemotherapy as an alternative adjuvant treatment.
Recently published data from phase III randomized trials suggested that taxane/platinum (TP)-based combination chemotherapy, such as paclitaxel/cisplatin, paclitaxel/carboplatin, and paclitaxel/cisplatin/bevacizumab, was the most effective treatment for metastatic or recurrent cervical cancer [25,26,27]. Despite no randomized trials of TP chemotherapy in the adjuvant setting, several retrospective studies demonstrated the efficacy and safety of postoperative TP for patients with early stage cervical cancer [28,29,30,31,32,33,34]. These studies strongly suggest that TP may be an alternative adjuvant treatment to RT. However, because the follow-up in these studies was relatively short (33–46.8 months), the value of adjuvant TP chemotherapy remains to be determined.
The aim of this study is to evaluate the long-term outcomes of patients with FIGO IB–IIB stage cervical cancer who received postoperative adjuvant TP after radical hysterectomy.
Patients and methods
Patients
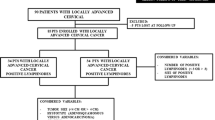
Permission to proceed with the data acquisition and analysis was obtained from the National Hospital Organization Shikoku Cancer Center’s Institutional Review Board. A list of 437 patients who received primary treatment for the International Federation of Gynecology and Obstetrics (FIGO) stage IB–IIB cervical cancer at National Hospital Organization Shikoku Cancer Center from January 2003 to December 2012 was generated from our institutional tumor registry. Through a chart review, 164 patients who were treated with radical hysterectomy were identified (Supplemental Fig. 1). Patients who received neoadjuvant chemotherapy, those who received non-radical surgery, and those who received RT or chemotherapy as their primary treatment were excluded. At our institution, the histological classification of cancer is performed by two independent pathologists, and histology, LVSI, tumor size, marginal status, parametrial involvement, deep stromal invasion (> 50%), and lymph node metastasis were routinely recorded. The patients were clinically staged according to the FIGO staging criteria.
Treatment
All the patients who were enrolled in the current study underwent with type C radical hysterectomy and pelvic lymphadenectomy [35]. The lymphadenectomy procedure included complete bilateral pelvic lymphadenectomy with the aim of removing all of the external iliac, internal iliac, common iliac, obturator, and presacral lymph nodes. When para-aortic lymph node (PALN) metastasis was suspected on the preoperative computed tomography scan or by intraoperative palpation, a para-aortic lymphadenectomy was performed. Seven patients with histologically confirmed PALN metastasis were not included in this study (Supplemental Fig. 1).
Postoperative adjuvant therapy is indicated when a patient’s pathological report displays any of the following high-risk prognostic factors: parametrial invasion, pelvic lymph node metastasis, or a positive surgical margin, or one of the following intermediate-risk prognostic factors: deep stromal invasion, LVSI, or a large tumor (over 4 cm in diameter). Pathological reports revealed at least one risk factor in 122 patients (Supplemental Fig. 1). We provided these patients with information on adjuvant RT or adjuvant chemotherapy. Patients could then choose the modality of adjuvant therapy.
Follow-up
Once treatment ended, the patients were followed up regularly by gynecological oncologists. The median duration of the follow-up was 82.4 months (range 7.1–176.1 months).
Statistical analysis
Overall survival (OS) was defined as the time from radical hysterectomy to death or the latest observation. Recurrence-free survival (RFS) was defined as the time from radical hysterectomy to the date of clinically proven recurrence. Univariate analyses were performed by comparing Kaplan–Meier curves using the log-rank test. The Cox proportional hazards regression model was employed to investigate predictors of survival. Kruskal–Wallis test and Mann–Whitney U test were used to compare groups. P values of < 0.05 were considered statistically significant. MedCalc (MedCalc Software, Mariakerke, Belgium) was used for all analyses.
