Abstract
Background
Anthracyclines are used to treat childhood acute lymphoblastic leukemia (ALL). Even when administered at low doses, these agents are reported to cause progressive cardiac dysfunction. We conducted a clinical trial comparing the toxicities of two anthracyclines, pirarubicin (THP) and daunorubicin (DNR), in the treatment of childhood ALL. The results from our study that relate to acute and late toxicities are reported here.
Methods
276 children with B-ALL were enrolled in the trial from April 1997 to March 2002 and were randomly assigned to receive a regimen including either THP (25 mg/m2 × 11) or DNR (30 mg/m2 × 11). Acute toxicity was prospectively assessed based on the National Cancer Institute Common Toxicity Criteria. Acute hematological toxicity was also examined via some parameters. Patients with event-free survival of >5 years were retrospectively surveyed for cardiac function at 5 and 10 years and at the most recent assessment more than 10 years from the onset of ALL.
Results
Acute hematological toxicity in the early phase was more significant in the THP arm. Based on ultrasound cardiography, cardiac function was impaired in both groups during the follow-up period, but there was no significant difference between the groups except for a greater decline in fractional shortening on ultrasound cardiography in the DNR arm.
Conclusions
While acute hematological toxicity was more significant in the THP arm, THP also appeared to be less cardiotoxic. However, the evaluation of late cardiotoxicity was limited because only a few subjects were followed beyond 10 years after ALL onset. Considering that the THP regimen produced an EFS rate comparable with that of the DNR regimen, the efficacy and toxicity of THP at reduced doses should be studied in order to identify potentially safer regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The outcome of acute lymphoblastic leukemia (ALL) in childhood has improved markedly over the last five decades, largely due to the evolution of chemotherapy and stratification of treatment intensity [1]. Anthracyclines are the agents used to treat childhood ALL [2]; however, late cardiotoxicity following anthracyclines is common and sometimes progressive [3]. Children receiving lower doses of these agents are reported to have subclinical abnormalities in myocardial function later in life, even when overt cardiac symptoms are absent [4]. Consequently, late cardiac dysfunction induced by anthracyclines is now a major concern in the long-term follow-up of childhood cancer survivors, which has led to several efforts to reduce cardiotoxicity. These include administration by prolonged infusion to lower the peak level, the use of liposomal preparations to deliver the drug more effectively to tumors, and co-administration of cardioprotective agents such as dexrazoxane [5–7]. New analogues with improved therapeutic indices are also being developed. 4′-O-tetrahydropyranyl doxorubicin (pirarubicin, THP) is a synthetic analogue of doxorubicin (ADR) containing a tetrahydropyranyl group at the 4′-position of the amino sugar. This agent was discovered and developed in Japan [8]. Compared to ADR, THP accumulates in tumor cells more rapidly and inhibits DNA and RNA synthesis at lower concentrations [9]. In animal models, THP has been reported to exert higher antitumor activity and lower cardiotoxicity than other anthracyclines [10–12]. Furthermore, Takagi and Oguro demonstrated that THP was effective for lymphoid malignancies in adults [13]. THP has been used in the front-line treatment of childhood ALL in Japan [14, 15], although DNR and ADR are being routinely used in other countries.
THP may be highly advantageous for treating children with cancer, but its clinical efficacy and safety have not been evaluated with well-designed clinical trials, especially in childhood ALL. The Japan Association of Childhood Leukemia Study (JACLS) conducted a randomized controlled trial of THP vs daunorubicin (DNR) in the treatment of childhood ALL to assess the efficacy and acute toxicity of each drug. In addition, we retrospectively conducted an observational study of late cardiac toxicity among long-term survivors in the trial.
Patients and methods
Patients
Between April 1997 and March 2002, we enrolled 286 consecutive children and adolescents aged 1–15 years with newly diagnosed B-ALL in a randomized controlled trial of two anthracyclines, DNR and THP. Patients were included if they were stratified into intermediate (IR) or high (HR) risk categories according to the ALL-97 study of JACLS [16]. IR was defined as patients aged 1–9 years with white blood cell (WBC) counts at diagnosis of between 10,000 and 49,999/µl, while HR included patients aged 1–9 years with WBC counts at diagnosis of between 50,000 and 99,999/µl, or those more than 10 years old with WBC counts at diagnosis of less than 50,000/µl. Standard-risk (SR) patients (aged between 1 and 9 years and with WBC counts at diagnosis of less than 10,000/µl) with the following findings were regarded as IR: t (1,19), splenomegaly beyond the umbilicus or lymph node enlargement >3 cm in diameter. SR and IR patients with central nervous system (CNS) involvement were regarded as HR. Among patients initially stratified into IR or HR, those showing M2/M3 marrow on day 14 were treated with an extremely high-risk (ER) protocol after induction therapy and excluded from the evaluations in the following phases. Patients with the Philadelphia chromosome or chromosome 11q23 translocation were not included in IR/HR. Mature B-cell leukemia patients were not included in this study.
