Abstract
Background
Although liver resection combined with preoperative chemotherapy is expected to improve outcomes of patients with resectable colorectal liver metastasis (CRLM), there is as yet insufficient clinical evidence supporting the efficacy of preoperative systemic chemotherapy. The aim of this phase II study was to assess the feasibility and efficacy of preoperative FOLFOX systemic chemotherapy for patients with initially resectable CRLM.
Methods
A prospective multi-institutional phase II study was conducted to evaluate the feasibility and efficacy of preoperative chemotherapy for resectable CRLM (ClinicalTrials.gov identifier number NCT00594529). Patients were scheduled to receive 6 cycles of mFOLFOX6 therapy before liver surgery. The primary endpoint was the macroscopic curative resection rate.
Results
A total of 30 patients were included in this study. Two patients who were diagnosed with hepatocellular and intrahepatic cholangiocellular carcinoma based on pathology were excluded from the analysis. More than half of the patients (57 %) had solitary liver metastasis. The completion rate of preoperative chemotherapy was 64.3 % and the response rate was 53.6 %. Two patients were unable to proceed to liver resections due to disease progression and severe postoperative complications following primary tumor resection. Macroscopic curative resection was obtained in 89.3 % of eligible patients. Postoperative mortality and severe complication (≥Gr. 3) rates were 0 and 11 %, respectively. The 3-year overall and progression-free survival rates were 81.9 and 47.4 %, respectively.
Conclusion
Our phase II study demonstrated the feasibility of liver resection combined with preoperative mFOLFOX6 therapy in patients with initially resectable CRLM. Further study is warranted to address the oncological effects of preoperative chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 40 % of patients with colorectal cancer (CRC) either already have liver metastases when the primary tumor is diagnosed (20 %) or they will develop colorectal liver metastases (CRLM) during follow-up (20 %) [1–3]. Surgical resection is regarded as the standard of care for CRLM patients and yields 5-year overall survival (OS) rates of 30–40 % [4–7]. However, up to 60 % of patients will relapse after surgery, despite the administration of adjuvant chemotherapy [8–11], and these recurrences are confined to the liver in half of the cases [12, 13].
One of the most promising treatment options to prolong the disease-free survival period is to eradicate micrometastases in the liver and other organs by liver resection combined with perioperative chemotherapy [9, 11, 14]. However, the optimal timing of the chemotherapy remains to be resolved. Preoperative chemotherapy for resectable CRLM has several advantages, including an assessment of the in vivo treatment response, downsizing of the metastases, and upfront treatment for micrometastatic disease. However, despite these potential advantages, several studies have indicated that systemic preoperative chemotherapy is associated with liver-specific toxicity characterized by pathological changes, such as steatosis, steatohepatitis and sinusoidal dilatation, and increased perioperative morbidity, and that it can even preclude resection [15–23]. To date, several reports evaluating the feasibility and efficacy of preoperative chemotherapy for resectable CRLM have been published, but the majority of these are retrospective studies, and there is a lack of evidence to draw any conclusion [24–28].
The aim of the prospective phase II study reported here was to assess the feasibility of liver resection, including a major hepatectomy, after preoperative FOLFOX systemic chemotherapy for patients with initially resectable CRLM.