Results
Patient characteristics
One-hundred and twenty-two patients who underwent radical hysterectomy and had any surgical-pathological risk factors were evaluated for the analysis. The clinical–pathological demographics of the patients are shown in Table 1. There were 82 (67.2%) patients who received TP as an adjuvant chemotherapy and 10 (8.2%) patients who received other regimens (irinotecan/nedaplatin or tegafur/uracil) (Table 1 and Supplemental Fig. 1). Twenty-five (20.5%) patients received adjuvant RT. The remaining 5 (4.1%) patients received no further therapy (NFT) at the patient’s request.
The median number of dissected lymph nodes was 38 (range 14–117) and 37 (30.3%) patients had lymph node metastasis. For the majority of the prognostic factors, such as age, FIGO stage, parametrial invasion, surgical margin, LVSI, and maximum tumor diameter, the distribution of patients was not significantly different according to the modality of adjuvant therapy (Table 1). However, the proportion of patients who had squamous cell carcinoma (SCC) was significantly higher in patients who received RT than in patients who received TP (P = 0.008). Patients who received other chemotherapies were more likely to have lymph node metastasis (P = 0.03) and shallow stromal invasion (P = 0.03) than patients who received TP.
Among 122 patients who were evaluated in the current study, 49 (40.2%) displayed high-risk prognostic factors. Meanwhile, 73 (59.8%) patients displayed intermediate-risk prognostic factors. The distribution of patients who displayed high- or intermediate-risk factors was not significantly different according to the modality of adjuvant therapy (Table 1).
Postoperative chemotherapy
In 9 of the 64 patients who initially received paclitaxel/cisplatin, the regimen was changed to paclitaxel/carboplatin because of inadequate renal function (8 patients) or severe gastrointestinal symptoms (1 patient). In 2 of the 18 patients who initially received paclitaxel/carboplatin, the regimen was changed to docetaxel/carboplatin because of an allergic reaction to paclitaxel or severe peripheral neuropathy. In our standard chemotherapy, paclitaxel/cisplatin consists of paclitaxel (175 mg/m2 on day 1) plus cisplatin (50 mg/m2 on day 1) triweekly, or paclitaxel (80 mg/m2 on day 1, 8, and 15) plus cisplatin (25 mg/m2 on day 1, 8, and 15) weekly. Paclitaxel/carboplatin consists of paclitaxel (175 mg/m2 on day 1) plus carboplatin (at area under the curve of 5 mg/mL/min on day 1) triweekly. Docetaxel/carboplatin consists of docetaxel (60 mg/m2 on day 1) plus carboplatin (at area under the curve of 5 mg/ml/min on day 1) triweekly. Nine patients received irinotecan/nedaplatin which consists of irinotecan (60 mg/m2 on day 1 and 8) plus nedaplatin (80 mg/m2 on day1) triweekly. One patient received tegafur/uracil (600 mg/day) for 90 days.
The median number of postoperative chemotherapy cycles was 5 (range 3–6). The total number of cycles of chemotherapy was 385; paclitaxel/cisplatin comprised 238 cycles, paclitaxel/carboplatin comprised 97 cycles, docetaxel/carboplatin comprised 8 cycles, and irinotecan/nedaplatin comprised 42 cycles.
Postoperative radiotherapy
Among 25 patients who received adjuvant RT, 14 patients were treated with external beam pelvic RT plus concurrent chemotherapy and 11 patients were treated with pelvic RT alone. The external irradiation was delivered to the whole pelvis at 1.8 Gy per fraction for a total of 28 fractions (50.4 Gy). Cisplatin (40 mg/m2) was employed as a radiosensitizing agent and administered intravenously during the course of pelvic RT.
Treatment outcomes
Among 122 patients who underwent radical hysterectomy and had surgical–pathological risk factors, 15 (12.3%) developed recurrent disease and 12 (9.8%) died of their disease after a median follow-up of 82.4 months. The estimated 5-year OS and RFS rates were 91.7 and 87.5%, respectively (Supplemental Figs. 2A and 2B).
