Abstract
Background
The present study summarizes the results of treatment in the form of disease-free survival and overall survival in bulky stage IB2 and locally advanced (stages II–IVA) squamous cell carcinoma of the uterine cervix. The treatment has been given in the form of NACT followed by CCRT in one arm and CCRT in the other arm.
Materials and Methods
This retrospective study analyzed 713 cervical cancer patients who were treated at our center during 2007 and 2008; out of 713 patients, data of 612 patients have been compared. The patients' data were analyzed retrospectively. Patients had undergone PF 28.6 %, TPF 21.5 %, and only CCRT 49.9 %. Majority of patients were in the age group 41–50 years, while stage wise, mainly stage IIIb and IIb. Disease-free survival was observed on the basis of stage and NACT. The survival analyses were performed using the Kaplan–Meier method. All statistical calculations were done with SPSS Statistics version 20.0.
Results
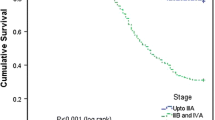
For cancer cervix NACT versus CCRT, the DFS rate was at 5 years (58.3 vs. 41.8 % p = 0.001). NACT followed by CCRT demonstrated significantly superior DFS as compared to definitive CCRT, respectively, TPF (hazard ratio (HR) = 0.248, 95 % confidence interval (CI) 0.123–0.500; p < 0.001), PF (HR = 0.445, 95 % CI 0.266–0.722; p = 0.002). The results of univariate stage, age, and multivariate study show that stage hemoglobin level, interval between external-intracavitary radiation, and type of neoadjuvant chemotherapy were the factors affected survival cervical patients treated with radiation. The grade 3/4 hematologic toxicities were more in the NACT group than CCRT (p < 0.001) while the non-hematological toxicity was not significant; the TPF group experienced more toxicity than PF (p = 0.029). This treatment regimen is feasible as evidenced by the acceptable toxicity of NACT and by the high compliance to radiotherapy. The grade 3/4 hematologic toxicities were more in NACT groups than CCRT (p < 0.001); the TPF group experienced more toxicity than PF (p = 0.029).
Conclusion
TPF/PF as NACT is feasible and produces impressive responses in cancer cervix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the fifth most common cancer in humans, the second most common cancer in women worldwide and the most common cancer cause of death in the developing countries. The worldwide incidence of cervical cancer is approximately 510,000 new cases annually, with approximately 288,000 deaths worldwide. [1] Cervical cancer is the second most common female cancer in women aged 15–44 years in India. 67,477 new cervical cancer deaths occur annually in India. India has a population of approximately 365.71 million women above 15 years of age, who are at risk of developing cervical cancer. The median age at first sexual intercourse for women is 25–49 years and for men it is 25–54 years. The current estimates indicate approximately 132,000 new cases diagnosed and 74,000 deaths annually in India, accounting to nearly one-third of the global cervical cancer deaths. [2] Indian women face a 2.5 % cumulative lifetime risk and 1.4 % cumulative death risk from cervical cancer. In India approximately 80 % present with locally advanced disease (FIGO IIB-IVA) due to lack of screening. [3] Our institute is a busy regional cancer institute that has registered 55,242 cases in last decade. Due to long waiting over the radiotherapy machines, often giving anterior chemotherapy is the only available option. The 5-year survival rate with radiotherapy (RT) is about 60 % in stage IIB, 30–35 % in Stage IIIB, and less than 15 % in stage IVA disease. Failure is accounted by local recurrence in 40–70 % and distant failure in about 20–25 % patients. [4] The large volume of primary tumor and presence of micrometastatic disease at diagnosis are important reasons for RT treatment failure. Following National Cancer Institute alert concurrent chemoradiotherapy (CCRT) became the standard of care which was proven to have benefit in subsequent meta-analyses also advanced cervical carcinoma at present. [5–7] The present study was conducted with the primary aim to compare the disease-free survival to neoadjuvant chemotherapy followed by CCRT and CCRT alone in bulky stage I (IB2) and locally advanced (stages II–IVA) squamous cell carcinoma of the uterine cervix. The secondary aim was to compare the toxicities of the treatment in the two arms (Figs. 1, 2).