Methods
Patients
Thirty patients were recruited from 12 affiliated hospitals between December 2007 and June 2010. To be eligible for enrolment, patients had to be between 20 and 80 years of age with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, histologically proven colorectal adenocarcinoma, previously untreated liver metastases of any size and any number that were potentially resectable, and no detectable extrahepatic tumors (except potentially resectable lung metastases). Liver metastases were regarded as unresectable in patients who were unlikely to tolerate a hepatectomy due to insufficient residual liver volume, require extended liver resection larger than a trisegmentectomy or hepatectomy with vascular reconstruction, or have overt hepatic hilar lymph node metastases. The primary tumor had to have been either already resected in a curative intent (pathological R0 resection) or judged to be curatively resectable (in the case of synchronous liver metastases) by each multi-disciplinary team at the treating hospital. Patients who had already undergone chemotherapy with oxaliplatin were excluded. We also excluded those patients with other cancerous lesions to be cured (with the exception of non-melanoma skin carcinoma or in situ cervical cancer), major hepatic insufficiency, an absolute neutrophil count of <1.5 × 109/L, platelet counts of <100 × 109/L, serum creatinine of >1.5 mg/dL, a grade of common toxicity criteria of >1 for peripheral neuropathy, uncontrolled congestive heart failure, angina pectoris, hypertension, arrhythmia, a history of significant neurological or psychiatric disorders, or active infection. Pregnant or breastfeeding women were also excluded. A clinical examination, chest radiography, spiral whole-body computed tomography (CT) scan with contrast medium, liver magnetic resonance imaging (MRI) examination, electrocardiogram, and standard laboratory work-up were performed prior to study entry. The trial was approved by the medical ethics committees of all participating centers. Written informed consent was obtained from all patients before study entry. This trial was registered with ClinicalTrials.gov, number NCT00594529.
Procedures
The decision-making flowchart of this study is shown in Fig. 1. In cases of synchronous CRLM, laparoscopic curative resection of the primary CRC was performed after study enrollment. Within about 2 weeks after the first-stage operation, preoperative chemotherapy using mFOLFOX6 for CRLM was initiated as previously described [29]. In cases of metachronous CRLM, mFOLFOX6 chemotherapy was commenced within 2 weeks after study entry. At the completion of 3 cycles of mFOLFOX6, the tumor response in the liver was assessed by contrast CT scan or MRI and was scored according to RECIST, version 3.0 (Response Evaluation Criteria in Solid Tumors), by the physicians in each institution [30]. When tumor response was assessed as complete response (CR), partial response (PR) or stable disease (SD), an additional 3 cycles were administered, adding up to a total of 6 cycles of mFOLFOX6. Within 2–4 weeks after the completion of the preoperative chemotherapy, the response of the CRLM was reassessed, and the patients whose CRLM were ultimately regarded as resectable proceeded to liver resection within 3–6 weeks after the last administration of preoperative chemotherapy or whenever they had completely recovered from the adverse effects of the chemotherapy and regained adequate liver function. If the CRLM were deemed as unresectable, subsequent treatment depended on the discretion of the treating medical oncologists. When a clinical CR was achieved by the preoperative chemotherapy, the intraoperative contrast-enhanced liver ultrasonography results were analyzed by physicians with knowledge of the pre-treated helical CT and MRI results, and all sites at which the liver tumors were considered to have disappeared were resected. CRLM were considered to have definitively disappeared when no lesions or abnormalities, such as calcification or heteroechogeneity, were observed at the site of the previous liver tumors, and the disappeared tumors were left in the remnant liver. In cases of progressive disease (PD) after 3 cycles of mFOLFOX6, the patients proceeded to liver resection when the liver lesions were still considered to be resectable; when the lesions were considered to be unresectable, the patients were treated at the physician’s discretion. When mFOLFOX6 therapy was discontinued due to adverse effects, a liver resection was planned within 3–6 weeks after the cessation of the preoperative treatment if the CRLM were regarded as resectable. After the curative liver resection for CRLM, the patients received 5-fluorouracil (5FU)-based adjuvant systemic chemotherapy.