Survival was analyzed using 4 subgroups divided by the modality of adjuvant therapy (TP, other chemotherapies, RT, and NFT). The estimated 5-year OS and RFS rates were, respectively, 95.1 and 90.2% in the TP group, 90.0 and 90.0% in the other chemotherapy group, 78.9 and 75.3% in the RT group, and 100 and 100% in the NFT group. No significant differences in OS and RFS were observed between the TP and other chemotherapy groups (OS, P = 0.81; RFS, P = 0.96), between the TP and RT groups (OS, P = 0.058; RFS, P = 0.053), and between the TP and NFT groups (OS, P = 0.53; RFS, P = 0.48) (Fig. 1a and b). Similar results were observed in the analysis of patients who displayed intermediate-risk factors (TP vs. other chemotherapy, OS, P = 0.53, RFS, P = 0.49; TP vs. RT, OS, P = 0.34, RFS, P = 0.70; TP vs. NFT, OS, P = 0.52, RFS, P = 0.44) (Fig. 1c and d). However, when analyzing only patients who displayed high-risk factors (Fig. 1e and f), both OS and RFS were significantly shorter in the RT group compared to the TP group (OS, P = 0.003; RFS, P = 0.006). No significant difference in survival was observed between the TP group and the other chemotherapy group (OS, P = 0.47; RFS, P = 0.38) (Fig. 1e and f). Table 2 shows univariate and multivariate analysis, investigating prognostic factors for survival in the high-risk patients. The univariate analysis identified surgical margin and modality of adjuvant therapy (TP vs. non-TP therapy [including RT and other chemotherapies]) as statistically significant variables for both OS and RFS. On the multivariate analysis, non-TP adjuvant therapy was independently associated with shorter OS and RFS (Table 2).
Survival curves in patients who received radical hysterectomy and displayed surgical–pathological risk factors using 4 subgroups according to the postoperative adjuvant therapy. a Overall survival (OS) in all patients. b Recurrence-free survival (RFS) in all patients. In the analysis of all patients, no significant difference of OS and RFS was observed in the subgroups. c OS in patients with intermediate-risk factor. d RFS in patients with intermediate-risk factor. In the analysis of patients with intermediate-risk factor, no significant difference of OS and RFS was observed in the subgroups. e OS in patients with high-risk factor. f RFS in patients with high-risk factor. In the analysis of patients with high-risk factor, both OS and RFS were significantly shorter in the RT group than TP group. No significant difference was observed between the TP and other chemotherapy groups. TP taxane/platinum, RT radiotherapy, NFT no further therapy
Prognostic factors for survival in patients who received postoperative TP
To investigate prognostic factors for survival in patients who received adjuvant TP, survival analyses were performed in the TP group (Fig. 2). OS and RFS were not significantly different between the intermediate- and high-risk patients (estimated 5-year OS rate 94.2% vs. 94.6%, hazard ratio [HR] 1.03, 95% confidence interval [CI] 0.23–4.62, P = 0.85; estimated 5-year RFS rate 88.5% vs. 89.2%, HR 1.14, 95% CI 0.34–3.76, P = 0.48) (Fig. 2a and b). As shown in Table 3, on univariate analysis in the TP group, a positive surgical margin and non-SCC histology were significantly associated with shorter OS and RFS. In a multivariate model, a positive surgical margin remained significantly correlated with shorter OS and RFS.
Pattern of recurrence
As shown in Table 4, recurrence was observed in 8 (9.8%) patients in the TP group, 1 (10.0%) patient in the other chemotherapy group, and 6 (24.0%) patients in the RT group. No recurrence was observed in the NFT group. The sites of recurrence are shown in Table 4. Recurrences were considered local if to the pelvis or vagina and distant if to extrapelvic locations. Local recurrence was seen in 2 (2.4%) patients, distant recurrence in 5 (6.1%) patients, and local and distant recurrence in 1(1.2%) patient in the TP group. One (10%) patient had local recurrence in the other chemotherapy group. Local recurrence was seen in 4 (16.0%) patients, distant recurrence in 1 (4.0%) patient, and local and distant recurrence in 1 (4.0%) patient in the RT group. The TP group had a significantly lower local recurrence than the RT group (Fig. 3a) (5-year cumulative recurrence rate 3.7 vs. 21.1%; HR 0.17, 95% CI 0.03–0.90, P = 0.006). Meanwhile, the frequency of distant recurrence in the TP group was similar to that in the RT group (Fig. 3b) (5-year cumulative recurrence rate 7.4 vs. 8.0%; HR 0.87, 95% CI 0.17–4.59, P = 0.87).