Materials and Methods
During the period from 2007 to 2008, 723 patients of cervical cancer were analyzed retrospectively. The patients whose detailed records were available for analysis were included in the study. Disease-free survival (DFS) was calculated on the basis of stage and NACT. The survival analyses were performed using the Kaplan–Meier method, and Cox Regression analysis was used to calculate hazards ratio. All statistical calculations were performed with SPSS Statistics version 20.0. The NACT was given as injection cisplatin 40 mg/m2 (d 1, 2), paclitaxel 175 mg/m2 (d 1), and 5-fluorouracil in 750 mg/m2 (d 1, 2, 3) in TPF arm while in PF arm injection cisplatin 40 mg/m2 (d 1, 2) and 5-fluorouracil in 750 mg/m2 (d 1, 2, 3). Chemotherapy was repeated after 3 weeks after checking complete blood count, liver function test, renal function test done before every cycle of chemotherapy. Two cycles of NACT were given. The chemotherapy was administered on an outpatient basis in a day care room. CTCAE toxicity criteria were used for monitoring and documentation of hematological toxicities. RT was started around third week of completing the second cycle of chemotherapy. CCRT treatment was same in both arm NACT and CCRT-alone arm. External beam radiation therapy was administered using cobalt 60 teletherapy machine. A dose of 50 Gy in 25 fractions in 5 weeks was given at a dose of 200 centi gray per fraction daily, for 5 days in a week. After a gap of 1 to 2 weeks, patients were reassessed for response and patient with good local response and preserved local anatomy were subjected to intracavitary brachytherapy using HDR system, Ir192 based, giving a separate dose for separate stages in fractionated schedules to point A. The RTOG toxicity criterion was used for radiation induced toxicities. [8] Patients were followed in an outpatient clinic monthly during first year, every 2 months during second year and every 3 months during third year. The patients were finally assessed at 36 months from the start of study. Tumor recurrence was defined as the presence of biopsy proven cancer 3 months after completion of RT. Patients with progressive/recurrent disease were offered palliative CT or symptomatic treatment depending on the ECOG performance status. The patients with isolated recurrent disease were offered salvage surgical intervention (Tables 1, 2, 3).
Results
Of the 713 patients, retrospective data analysis of 612 patients belonged to FIGO stage IB2 (4 %), IIA (6.8 %), IIB (19.6 %), IIIA (7.5 %), IIIB (53.3 %), IVA (4.3 %), and 101 patients lost to treatment follow-up. In each arm, patients were, respectively, in PF 28.6 %, TPF 21.5 %, and only CCRT 49.9 %. The majority of patients were in the age group 41–50 years, while stage wise, of stage IIIB (53.3 %) and IIB (21.6 %). In our patient cohort, the DFS for NACT followed by CCRT was 58.3 % as against 41.8 % for CCRT alone (p = 0.001). Considering as univariate analysis, NACT followed by CCRT demonstrated significantly superior DFS as compared to definitive CCRT, respectively, TPF [hazards ratio (HR) = 0.248, 95 % confidence interval (CI) 0.123–0.500; p < 0.001], PF (HR = 0.445, 95 % CI 0.266–0.722; p = 0.002). Median OS was 48.00 months in CCRT while in NACT it was 52.00 months. Thus, the OS was superior by 8.34 % in the NACT arm (p value 0.689). The multivariate analyses suggest, the factors that were statistically affected survival of cervical cancer included hemoglobin level (p value < 0.016) and interval between external beam radiation and intracavitary radiation (p value < 0.028). Patients with low hemoglobin level (≤8 g/dl) were associated with 1.58-fold mortality risk compared with patients who had level >10 g/dl (95 % CI 1.04–2.06). The interval between external and intracavitary radiation >4 weeks was associated with 1.96-fold mortality risk compared with patients who had duration ≤1 week (95 % CI 1.48–2.34). The results of univariate stage, age, and multivariate study show that stage hemoglobin level, interval between external-intracavitary radiation, and type of neoadjuvant chemotherapy were the factors affected survival cervical patients treated with radiation. The grade 3/4 hematologic toxicities were more in the NACT group than CCRT (p < 0.001) while the nonhematological toxicity was not significant; the TPF group experienced more toxicity than PF (p = 0.029). This treatment regimen is feasible as evidenced by the acceptable toxicity of NACT and by the high compliance to radiotherapy. In addition, patients between 41–50 years of age responded better with NACT arm (median DFS 51.00 months) versus CCRT (median DFS 45.00 months), (HR = 1.48, 95 % CI 1.19–1.85; p < 0.001). The chemotherapy was well tolerated. There was no evidence that CT enhanced the acute and late side effects of subsequent RT.
