Abstract
Background
Programmed cell death ligand 1 (PD-L1) regulates immune responses through interaction with its receptor. PD-L1 is not only a predictor of poor prognosis but also a new therapeutic target in several malignancies. Neoadjuvant chemoradiotherapy (CRT) is an effective tool for local control of rectal cancer, but the disease recurrence rate remains high. The aim of this study was to retrospectively evaluate the correlation between PD-L1 expression and clinicopathological variables in rectal cancer after neoadjuvant CRT.
Materials and methods
A total of 90 rectal cancer patients who underwent neoadjuvant CRT were enrolled in this study. We evaluated PD-L1 expression using immunohistochemistry. Moreover, we investigated the correlation between PD-L1 expression and tumor-infiltrating T cells, and between CD8- and Foxp3-positive cells.
Results
Patients with high PD-L1 expression more frequently had vascular invasion and tumor recurrence compared to patients with low PD-L1 expression (P = 0.0225 and P = 0.0051). High PD-L1 expression was significantly associated with poor recurrence-free and overall survival (P = 0.0027 and P = 0.0357). Multivariate analysis revealed lymph node metastasis and high PD-L1 expression as independent risk factors for tumor recurrence (P = 0.0102 and P = 0.0374). Numbers of infiltrating CD8-positive cells in patients with high PD-L1 expression were significantly lower than in patients with low PD-L1 expression (P = 0.0322).
Conclusion
Our data suggest that inhibition of PD-L1 may be a new immunotherapeutic strategy to reduce tumor recurrence and improve prognosis in patients with rectal cancer after neoadjuvant CRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rectal cancer is one of the most common types of cancer in Japan and other developed nations. The introduction of preoperative chemoradiotherapy (CRT) and total mesorectal excision (TME) for the management of locally advanced rectal cancer has decreased the local recurrence rate [1–4]. However, preoperative CRT does not improve survival, and disease recurrence remains the major cause of mortality in rectal cancer patients [5]. Identification of predictors of disease recurrence and poor prognosis would aid in the successful treatment of these patients and in the discovery of new therapeutic strategies.
Interactions between the programmed cell death 1 (PD-1) receptor and its ligands (PD-L1/2) have been suggested as a potential mechanism of tumor immune tolerance and escape [6, 7]. Recent data show that high PD-L1 levels in tumor cells are associated with increased aggressiveness and poor prognosis in many malignancies [8]. PD-L1 is expressed by T and B cells, macrophages, and dendritic cells, and is up-regulated upon activation by interferons [9, 10]. Up-regulation of PD-1 ligands has been reported in many malignancies including melanoma, lung cancer, renal cell carcinoma, ovarian cancer, colorectal cancer [11], breast cancer [12], and osteosarcoma [13], and has been suggested to play a central role in tumor−immune system interactions [14, 15]. The expression of PD-L1 on tumor cells was shown to suppress the cytotoxic activity of CD8-positive T cells [16, 17], and functional PD-L1 expressed on transformed cells suppresses T-cell interleukin-2 (IL-2) production, which further inhibits T-cell proliferation and survival [18]. Therefore, inhibition of PD-L1 expression can improve the ability of T cells to attack tumor cells.
Currently, preclinical and clinical studies are ongoing using anti-PD-1/PD-L1 agents such as nivolumab as a new immunotherapeutic target for several malignancies [7]. Therefore, in the present study, we retrospectively investigated the association between PD-L1 expression and clinicopathological variables in rectal cancer after neoadjuvant CRT. Moreover, we evaluated the correlation between PD-L1 expression and tumor-infiltrating T cells (TILs), and between CD8- and Foxp3-positive cells.
Materials and methods
Patients and specimens
From August 2003 to May 2014, 100 patients with rectal cancer received preoperative CRT followed by surgery at Mie University Hospital (Mie, Japan). Of these, 4 patients exhibited a complete pathological response and were excluded from participation. Samples from 6 cases were in poor condition or not available. Finally, a total of 90 patients were enrolled in this study. The study design was approved by the ethics review board of Mie University Hospital. All patients provided informed consent to allow the use of their tissues in this study.
5-Fluorouracil (5-FU)-based CRT regimen
Patients with rectal cancer were treated with a short course (20 Gy in 4 fractions) or a long course (45 Gy in 25 fractions) of radiotherapy using a 4-field box technique with concurrent chemotherapy to take advantage of 5-FU radiosensitization, as described previously [19]. In total, 43 patients received short-course radiotherapy with chemotherapy for 1 week. The remaining 47 patients received long-course radiotherapy with chemotherapy for 4 weeks. The time interval between preoperative CRT and surgery was 2–3 weeks for short-course radiotherapy and 4–6 weeks for long-course radiotherapy. Patients underwent standard surgery, including TME, and received 5-FU-based adjuvant chemotherapy following surgery for 6 months to 1 year.