Cumulative incidence curves for local (a) and distant pelvic recurrence (b) based on adjuvant treatment types. The TP group had a significantly lower local recurrence than the RT group. Meanwhile, the frequency of distant recurrence in the TP group was similar to that in the RT group. TP taxane/platinum chemotherapy, RT radiotherapy
Surgical–pathological risk factors in patients who developed recurrence are also shown in Table 4; a positive surgical margin was seen in 1 patient in the TP group and 1 patient in the RT group.
Adverse effects of postoperative TP
Overall, postoperative TP was well tolerated. The most frequently observed grade 3–4 hematological toxicity was neutropenia (19 patients, 29.7%). Thirteen (20.3%) patients developed grade 3–4 anemia and 2 (3.1%) patients developed grade 3–4 thrombocytopenia. Bowel obstruction was the only grade 3–4 non-hematological toxicity (grade 3 in 1 patient, 1.6%).
Discussion
The current study demonstrated that postoperative adjuvant TP chemotherapy improved the survival outcome for patients with FIGO IB–IIB stage cervical cancer who had been treated with radical hysterectomy.
Based on the results of recent studies investigating the efficacy of systemic chemotherapy as an adjuvant treatment for early stage cervical cancer, the survival outcome for patients who received adjuvant chemotherapy is similar to that of patients who underwent adjuvant RT or concurrent chemoradiotherapy (CCRT) [28, 31, 33, 34]. A large-scale retrospective study, in which 1074 patients with node-positive stage IB–IIB cervical cancer who underwent radical hysterectomy received postoperative chemotherapy, RT, or CCRT, showed that those who received postoperative chemotherapy exhibited similar survival outcomes to those who received CCRT [36]. Other studies also reported that patients receiving adjuvant chemotherapy had an equivalent survival outcome to those receiving adjuvant RT or CCRT (3-year OS 100% [33], 5-year OS 86.5% [31], 5-year OS 95.5% [37], 4-year OS 76.0% [28]). In the current study, stage IB–IIB cervical cancer patients with surgical–pathological risk factors who received postoperative TP had an estimated 5-year OS of 95.1%. These results strongly support the efficacy of adjuvant chemotherapy for patients with FIGO IB–IIB stage cervical cancer who had been treated with radical hysterectomy. Moreover, the long-term good survival outcome and less severe adverse events in the current study indicate that TP chemotherapy has activity and tolerance not only for recurrent or advanced disease but also in the adjuvant setting for cervical cancer.
The pattern of recurrence in patients who underwent radical hysterectomy and received adjuvant chemotherapy has been reported in several studies, showing that adjuvant chemotherapy was effective in regional tumor control as well as distant control [28,29,30,31,32, 34]. In contrast, a recent study reported that the utility of adjuvant chemotherapy was independently associated with decreased distant recurrence, but it was also associated with increased local recurrence compared with adjuvant RT in patients with node-positive cervical cancer [36]. However, a TP regimen was not used as adjuvant chemotherapy in all patients in these studies. In the current study, the TP group showed significantly fewer local recurrence than the RT group. Despite the small sample size, these findings could indicate the efficacy of adjuvant TP on regional tumor control in patients who undergo radical hysterectomy. Recently, neoadjuvant chemotherapy has been tried before surgery in bulky or locally advanced cervical cancer in an attempt to reduce tumor volume. Several studies reported the efficacy of TP in the neoadjuvant setting, where it showed a response rate of 90–95% [38,39,40]. The biological mechanism underlying how TP handle tumor regrowth in the pelvis is unclear. However, these findings indicate that TP may be active in pelvic lesions in cervical cancer.