Institutional review boards at all participating institutions approved the study, and each patient or guardian provided informed consent before the initiation of treatment.
Treatment
Details of the treatment regimens are shown in Table 1. Both IR and HR patients received the same regimen except for cranial irradiation in HR from the 52nd to the 54th week. Two doses of triple intrathecal chemotherapy were added in HR patients during cranial irradiation. The regimens comprised induction, consolidation, sanctuary, re-induction, and maintenance therapies. Total treatment duration was 98 weeks, consisting of a 20-week early phase and a 78-week maintenance phase. The patients were assigned to receive a total of 11 doses (2 doses in induction, 2 in consolidation, 2 in re-induction, and 5 in maintenance therapies) of DNR at 30 mg/m2 or THP at 25 mg/m2. Previously, THP was used at a dose of 20–30 mg/m2 in treatment regimens for ALL [14, 15]. In this study, we selected a dose of 25 mg/m2 for THP. Cumulative doses of the anthracyclines were 330 mg/m2 in the DNR arm and 275 mg/m2 in the THP arm.
Evaluation
The primary endpoint of the randomized controlled trial was the event-free survival (EFS) rate, while the secondary endpoints were the overall survival rate and the incidence of acute toxicity. Patient response and evidence of acute toxicity were closely monitored at each center during the treatment. Acute toxicity in each treatment phase was evaluated with the WHO grading system until June 2003, and then with the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0. Toxicities graded by the WHO system were regraded according to the NCI-CTC. Acute hematological toxicity in each treatment phase was evaluated for the following variables: days of fever over 38 °C, neutrophil count less than 500/µl, administration of granulocyte-colony-stimulating factor, and the number of platelet or packed red blood cell transfusions. Patients with EFS exceeding 5 years were retrospectively surveyed for cardiac function at 5 and 10 years and at the latest assessment beyond 10 years from the onset of ALL in an additional observation study. To determine EFS, we used the database of the JACLS data center, which was last updated in 2013. Patients who had any event or loss to follow-up after 5 years of follow-up were excluded from the survey at the event or the loss. The survey included the occurrence of any cardiac symptoms, brain natriuretic peptide (BNP) level in serum, corrected QT interval (QTc) on electrocardiogram (ECG), or fractional shortening (FS) and ejection fraction (EF) of the left ventricule measured with ultrasound cardiography (UCG). The survey was conducted in 2011 and updated in 2015 by collecting the data measured at each institute.
Statistics
Because this trial was our first collaborative study involving four regional groups, the assignment to treatment groups was randomized using a minimization procedure stratified by age range and region for each participating regional group.
The chi-square or Fisher’s exact probability test was used for binary data comparisons when analyzing patient characteristics or toxicity.
Student’s t test was used to compare means of the parameters of myelosuppression. Cardiac functions were assessed in each group by comparing the values at each follow-up point with those at onset. Differences were analyzed for statistical significance with the paired t test. All P values were two-sided and results were considered significant if the P value was less than 0.05. These analyses were carried out using STATA ver.13 (STATA Corp., College Station, TX, USA) or GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics
Of the 286 patients, 144 were randomly assigned to the DNR arm and 132 to the THP arm. Three patients were inappropriately stratified to other risk groups and treated with unscheduled protocols. Seven patients refused randomization. Among them, four were treated with the THP regimen and three received the DNR regimen. Six patients assigned to the THP arm and seven to the DNR arm who showed M2 or M3 marrow on day 14 were treated with the ER regimen after induction therapy. One patient in the THP arm was intentionally treated with the SR regimen. Although these 14 patients were analyzed according to the originally assigned arm on an intent-to-treat principle for EFS and OS evaluations, the toxicity assessment was done in the regimens actually implemented for each patient. Median follow-up for the DNR arm was 124 (range 9–191) months, and that for the THP arm was 126 (range 2–186) months. Age at diagnosis, gender ratio, and regimen applied (IR/HR) did not differ significantly between the two groups. The male-to-female ratio was 73/71 in the DNR arm and 70/62 in the THP arm. The mean (SE) of age at diagnosis was 7.04 (0.38) in the DNR arm and 6.31 (0.39) in the THP arm. The number of patients treated with the HR protocol was 68 out of 144 with the DNR regimen and 54 out of 132 with the THP regimen. The number of patients with EFS more than 5 years was 105 in the DNR arm and 99 in the THP arm.