Clinical and pathological responses to CRT
Clinical responses following preoperative CRT were evaluated by barium enema, endoscopy, computed tomography, and magnetic resonance imaging. Clinical responses were graded as complete responses, partial responses, no change, or progressive disease. The tumor regression grade (TRG) was evaluated using the 3-point Ryan TRG system [20]. In the 3-point Ryan system, a TRG of 1 (no viable cancer cells) and 2 (single or small groups of cancer cells) are combined into one category (TRG1), while a TRG of 3 (residual cancer outgrown by fibrosis) constitutes TRG2, and a TRG of 4 (significant fibrosis outgrown by cancer) and 5 (no fibrosis with extensive residual cancer) are combined into TRG3.
PD-L1, CD8, and Foxp3 immunohistochemistry
Formalin-fixed paraffin-embedded specimens were sliced into 5-μm sections. After deparaffinization and dehydration, the sections were placed in 10 mM sodium citrate buffer (pH 6.0) and autoclaved at 121 °C for 10 min for antigen retrieval. Sections were incubated in 3 % hydrogen peroxide for 10 min, blocked by normal goat serum (Vector Laboratories Inc., Burlingame, CA, USA) for 1 h, and incubated with primary antibody overnight at 4 °C for PD-L1 and Foxp3 and for 1 h at room temperature for CD8. Monoclonal mouse anti-human PD-L1 (CD274) antibody (clone 27A2; LifeSpan BioSciences, Seattle, WA, USA; dilution 1:100), monoclonal rabbit anti-human CD8 antibody (clone EP1150; GeneTex, San Antonio, TX, USA; dilution 1:1,000) for cytotoxic T cells, and monoclonal mouse anti-human Foxp3 antibody (clone 236A/E; Abcam, Cambridge, UK; dilution 1:100) for regulatory T cells were used as primary antibodies at a dilution of 1:100, together with a labeled streptavidin–biotin system (EnVision + DualLink System-HRP; Dako Cytomation, Glostrup, Denmark). Antibody binding was visualized using 3,3′-diaminobenzidine (Dako Cytomation). Negative controls using preimmune immunoglobulin were performed simultaneously.
Evaluation of PD-L1 immunostaining and counts of CD8- and Foxp3-positive cells
PD-L1 expression was evaluated under a light microscope (BX50; Olympus, Tokyo, Japan). The staining intensity was scored as 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. We defined a staining intensity score of 2 or 3 as high PD-L1 expression. The numbers of CD8- and Foxp3-positive cells per five fields at a magnification of 400× were counted under light microscopy, and median counts were recorded. Each sample was evaluated in a blinded manner by two investigators (KM and SI) who did not have any clinical or pathological information regarding the origin of the samples.
Statistical analyses
Statistical analyses were performed using Stat View 5.0 for Windows (SAS Institute Inc., Cary, NC, USA). Significant differences were analyzed using the chi-squared test. Correlation between continuous and categorical variables was evaluated using the Mann–Whitney U test for two groups. The correlations between variables were assessed with the Spearman rank correlation coefficient. Recurrence-free survival (RFS) for 82 patients with R0 resection, which means no microscopic residual tumor cells and overall survival (OS) for all patients were calculated from the date of surgery to the date of disease recurrence or patient death, respectively. RFS and OS probabilities were calculated using the Kaplan–Meier method, and intergroup differences were determined using the log-rank test. Logistic regression analysis was used to evaluate whether PD-L1 expression predicted tumor recurrence as the final analysis. The influence of prognostic predictors identified via univariate analysis was assessed by multivariate analysis using Cox’s proportional hazards model. P < 0.05 was considered to indicate statistical significance.
Results
Patients and tumor characteristics
The study included 64 men and 26 women, with a median age of 64 years (range 33–80 years). Post-CRT pathological T stages were ypT0 (n = 5), ypT1 (n = 10), ypT2 (n = 25), ypT3 (n = 47), and ypT4 (n = 3). In total, 31 patients (34.4 %) presented with lymph node metastases. Eighty tumors (88.9 %) had well-differentiated or moderately differentiated adenocarcinoma histology. Overall, 24 (29.2 %) of 82 patients who received therapy with curative intent experienced tumor recurrence—local recurrence alone occurred in 8 patients (9.7 %), while distant recurrence occurred in 16 patients (19.5 %) (Table 1). The median follow-up period was 46 months (range 2–139 months).
PD-L1 immunohistochemical findings
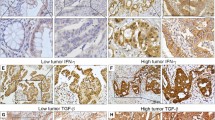
PD-L1 expression was observed in the cytoplasm and nucleus of cancer cells (Fig. 1). CD26 staining scores were 0 in 6 patients, 1 in 48 patients, 2 in 26 patients, and 3 in 10 patients. Thirty-six patients (40 %) were included in the high PD-L1 expression group.
Correlation between PD-L1 expression and clinicopathological variables
Table 1 shows the correlation between PD-L1 and clinicopathological variables. Patients with high PD-L1 expression more frequently had vascular invasion compared to patients with low PD-L1 expression (P = 0.0225). High PD-L1 expression was significantly associated with tumor recurrence in patients who received treatment with curative intent (P = 0.0051).