In previous studies, the 5-year survival of early stage cervical cancer patients who have high-risk factors and received adjuvant CCRT was 71–81% [9, 41, 42]. In the current study, high-risk patients received TP had similar survival outcomes to those with intermediate-risk factors; the estimated 5-year OS and RFS were 94.6 and 89.2%, respectively. Furthermore, in patients who displayed high-risk factors, the multivariate analysis revealed that non-TP adjuvant therapy was independently associated with shorter survival. Our findings may indicate the impact of adjuvant TP chemotherapy on patients with high-risk factors, as well as adjuvant CCRT. However, it is possible that the good outcome in the TP group was not caused by the benefit of adjuvant TP, but by the effect of surgical treatment. In the current study, the median number of resected lymph nodes was 38. Generally, the number of resected lymph nodes in systematic lymphadenectomy for cervical cancer has been reported to be 13 to 56.4 [43]. A significant relationship between the number of resected lymph nodes and survival outcome has also been reported [44]. It is possible that the good outcome in the TP group was mainly because of the quality of surgery in the current study.
Interestingly, a positive surgical margin was the only independent prognostic factor correlated with shorter OS and RFS in patients who received adjuvant TP. To our knowledge, no factor has been detected that is associated with poor survival in patients who received adjuvant chemotherapy following radical hysterectomy. Our finding indicates that surgically treated patients who display a positive surgical margin may need to receive additional treatment, such as CCRT. A recent study reported that paclitaxel/carboplatin-based CCRT followed by paclitaxel/carboplatin-based consolidation chemotherapy was feasible and effective in patients with surgically treated early stage cervical cancer with high-risk factors [45]. The use of paclitaxel/carboplatin concurrently with RT or as consolidation chemotherapy might be effective in this population.
The limitations of the current study need to be addressed. The first is that it involved a relatively small sample size and was retrospective. Potential biases may have influenced the results, such as the heterogeneity of the patient population and selection bias exercised by physicians. Secondly, although the current study showed the promising activity of postoperative TP, it remains uncertain whether patients with intermediate-risk factors could obtain a survival benefit with adjuvant chemotherapy. The GOG92 study, in which adjuvant RT versus no further treatment was tested, showed that adjuvant RT was significantly associated with prolonged RFS [46]. However, the improvement in OS did not reach statistical significance [46]. The results of this study indicate that the role of adjuvant therapy for patients with intermediate-risk factors is still controversial. The role of adjuvant RT/CCRT in intermediate-risk patients is currently being evaluated in an international phase III randomized trial (GOG263) [47].
In summary, postoperative TP could be an alternative adjuvant treatment for patients with FIGO IB–IIB stage cervical cancer who are treated with radical hysterectomy. Future randomized trials are needed to verify the efficacy of this treatment.