Acute toxicity
Acute hematological toxicity for each treatment phase is shown in Table 2. An anthracycline was included in the induction, consolidation, re-induction, and maintenance therapies, but not in sanctuary therapy. Duration of fever >38 °C was significantly longer in the THP arm than the DNR arm during induction. Significantly more patients in the THP arm had more prolonged neutrocytopenia than those in the DNR arm after the consolidation or re-induction phases. Blood component transfusion was more frequent in the THP arm than in the DNR arm throughout the early phase. There was no significant difference in any variable related to myelotoxicity during the maintenance phase. No other acute toxicities differed significantly between the two groups in any of the therapy phases except for more frequent nausea and vomiting in patients receiving DNR during sanctuary therapy (P < 0.01, Table 3). A total of 8 and 23 severe infections were observed through the entire course of treatment with DNR and THP, respectively (P = 0.02). l-Asparaginase (LASP)-induced pancreatitis was similarly noted (8 patients with DNR and 7 with THP, P = 0.93). Two DNR patients developed acute cardiac complications. One presented with an arrhythmia after the first dose of DNR in induction therapy, and another developed heart failure associated with infection during consolidation therapy.
Late cardiac toxicity
During their long-term follow-up, the subjects were asked to pay yearly visits to their clinics to undergo laboratory tests, but a considerable number of patients were lost to follow-up over the 10 years. No patients with cardiac symptoms were reported in either arm during the follow-up period. Table 4 shows cardiac function parameters during long-term follow-up. In comparison of the two arms, BNP at 10 years was significantly lower in THP-arm.
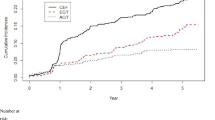
The four parameters evaluated at each follow-up visit were compared with the baseline data at onset. The values of QTc and FS over the 10-year follow-up period were significantly impaired compared to the baseline data in the DNR arm, while QTc was significantly prolonged during the follow-up period in the THP arm (Fig. 1). EF gradually decreased in both groups, but not statistically significantly at any time point. There was no distinctive trend in BNP.
Changes in cardiac function parameters. a Fractional shortening of left ventricle on ultrasound cardiography. b Ejection fraction of left ventricle on ultrasound cardiography. c Corrected QT interval on electrocardiogram. d Brain natriuretic peptide in serum. Each point is a value for a particular subject, and each bar indicates the mean value. Statistical analysis was performed with the paired t test
Discussion
Anthracyclines have been used to treat childhood ALL since the 1960s, most commonly in remission induction therapy as the fourth drug following vincristine, steroid, and LASP. Although the benefit of adding anthracyclines to remission induction therapy is debatable when treating standard-risk patients [17, 18], induction therapy intensified with anthracyclines was reported to produce better outcomes in high-risk patients [19]. These findings may be partially due to the fact that anthracyclines are highly myelotoxic and potentially increase infectious morbidity or mortality during the early stages of treatment. Some major study groups have also used anthracyclines as an agent for delayed intensification [20, 21]. Therefore, this trial was designed for patients who were expected to benefit from anthracyclines.
As outcomes for childhood cancer patients have improved, the late effects of treatment have assumed increased importance in the long-term follow-up. Anthracycline-induced cardiomyopathy is a serious sequela which may impair the quality of life or sometimes cause early death [22, 23]. Late-onset heart failure was reported to occur 4–10 years after the administration of anthracyclines at cumulative doses of 390–450 mg/m2 in children treated for cancer [24]. Lipshlutz et al. reported that increased afterload, decreased contractility, or both were detected in 65% of patients with a mean follow-up of 6.4 years after completing anthracycline therapy for childhood ALL at a cumulative dose of 228–550 mg/m2 [25]. They also indicated that the abnormalities were progressive in 71% of these patients. Recently, it was reported that ALL survivors treated with anthracyclines at cumulative doses below 300 mg/m2 also had some measurable signs of cardiac dysfunction on UCG [4]. The authors expressed their concern that, with prolonged follow-up, even low doses of anthracyclines may cause cardiac dysfunction. Substitution of ADR or DNR with a less cardiotoxic anthracycline should help to reduce cardiac complications. The present trial was conducted to test this hypothesis.