Discussion
The primary objectives of NACT in the treatment of cervical cancer include improvement in tumor characteristics along with CCRT prolonged disease-free survival. The impact on survival of this relatively new approach is still a matter of discussion, and different treatment strategies may be considered. Some studies have remarked that NACT followed by RT has yielded neither higher response rates nor longer survival, probably due to selective resistance to radiation after chemotherapy. Zanetta et al. [9] have concluded that the response to NACT may perform as an important prognostic factor, guiding the direction to subsequent therapy. [10] The response to NACT identifies few patients who are destined to fare better than non-responders but this has been questioned. However, as a group, those receiving NACT have demonstrated improved progression-free and overall survival.
A meta-analysis by Tierney et al. was based on 2074 patients from 18 trials; the median follow-up in all trials was 5.7 years for surviving patients. Around 70 % of patients had advanced disease (stage II or III). The study results showed that the addition of NACT to local therapy (mainly radiotherapy) did not have any impact on overall survival (HR = 1.05; 95 % CI 0.94–1.19), disease-free survival (HR = 1.00; 95 % CI 0.88–1.14), or loco-regional disease-free survival (HR = 1.03; 95 % CI 0.9–1.17). However, a highly significant level of statistical heterogeneity was evident for each of the outcomes measured; viz. p value for survival was 0.0003. It was suggested that chemotherapy may select the radioresistant cellular clones due to cross-resistance between certain chemotherapy agents and radiotherapy. Finally, NACT may optimize a patient’s pathologic risk factors, introducing the option of fertility-sparing treatment to a patient who would otherwise not be a candidate. In this setting, NACT offers benefits other than an equivalent oncologic outcome. In our study, the DFS in NACT group for stage IIIB is significantly improved compared to the CCRT-alone group with acceptable toxicity. Significantly survival benefit was due to maximum number of patients in stage IIIB. The stage of the disease and extent of parametrial involvement had superior response to NACT arm. Tumor size and parametrial involvement have been reported to be important predictors of CT response in earlier studies. [11–13] Also the patients between 41–50 years of age responded better with NACT. According to McCormack, the overall and progression-free survivals at 3 years were 67 % (95 % CI 51–79) and 68 % (95 % CI 51–79), respectively, after use of weekly NCAT for 6 weeks. The Grade 3 and 4 toxicities were 20 % during NACT—11 % were hematological and 9 % were non-hematological—and 52 % during CRT—41 % were hematological and 22 % were non-hematological [14].
Conclusion
TPF/PF as NACT is feasible and produces significantly better responses in our study. This study suggests that TPF regimen is better than PF. TPF can be used as an optional NACT, but the hematological toxicity is more in taxane-based regimen. Combination neoadjuvant chemotherapy with paclitaxel and cisplatin may improve long-term survival of patients with cervical cancer. The multivariate study results show that hemoglobin level, interval between external-intracavitary radiation, and type of neoadjuvant chemotherapy were the factors also affected survival in cervical patients treated with radiation. As our study is a retrospective study, more prospective randomized studies are required to confirm the results.
Abbreviations
- CCRT:
-
Concurrent chemo-radiotherapy
- CI:
-
Confidence interval
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- DFS:
-
Disease-free survival
- ECOG:
-
Eastern Cooperative Oncology Group
- FIGO:
-
Federation of Gynecology and Obstetrics
- HR:
-
Hazards ratio
- NACT:
-
Neo-adjuvant chemotherapy
- PF:
-
Platin/5-FU
- RT:
-
Radiotherapy
- RTOG:
-
Radiation Therapy Oncology Group
- TPF:
-
Taxol/Platin/5-FU
References
Sankaranarayanan R, Ferlay J. Worldwide burden of gynecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–25.
WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre): Summary report on HPV and cervical cancer statistics in India 2007. http://www.who.int/hpvcentre. Assessed 1 May 2008.