References
Organization WH World Health Organization. http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed 24 May 2017
Cancer Research UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer. Accessed 28 May 2017
National Cancer Institute. Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/statfacts/html/cervix.html. Accessed 25 May 2017
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, Cervical Cancer. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed 30 May 2017
Peters WA 3rd, Liu PY, Barrett RJ 2nd et al (2000) Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18(8):1606–1613
Delgado G, Bundy B, Zaino R et al (1990) Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 38(3):352–357
Estape RE, Angioli R, Madrigal M et al (1998) Close vaginal margins as a prognostic factor after radical hysterectomy. Gynecol Oncol 68(3):229–232
Sedlis A, Bundy BN, Rotman MZ et al (1999) A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol 73(2):177–183
Monk BJ, Wang J, Im S et al (2005) Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol 96(3):721–728
Fuller AF Jr, Elliott N, Kosloff C et al (1989) Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage IB and IIA carcinoma of the cervix. Gynecol Oncol 33(1):34–39
Soisson AP, Soper JT, Clarke-Pearson DL et al (1990) Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol 37(3):390–395
Stock RG, Chen AS, Flickinger JC et al (1995) Node-positive cervical cancer: impact of pelvic irradiation and patterns of failure. Int J Radiat Oncol Biol Phys 31(1):31–36
Sevin BU, Lu Y, Bloch DA et al (1996) Surgically defined prognostic parameters in patients with early cervical carcinoma. A multivariate survival tree analysis. Cancer 78(7):1438–1446
Snijders-Keilholz A, Hellebrekers BW, Zwinderman AH et al (1999) Adjuvant radiotherapy following radical hysterectomy for patients with early-stage cervical carcinoma (1984–1996). Radiother Oncol 51(2):161–167
Marchiole P, Buenerd A, Benchaib M et al (2005) Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgico-pathological study. Gynecol Oncol 97(3):727–732
Van de Putte G, Lie AK, Vach W et al (2005) Risk grouping in stage IB squamous cell cervical carcinoma. Gynecol Oncol 99(1):106–112
Chernofsky MR, Felix JC, Muderspach LI et al (2006) Influence of quantity of lymph vascular space invasion on time to recurrence in women with early-stage squamous cancer of the cervix. Gynecol Oncol 100(2):288–293
Ebina Y, Yaegashi N, Katabuchi H et al (2015) Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol 20(2):240–248
Colombo N, Carinelli S, Colombo A et al (2012) Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(7):vii27–vii32
Beckmann MW, Mallmann P, Uterus Commission of the Gynecological Oncology Working G (2009) Interdisciplinary S2k guideline on the diagnosis and treatment of cervical carcinoma. J Cancer Res Clin Oncol 135(9):1197–1206
Landoni F, Maneo A, Colombo A et al (1997) Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 350(9077):535–540
Ryu SY, Park SI, Nam BH et al (2011) Is adjuvant chemoradiotherapy overtreatment in cervical cancer patients with intermediate risk factors? Int J Radiat Oncol Biol Phys 79(3):794–799
Mabuchi S, Okazawa M, Isohashi F et al (2011) Radical hysterectomy with adjuvant radiotherapy versus definitive radiotherapy alone for FIGO stage IIB cervical cancer. Gynecol Oncol 123(2):241–247
Undurraga M, Loubeyre P, Dubuisson JB et al (2010) Early-stage cervical cancer: is surgery better than radiotherapy? Expert Rev Anticancer Ther 10(3):451–460
Tewari KS, Sill MW, Long HJ 3rd et al (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370(8):734–743
Monk BJ, Sill MW, McMeekin DS et al (2009) Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 27(28):4649–4655
Kitagawa R, Katsumata N, Shibata T et al (2015) Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol 33(19):2129–2135
Takekuma M, Kasamatsu Y, Kado N et al (2016) Adjuvant chemotherapy versus concurrent chemoradiotherapy for high-risk cervical cancer after radical hysterectomy and systematic lymphadenectomy. Int J Clin Oncol 21(4):741–747
Lee KB, Lee JM, Ki KD et al (2008) Comparison of adjuvant chemotherapy and radiation in patients with intermediate risk factors after radical surgery in FIGO stage IB-IIA cervical cancer. Int J Gynecol Cancer 18(5):1027–1031