Although THP has been used to treat both adult and childhood leukemia in Japan for more than 20 years [14, 15, 26–28], only a few clinical trials have evaluated the late cardiotoxicity of THP [29]. Moreover, there has been no well-designed clinical trial comparing its efficacy and toxicity with those of other anthracyclines used to treat childhood ALL. Our study was designed as a randomized controlled trial to compare EFS and acute toxicity between the THP and DNR arms. We also performed a longitudinal observational study of long-term cardiac function in these cohorts.
This study proved that the THP regimen produced an EFS comparable to that achieved with the DNR regimen (EFS rate at 10 years was 73.3% for the DNR regimen and 69.5% for the THP regimen, P = 0.60). These results suggest that the antileukemic activity of multiagent combination chemotherapy for childhood ALL is preserved when DNR is substituted with THP. However, myelosuppression was more severe with the THP regimen at the planned dose. This acute toxicity may underlie the treatment-related deaths in two patients treated with the THP regimen. Especially in induction and re-induction therapies, combining THP with DEX can potentially lead to serious infections. Oral mucositis was also found more in patients with THP, and may partially contribute to the infectious complications seen with this anthracycline.
In the survey of long-term cardiac function, it was hard to recruit a sufficient number of subjects to facilitate a valid assessment because a longitudinal survey of the cohorts beyond 10 years after ALL onset was attempted. In addition, the assessment was retrospective and not centralized. Although there are some limitations of this survey, we did find some trends for changes in cardiac parameters among the ALL survivors. Impairment of parameters on UCG or ECG was observed in both arms, while a decrease in FS at assessments performed beyond 10 years after ALL onset was only observed in the DNR arm (P < 0.05). These results suggest a possible association between THP regimen and decreased risk of cardiotoxicity during longer follow-up periods, although we cannot draw any definite conclusion based on such a small sample size.
A recent report indicated that DNR was less cardiotoxic than ADR in survivors of childhood cancer [30]. The DNR to ADR cardiotoxicity equivalence ratio was estimated to be between 0.4 and 0.5 in the report. The ratio of THP to ADR seems to be similar to or less than that of DNR to ADR.
In conclusion, acute bone marrow suppression was more severe in patients on the THP regimen, while this regimen produced an EFS rate comparable to that achieved with the DNR regimen. Based on these observations, future trials should assess the efficacy and toxicity of THP at doses lower than 25 mg/m2 to minimize early complications. If we can reduce the dosage without decreasing the efficacy, a further reduction in the risk of cardiotoxicity may be realized.
References
Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. N Engl J Med 373:1541–1552
Hitchcock-Bryan S, Gelber R, Cassady JR et al (1986) The impact of induction anthracycline on long-term failure-free survival in childhood acute lymphoblastic leukemia. Med Pediatr Oncol 14:211–215
Lipshultz SE, Lipsitz SR, Sallan SE et al (2005) Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 23:2629–2636
Rathe M, Carlsen NL, Oxhøj H (2007) Late cardiac effects of anthracycline containing therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 48:663–667
Lipshultz SE, Giantris AL, Lipsitz SR et al (2002) Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 acute lymphoblastic leukemia protocol. J Clin Oncol 20:1677–1682
Russo D, Piccaluga PP, Michieli M et al (2002) Liposomal daunorubicin (DaunoXome) for treatment of poor-risk acute leukemia. Ann Hematol 81:462–466
Asselin BL, Devidas M, Chen L et al (2016) Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-Cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 34:854–862
Umezawa H, Takahashi Y, Kinoshita M et al (1979) Tetrahydropyranyl derivatives of daunomycin and adriamycin. J Antibiot (Tokyo) 32:1082–1084
Munck JN, Fourcade A, Bennoun M et al (1985) Relationship between the intracellular level and growth inhibition of a new anthracycline 4′O-tetrahydropyranyl-Adriamycin in Friend leukemia cell variants. Leuk Res 9:289–296