Surgical exploration consisted of inspection of the peritoneal cavity to exclude extrahepatic involvement and the histological examination of frozen sections of any suspicious lesion. The liver was then mobilized and explored by contrast-enhanced ultrasonography to detect and localize all CRLM. The type and extent of the curative liver resection (wedge resection, or monosegmentectomy or plurisegmentectomy) were decided upon by the surgeon of each multidisciplinary team at the treating hospital but were modified if previously undetected deposits were discovered perioperatively or if the tumor was larger than expected. Surgical R0 and R1 resections (sR0 and sR1) were defined as the absence or presence of tumor exposure on the surgical margin, respectively, and surgical R2 resections (sR2) as the presence of macroscopic residual tumors. A liver resection combined with even a single radiofrequency ablation (RFA) was regarded as non-curative and expressed as sRX in this study. Pathological response to chemotherapy was evaluated by the pathologists in each institution as follows: grade 0, no definite response identified; grade 1a, less than one-third of cancer cells were degenerated and necrotic; grade 1b, less than two-thirds and more than one-third of cancer cells were degenerated and necrotic; grade 2, more than two-thirds of cancer cells were degenerated and necrotic; grade 3, no viable cancer cells were observed (CR). The grades of the postoperative complications associated with liver resection were evaluated according to the Clavien–Dindo classification [31].
The primary trial endpoint was the rates of macroscopically curative resection (sR0 and sR1), which were assessed intraoperatively as a complete resection without any macroscopic residual tumors. The secondary endpoints included the completion rates of preoperative chemotherapy, the response rates to preoperative chemotherapy, treatment-related adverse effects, the perioperative morbidity and mortality rates associated with liver resections, and patient survival [progression-free survival (PFS) and OS].
Sample size and statistical analysis
The sample size was determined by defining a threshold and expected curative resection rates as 70 and 90 %, respectively, and by setting both alpha and beta errors at 0.10, thus requiring 25 patients for proper statistical analysis. Taking into consideration that some patients might be excluded from the analysis, we enrolled 30 patients to fulfill the target requirement of 25 patients. The PFS rates were estimated by the Kaplan–Meier method using SPSS software, version 17.0 (IBM Corp., Armonk, NY).
Results
Patient characteristics
Of the 30 patients initially recruited to the study, 28 were eligible for enrolment since two patients were ultimately diagnosed with hepatocellular carcinoma (HCC) and intrahepatic cholangiocellular carcinoma (ICC), respectively, based on pathological examination of resected liver lesions (Table 1). There were 21 synchronous and seven metachronous liver metastases, which were detected in at stage II in four patients and at stage III in three patients (TNM classification of malignant tumours, 7th edn). Among these, 57 % patients had a solitary liver metastasis, and at most six liver metastases were resected in 2 patients. The median diameter of the largest tumor was 21.5 (11.3–100) mm.
Preoperative chemotherapy and response rate
Among the 28 eligible patients, one patient did not receive preoperative chemotherapy due to major anastomotic leakage after primary tumor resection (low anterior resection) of a rectal cancer, leading to a deterioration of the systemic condition. Consequently, 27 patients underwent preoperative chemotherapy.
Of the 28 eligible patients, 18 completed six cycles of mFOLFOX6, resulting in a completion rate of 64.3 %. Ten patients failed to complete the chemotherapy due to PD (3 cases), severe adverse effects (3 cases), clinician’s discretion (2 cases), patient’s rejection of further chemotherapy (1 case), and poor performance status following the primary tumor resection (1 case), as described previously. The details and frequencies of the adverse effects of preoperative chemotherapy (Table 2) were compatible with those previously reported in the treatment for metastatic diseases [29].
The reduction rate of target lesion at the best response of each patient is shown in Fig. 2. The median reduction rate was 30.4 %, and the overall response rate (CR + PR) was 51.9 %. As mentioned previously, three patients were assessed with PD (11 %), namely, two patients with progression of metastatic lesion and one patient with development of new lesions. During preoperative chemotherapy, one of these three patients chose to undergo subsequent therapeutic conversion to hepatic arterial infusion and the remaining 2 patients proceeded to liver resections, resulting in pathological R0 resections.