FIGO staging for cancer cervix uteri. UICC manual for classification of malignant tumors. Berlin: Springer; 1987.
Woo YJ, Byun JM, Jeong DH, et al. Prognosis of stage IIb cervical cancer among treatment regimens: radical hysterectomy versus neoadjuvant chemotherapy followed by radical hysterectomy versus concurrent chemoradiotherapy. Korean J Obstet Gynecol. 2012;55:913–9.
NCI Issues Clinical Announcement on Cervical Cancer, Chemotherapy Plus Radiation Improves Survival: http://www.nih.gov/news/pr/feb99/nci-22.htm.
Lukka H, Hirte H, Fyles A, et al. Concurrent cisplatin-based chemotherapy plus RT for cervical cancer: a meta-analysis. Clin Oncol. 2002;14:203–12.
Vikas Fotedar V, Seam RK, Gupta MK, et al. Neoadjuvant chemotherapy followed by radiotherapy versus radiotherapy alone in locally advanced carcinoma cervix: a prospective randomized study. J Dental Med Sci. 2013;4:58–63.
Saha A, Mukherjee A. Role of neoadjuvant chemotherapy in cancer cervix: a brief review. Clin Cancer Investig J. 2013;2:281–6.
Turan T, Yıldırım BA, Tulunay G, et al. Experience in stage IB2 cervical cancer and review of treatment. J Ger Gynecol Assoc. 2010;11:27–37.
Zanetta G, Lissoni A, Pellegrino A, et al. Neoadjuvant chemotherapy in locally advanced uterine cervical cancer: correlation between pathological response and survival. Proc Am Soc Clin Oncol. 1998;17:352–5.
Taneja A, Rajaram S, Agarwal S, et al. “Quick Cycle” neoadjuvant chemotherapy in squamous cell carcinoma of cervix. Indian J Pharmacol. 2005;37:320–4.
Sardi JE, Giaroli A, Sananes C, et al. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: the final results. Gynecol Oncol. 1997;67:61–9.
Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Metaanalysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer. 2003;39:2470–86.
McCormack M, Kadalayil L, Hackshaw A, et al. A phase II study of weekly neoadjuvant chemotherapy followed by radical chemoradiation for locally advanced cervical cancer. British J of Cancer. 2013;108:2464–9.
Acknowledgments
The authors would like to thank the Department of Oncology. The authors also express their gratitude to Teachers and PG Students of the department: Dr. Saroj, Dr. Kamlesh Harsh, Dr. Sitaram, Dr. Raj K Nirban, Dr. Parmilla Khatri, Dr. Guman Singh, Dr. Murali, Dr. Tanya, and Dr. Rajesh.
Compliance with ethical requirements and Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper. The research is independent and impartial. The author(s) declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Satya Narayan is a Resident at Acharya Tulsi Regional Cancer Treatment and Research Institute; Akhil Kapoor is a Resident at Acharya Tulsi Regional Cancer Treatment and Research Institute; Mukesh Singhal is a Resident at Acharya Tulsi Regional Cancer Treatment and Research Institute; Ramesh Purohit is a Resident at Acharya Tulsi Regional Cancer Treatment and Research Institute; Neeti Sharma is an Assosiate Professor at Acharya Tulsi Regional Cancer Treatment and Research Institute; Rajani Sharma is a Health Professional at PBM Hospital; Narendra Kumar is an MBBS Student at Sardar Patel Medical College; Shankar Lal Jakhar is an Assistant Professor at Acharya Tulsi Regional Cancer Treatment and Research Institute; Surendra Beniwal is an Assistant Professor Acharya Tulsi Regional Cancer Treatment and Research Institute; Harvindra Singh Kumar is a Senior Professor at Acharya Tulsi Regional Cancer Treatment and Research Institute; and Ajay Sharma is a Senior Professor at Acharya Tulsi Regional Cancer Treatment and Research Institute.
Rights and permissions
About this article
Cite this article
Narayan, S., Sharma, N., Kapoor, A. et al. Pros and Cons of Adding of Neoadjuvant Chemotherapy to Standard Concurrent Chemoradiotherapy in Cervical Cancer: A Regional Cancer Center Experience. J Obstet Gynecol India 66, 385–390 (2016). https://doi.org/10.1007/s13224-015-0698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-015-0698-5