Tsuruo T, Iida H, Tsukagoshi S et al (1982) 4′-O-tetrahydropyranyladriamycin as a potential new antitumor agent. Cancer Res 42:1462–1467
Hirano S, Wakazono K, Agata N et al (1994) Comparison of cardiotoxicity of pirarubicin, epirubicin and doxorubicin in the rat. Drugs Exp Clin Res 20:153–160
Koh E, Ueda Y, Nakamura T et al (2002) Apoptosis in young rats with adriamycin-induced cardiomyopathy—comparison with pirarubicin, a new anthracycline derivative. Pediatr Res 51:256–259
Takagi T, Oguro M (1987) (2″-R)-4′-O-tetrahydropyranyladriamycin, a new anthracycline derivative; its effectiveness in lymphoid malignancies. Cancer Chemother Pharmacol 20:151–154
Hara J, Park YD, Yoshioka A et al (2001) Intensification of chemotherapy using block therapies as consolidation and reinduction therapies for acute lymphoblastic leukemia during childhood. Int J Hematol 74:165–172
Igarashi S, Manabe A, Ohara A et al (2005) No advantage of dexamethasone over prednisolone for the outcome of standard- and intermediate-risk childhood acute lymphoblastic leukemia in the Tokyo Children’s Cancer Study Group L95-14 protocol. J Clin Oncol 23:6489–6498
Suzuki N, Yumura-Yagi K, Yoshida M et al (2010) Outcome of childhood acute lymphoblastic leukemia with induction failure treated by the Japan Association of Childhood Leukemia Study (JACLS) ALL F-protocol. Pediatr Blood Cancer 54(1):71–78
Tubergen DG, Gilchrist GS, O’Brien RT et al (1993) Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Childrens Cancer Group phase III trial. J Clin Oncol 11:527–537
Harms DO, Janka-Schaub GE (2000) Co-operative Study Group for Childhood Acute Lymphoblastic Leukemia (COALL): long-term follow-up of trials 82, 85, 89 and 92. Leukemia 14:2234–2239
Gaynon PS, Steinherz PG, Bleyer WA et al (1993) Improved therapy for children with acute lymphoblastic leukemia and unfavorable presenting features: a follow-up report of the Children’s Cancer Group Study CCG-106. J Clin Oncol 11:2234–2242
Nachman JB, Sather HN, Sensel MG et al (1998) Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med 338:1663–1671
Schrappe M, Reiter A, Zimmermann M et al (2000) Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Münster. Leukemia 14:2205–2222
Kaneko S, Tham EB, Haykowsky MJ et al (2016) Impaired left ventricular reserve in childhood cancer survivors treated with anthracycline therapy. Pediatr Blood Cancer 63:1086–1090
Armstrong GT, Ross JD (2014) Late cardiotoxicity in aging adult survivors of childhood cancer. Prog Pediatr Cardiol 36:19–26
Goorin AM, Chauvenet AR, Perez-Atayde AR et al (1990) Initial congestive heart failure, six to ten years after doxorubicin chemotherapy for childhood cancer. J Pediatr 116:144–147
Lipshultz SE, Colan SD, Gelber RD et al (1991) Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 324:808–815
Taga T, Watanabe T, Tomizawa D et al (2016) Preserved high probability of overall survival with significant reduction of chemotherapy for myeloid leukemia in Down syndrome: a nationwide prospective study in Japan. Pediatr Blood Cancer 63:248–254
Takamatsu Y, Suzumiya J, Utsunomiya A et al (2010) THP-COP regimen for the treatment of peripheral T-cell lymphoma and adult T-cell leukemia/lymphoma: a multicenter phase II study. Eur J Haematol 84:391–397
Kudo K, Kojima S, Tabuchi K et al (2007) Prospective study of a pirarubicin, intermediate-dose cytarabine, and etoposide regimen in children with Down syndrome and acute myeloid leukemia: the Japanese Childhood AML Cooperative Study Group. J Clin Oncol 25:5442–5447
Shimomura Y, Baba R, Watanabe A et al (2011) Assessment of late cardiotoxicity of pirarubicin (THP) in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 57:461–466
Feijen EA, Leisenring WM, Stratton KL et al (2015) Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol 33:3774–3780
Acknowledgements
We thank all of the patients who participated in this trial and all of the research staff at study centers who helped to recruit patients and provide data. This work was partly supported by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Hori, H., Kudoh, T., Nishimura, S. et al. Acute and late toxicities of pirarubicin in the treatment of childhood acute lymphoblastic leukemia: results from a clinical trial by the Japan Association of Childhood Leukemia Study. Int J Clin Oncol 22, 387–396 (2017). https://doi.org/10.1007/s10147-016-1062-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1062-1