Hepatectomy
Among the 28 eligible patients, 26 underwent protocol hepatectomy. Of the two patients who did not undergo protocol hepatectomy, one had progression of liver metastasis and one had a poor performance status following the previous surgery. In terms of the type of liver resections, more than half of the patients underwent partial hepatectomy (n = 18), and five patients underwent resection of two or more segments (Table 3). Median operative time and blood loss were 279 (range 113–795) min and 650 (range 122–2880) g, respectively. Of the 26 eligible patients who underwent protocol hepatectomy, the number of patients with sR0, sR1, sR2, and sRX was 24, 1, 0, and 1, respectively, resulting in 89.3 % (25/28) (95 % confidence interval 71.8–97.7 %) of macroscopically curative resections (sR0 and sR1), which was significantly higher than the threshold curative resection rate (70 %) (P = 0.02). This result met the primary endpoint of this clinical study. One patient was judged as sRX because of utilization of intraoperative RFA for curative intent. The pathologically curative resection rates were the same as the macroscopically curative resection rates. Hepatectomy-associated complications in 28 patients (including patients with HCC and ICC) are shown in Table 4. Postoperative hyperbilirubinemia was evident in 11 of the 28 patients (39 %) who underwent liver resections. With the except of grade 3 complications, nearly 90 % of the patients tolerated the hepatectomies without major complications, and there was no perioperative mortality in this series. A macroscopic blue liver was evident during the operation in eight of the 26 hepatectomized patients. There was no significant correlation between the presence of blue liver and the incidence of postoperative complications (data not shown). Among the 26 resected CRLM specimens, pathological response was determined as grade 0 in six patients, grade 1a in 11 patients, grade 1b in five patients, grade 2 in one patient, and grade 3 in one patient (data not available for 2 patients). There were no significant relationships between the pathological responses and the degree of postoperative complications (data not shown).
Postoperative adjuvant treatment and long-term outcomes
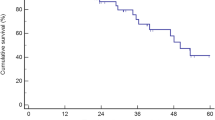
After the hepatectomies, 5FU-based adjuvant chemotherapy was administered to 22 of the 26 patients (mFOLFOX6 to 10 patients, capecitabine to 2 patients, S-1 to 3 patients, sLV5FU2 to 4 patients, UFT + calcium folinate to 3 patients). Following surgical removal of CRLM, 14 of the 26 patients developed recurrences in the residual liver (8 patients), lung (3 patients), and lymph nodes (3 patients), and seven patients died during the study period due to the deterioration of the recurrent lesions (5 patients) or irrelevant diseases (2 patients). The 3-year OS and PFS rates were 81.9 and 47.4 %, respectively (Fig. 3). For the two patients with PD during the preoperative mFOLFOX6 therapy who eventually proceeded to liver resections, one patient underwent oral administration of S-1 after the hepatectomy, but at about 3 months postoperatively recurrent tumors were detected in the residual liver along with possible lymph node metastasis at the liver hilum. The other patient completed a 6-month period of postoperative oral administration of capecitabine, but the patient developed a recurrence in the residual liver at about 1 year after the hepatectomy.
Discussion
Two major issues should be considered in any discussion of the clinical relevance of preoperative chemotherapy for resectable CRLM, namely: Does preoperative chemotherapy compromise the safety of hepatectomy? Does preoperative chemotherapy improve oncological outcomes?. The feasibility and efficacy of preoperative chemotherapy for resectable CRLM has been assessed in several studies [25–28, 32]. However, the majority of these studies were retrospective, with the exception of the EORTC Intergroup Trial [33, 34]. Therefore, it remains difficult to draw any definitive conclusion [32].
The adverse effects of chemotherapeutic regimens containing 5-FU, oxaliplatin, and/or irinotecan on non-tumor liver parenchyma have are known, and the effect of preoperative chemotherapy on early post-hepatectomy morbidity rates has remained a matter of debate. The EORTC Intergroup trial [33] demonstrated an increased rate of postoperative complications in the preoperative chemotherapy group (25 %) compared with the surgery-alone group (16 %), specifically showing a doubling in the rates of biliary fistula and hepatic failure. In contrast, several authors have found that the administration of preoperative chemotherapy did not worsen postoperative outcomes [24, 25]. The results of our study show that liver resection after preoperative systemic chemotherapy was feasible in our patient cohort, with no mortality and acceptable rates of postoperative complications (11 % of patients were ≥grade 3). Because postoperative morbidity depends on the extent of hepatectomy [35, 36], we consider that one of the reasons for these good results was that the majority of our patients underwent partial liver resection (64 %). Our results indicate the feasibility of preoperative chemotherapy for resectable CRLM. Unfortunately, we were unable to draw definite conclusions regarding the specific association between oxaliplatin use and perioperative complications since detailed pathological analyses of the abnormalities of non-tumor liver parenchyma were difficult in some cases due to only a small sample of normal liver tissue being available for assessment.