Hosaka M, Watari H, Kato T et al (2012) Clinical efficacy of paclitaxel/cisplatin as an adjuvant chemotherapy for patients with cervical cancer who underwent radical hysterectomy and systematic lymphadenectomy. J Surg Oncol 105(6):612–616
Li S, Hu T, Chen Y et al (2013) Adjuvant chemotherapy, a valuable alternative option in selected patients with cervical cancer. PLoS One 8(9):e73837
Takeshima N, Umayahara K, Fujiwara K et al (2006) Treatment results of adjuvant chemotherapy after radical hysterectomy for intermediate- and high-risk stage IB-IIA cervical cancer. Gynecol Oncol 103(2):618–622
Hosaka M, Watari H, Takeda M et al (2008) Treatment of cervical cancer with adjuvant chemotherapy versus adjuvant radiotherapy after radical hysterectomy and systematic lymphadenectomy. J Obstet Gynaecol Res 34(4):552–556
Jung PS, Kim DY, Lee SW et al (2015) Clinical role of adjuvant chemotherapy after radical hysterectomy for FIGO stage IB-IIA cervical cancer: comparison with adjuvant RT/CCRT using inverse-probability-of-treatment weighting. PLoS One 10(7):e0132298
Querleu D, Morrow CP (2008) Classification of radical hysterectomy. Lancet Oncol 9(3):297–303
Matsuo K, Shimada M, Aoki Y et al (2017) Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: systemic chemotherapy versus pelvic irradiation. Int J Cancer 141(5):1042–1051
Li L, Song X, Liu R et al (2016) Chemotherapy versus radiotherapy for FIGO stages IB1 and IIA1 cervical carcinoma patients with postoperative isolated deep stromal invasion: a retrospective study. BMC Cancer 16:403
Duenas-Gonzalez A, Lopez-Graniel C, Gonzalez-Enciso A et al (2003) A phase II study of multimodality treatment for locally advanced cervical cancer: neoadjuvant carboplatin and paclitaxel followed by radical hysterectomy and adjuvant cisplatin chemoradiation. Ann Oncol 14(8):1278–1284
Park DC, Kim JH, Lew YO et al (2004) Phase II trial of neoadjuvant paclitaxel and cisplatin in uterine cervical cancer. Gynecol Oncol 92(1):59–63
Tanioka M, Yamaguchi S, Shimada M et al (2017) Cisplatin with dose-dense paclitaxel before and after radical hysterectomy for locally advanced cervical cancer: a prospective multicenter phase II trial with a dose-finding study. Med Oncol 34(8):134
Okazawa M, Mabuchi S, Isohashi F et al (2013) Impact of the addition of concurrent chemotherapy to pelvic radiotherapy in surgically treated stage IB1-IIB cervical cancer patients with intermediate-risk or high-risk factors: a 13-year experience. Int J Gynecol Cancer 23(3):567–575
Mabuchi S, Okazawa M, Isohashi F et al (2011) Postoperative whole pelvic radiotherapy plus concurrent chemotherapy versus extended-field irradiation for early-stage cervical cancer patients with multiple pelvic lymph node metastases. Gynecol Oncol 120(1):94–100
Sakuragi N (2007) Up-to-date management of lymph node metastasis and the role of tailored lymphadenectomy in cervical cancer. Int J Clin Oncol 12(3):165–175
Pieterse QD, Kenter GG, Gaarenstroom KN et al (2007) The number of pelvic lymph nodes in the quality control and prognosis of radical hysterectomy for the treatment of cervical cancer. Eur J Surg Oncol 33(2):216–221
Mabuchi S, Isohashi F, Yokoi T et al (2016) A phase II study of postoperative concurrent carboplatin and paclitaxel combined with intensity-modulated pelvic radiotherapy followed by consolidation chemotherapy in surgically treated cervical cancer patients with positive pelvic lymph nodes. Gynecol Oncol 141(2):240–246
Rotman M, Sedlis A, Piedmonte MR et al (2006) A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 65(1):169–176
NCT01101451. https://clinicaltrials.gov/ct2/show/NCT01101451. Accessed 11 June 2017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2018_1249_MOESM1_ESM.pdf
Supplemental Figure 1. Study selection schema. RT, radiotherapy; PALN, para-aortic lymph node. Supplemental Figure 2. Survival curves in 122 patients who were treated with radical hysterectomy and displayed surgical-pathological risk factors. A, overall survival (OS). B, recurrence-free survival (RFS). The estimated 5-year OS and RFS rates were 91.7% and 87.5%, respectively (PDF 73 kb)
About this article
Cite this article
Okazawa-Sakai, M., Yokoyama, T., Fujimoto, E. et al. Long-term outcomes of postoperative taxane/platinum chemotherapy for early stage cervical cancer: a retrospective study. Int J Clin Oncol 23, 715–725 (2018). https://doi.org/10.1007/s10147-018-1249-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1249-8