The benefit of preoperative chemotherapy is also still being debated. We achieved a curative resection rate of 89.6 %, which is comparable or even better than the rates observed in previous studies, with margin-positive rates ranging from 5 to 19 % [32]. In terms of survival benefits, the EORTC study demonstrated an absolute increase of 9.1 % in PFS at 3 years in the combined treatment group compared with the surgery-alone group in the eligible patient cohort, indicating the beneficial effect of perioperative chemotherapy. However, no significant increase in OS was found. Our long-term oncological outcomes (3-year PFS of 47.4 %, 3-year OS of 81.9 %) were also better than those described in previous reports [32], but we believe that it is difficult to draw any conclusion regarding OS and PFS from our results because of the small sample size and the inclusion of patients with relatively small and few CRLM (median maximum diameter 21.5 mm, median number of metastases 1).
Given the trade-off between toxicity and benefits of preoperative chemotherapy for resectable CRLM, the optimal selection of patients who will benefit from preoperative chemotherapy is another matter of considerable interest. Adam et al. [37] and Okuno et al. [38] reported that patients with solitary CRLM may not be good candidates for preoperative chemotherapy because no improvement of long-term oncological outcomes was observed in this group. Further, Zhu et al. demonstrated that the clinical benefit of preoperative chemotherapy is limited and depends on the risk of tumor recurrence [39].
Our study has several limitations, such as the small sample size and inclusion of patients with relatively small and few CRLM. Nevertheless, we believe that our results will be useful for clarifying the role of preoperative chemotherapy for patients with resectable CRLM.
In conclusion, our phase II study demonstrated the feasibility of liver resection combined with preoperative mFOLFOX6 therapy in patients with initially resectable CRLM. Further studies are warranted to examine the oncological effects of the preoperative chemotherapy.
References
Cummings LC, Payes JD, Cooper GS (2007) Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer 109:718–726
Leporrier J, Maurel J, Chiche L et al (2006) A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 93:465–474
Manfredi S, Lepage C, Hatem C et al (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244:254–259
Scheele J, Stangl R, Altendorf-Hofmann A (1990) Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 77:1241–1246
Fong Y, Fortner J, Sun RL et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318
Choti MA, Sitzmann JV, Tiburi MF et al (2002) Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 235:759–766
Simmonds PC, Primrose JN, Colquitt JL et al (2006) Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 94:982–999
Nordlinger B, Guiguet M, Vaillant JC et al (1996) Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 77:1254–1262
Portier G, Elias D, Bouche O et al (2006) Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 24:4976–4982
Parks R, Gonen M, Kemeny N et al (2007) Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg 204:753–761
Mitry E, Fields AL, Bleiberg H et al (2008) Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 26:4906–4911
Fong Y, Cohen AM, Fortner JG et al (1997) Liver resection for colorectal metastases. J Clin Oncol 15:938–946
Nakajima Y, Nagao M, Ko S et al (2001) Clinical predictors of recurrence site after hepatectomy for metastatic colorectal cancer. Hepato-gastroenterol 48:1680–1684
Kobayashi A, Hasegawa K, Saiura A et al (2014) A randomized controlled trial evaluating efficacy of adjuvant oral uracil-tegafur (UFT) with leucovorin (LV) after resection of colorectal cancer liver metastases: The UFT/LV study. J Clin Oncol 32:5s (suppl;abstr 3584)
Cleary JM, Tanabe KT, Lauwers GY et al (2009) Hepatic toxicities associated with the use of preoperative systemic therapy in patients with metastatic colorectal adenocarcinoma to the liver. Oncologist 14:1095–1105
Vauthey JN, Pawlik TM, Ribero D et al (2006) Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24:2065–2072
Aloia T, Sebagh M, Plasse M et al (2006) Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol 24:4983–4990
Rubbia-Brandt L, Audard V, Sartoretti P et al (2004) Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 15:460–466
Nakano H, Oussoultzoglou E, Rosso E et al (2008) Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 247:118–124
Zorzi D, Laurent A, Pawlik TM et al (2007) Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 94:274–286
Karoui M, Penna C, Amin-Hashem M et al (2006) Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 243:1–7
Fernandez FG, Ritter J, Goodwin JW et al (2005) Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg 200:845–853
Parikh AA, Gentner B, Wu TT et al (2003) Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg 7:1082–1088
Spelt L, Hermansson L, Tingstedt B et al (2012) Influence of preoperative chemotherapy on the intraoperative and postoperative course of liver resection for colorectal cancer metastases. World J Surg 36:157–163
Araujo R, Gonen M, Allen P et al (2013) Comparison between perioperative and postoperative chemotherapy after potentially curative hepatic resection for metastatic colorectal cancer. Ann Surg Oncol 20:4312–4321
Cucchetti A, Ercolani G, Cescon M et al (2012) Safety of hepatic resection for colorectal metastases in the era of neo-adjuvant chemotherapy. Langenbeck Arch Surg 397:397–405
Pinto Marques H, Barroso E, de Jong MC et al (2012) Peri-operative chemotherapy for resectable colorectal liver metastasis: does timing of systemic therapy matter? J Surg Oncol 105:511–519
Reddy SK, Tsung A, Marsh JW et al (2012) Does neoadjuvant chemotherapy reveal disease precluding surgical treatment of initially resectable colorectal cancer liver metastases? J Surg Oncol 105:55–59
Matsumoto S, Nishimura T, Kanai M et al (2008) Safety and efficacy of modified FOLFOX6 for treatment of metastatic or locally advanced colorectal cancer. A single-institution outcome study. Chemotherapy 54:395–403
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Nigri G, Petrucciani N, Ferla F et al (2015) Neoadjuvant chemotherapy for resectable colorectal liver metastases: what is the evidence? Results of a systematic review of comparative studies. Surgeon 13:83–90
Nordlinger B, Sorbye H, Glimelius B et al (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371:1007–1016
Nordlinger B, Sorbye H, Glimelius B et al (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–1215
Ribeiro HS, Costa WL Jr, Diniz AL et al (2013) Extended preoperative chemotherapy, extent of liver resection and blood transfusion are predictive factors of liver failure following resection of colorectal liver metastasis. Eur J Surg Oncol 39:380–385
Kenjo A, Miyata H, Gotoh M et al (2014) Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg 218:412–422
Adam R, Bhangui P, Poston G et al (2010) Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg 252:774–787
Okuno M, Hatano E, Seo S et al (2014) Indication for neoadjuvant chemotherapy in patients with colorectal liver metastases based on a nomogram that predicts disease-free survival. J Hepato-biliary-pancreatic Sci 21:881–888
Zhu D, Zhong Y, Wei Y et al (2014) Effect of neoadjuvant chemotherapy in patients with resectable colorectal liver metastases. PLoS One 9:e86543
Acknowledgments
We thank all patients and their relatives for their willingness to participate in the study and the study team at the seven facilities for their cooperation with case registration. We also thank Ms. Yukari Hayashi and Ms. Sayaka Tokuda for great assistance in data management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no potential conflicts of interest.
About this article
Cite this article
Nagayama, S., Hasegawa, S., Hida, K. et al. Multi-institutional phase II study on the feasibility of liver resection following preoperative mFOLFOX6 therapy for resectable liver metastases from colorectal cancers. Int J Clin Oncol 22, 316–323 (2017). https://doi.org/10.1007/s10147-016-1050-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1050-5