Survival analysis based on PD-L1 expression
High PD-L1 expression was significantly associated with poor RFS and OS (P = 0.0027 and P = 0.0357, respectively) (Fig. 2). Cox’s univariate proportional hazards analysis of RFS indicated that serosal invasion, lymph node metastasis, vascular invasion, and high PD-L1 expression were significantly associated with tumor recurrence (P = 0.0195, P = 0.0002, P = 0.0050, and P = 0.0047, respectively). Lymph node metastasis and high PD-L1 expression were identified as independent risk factors for tumor recurrence in multivariate analysis (P = 0.0051 and P = 0.0249, respectively) (Table 2A). Lymph node metastasis and high PD-L1 expression were significantly associated with poor survival in the Cox’s univariate proportional hazards analysis of OS (P = 0.0072 and P = 0.0410, respectively). Moreover, both features were identified in multivariate analysis as independent prognostic factors in patients with rectal cancer after neoadjuvant CRT (P = 0.005 and P = 0.0279, respectively) (Table 2B).
Correlation between PD-L1 expression and tumor-infiltrating lymphocytes
The median number of infiltrating CD8- and Foxp3-positive cells were 13 (range 1–90) and 22 (range 1–78), respectively. A significant positive correlation was observed between the numbers of infiltrating CD8- and Foxp3-positive cells (Spearman’s ρ 0.369, P = 0.0005) (Fig. 3a). Numbers of infiltrating CD8-positive cells in patients with high PD-L1 expression were significantly lower than those in patients with low PD-L1 expression (P = 0.0322) (Fig. 3b). However, no significant correlation between PD-L1 expression and the number of infiltrating Foxp3-positive cells was observed (Fig. 3c).
Positive correlation between infiltrating CD8- and Foxp3-positive cell count (a). Correlation of PD-L1 expression with infiltrating CD8- (b) and Foxp3-positive (c) cell count. Patients with low PD-L1 expression had significantly more infiltrating CD8-positive T cells, but no significant correlation was observed between Foxp3-positive T cells and PD-L1 expression
Discussion
In the present study, high PD-L1 expression was significantly associated with tumor recurrence and poor prognosis in rectal cancer after neoadjuvant CRT. Our present data are also consistent with previous reports in rectal cancer patients treated with neoadjuvant CRT. To the best of our knowledge, this is the first report about PD-L1 in rectal cancer after neoadjuvant CRT.
TILs are widely considered to reflect primary host immune responses against solid tumors. Several authors have reported that a high density of TILs predicts a favorable clinical outcome in colorectal cancer [21–23]. We previously investigated the correlation between TILs and clinicopathological variables in 157 patients with stage I−III colorectal cancer who did not receive preoperative therapy and showed that a low number of infiltrating CD8-positive cells was an independent predictive factor for tumor recurrence. Additionally, we observed that a significant positive correlation existed between CD8- and Foxp3-positive cell counts (P = 0.0018) [23]. Similarly, in rectal cancer after neoadjuvant CRT, a significant positive correlation was observed between CD8- and Foxp3-positive cell counts. When the correlation between infiltrating CD8- and Foxp3-positive cells with clinical outcomes was investigated in rectal cancer patients after neoadjuvant therapy, high levels of both infiltrating CD8- and Foxp3-positive cells were significantly associated with tumor recurrence and poor prognosis, and a high level of infiltrating CD8-positive cells was an independent prognostic factor for RFS and OS (data not shown). These results also implicate a role for TILs in tumor recurrence and survival in rectal cancer after neoadjuvant CRT. Therefore, the control of PD-L1 expression may contribute to improvement of the therapeutic effect of neoadjuvant CRT in rectal cancer patients.
PD-L1 expression was negatively correlated with infiltrating CD8-positive cells; however, no significant correlation was observed between Foxp3-positive cells and PD-L1 expression. Although the interaction between PD-L1 and regulatory T cells has not been well-elucidated, Amarnath et al. reported that conventional T cells or irradiated K562 myeloid tumor cells overexpressing PD-L1 converted Th1-specific T-box 1 transcriptional factor-positive Th1 cells into Foxp3-positive regulatory T cells in an in vivo study [24]. Although its mechanism may contribute to the suppression of rejection or graft-versus-host disease after transplantation, inhibition of PD-L1 (which can suppress conversion of Foxp3-positive cells) for colorectal cancer may have adverse effects, as several reports have shown the impact of high numbers of infiltrating Foxp3-positive cells on favorable clinical outcome in colorectal cancers [25–27]. Therefore, further analysis is required to clarify the interaction and balance between PD-L1 and host immunity in colorectal cancer, including the influence of preoperative therapies on PD-L1, even though several clinical trials evaluating PD-1/PD-L1 antibodies in colorectal cancer are currently ongoing.
Shinto et al. reported that the number of CD8-positive cells in stromal tissue was increased by neoadjuvant CRT and a high level of stromal CD8-positive cells after CRT was associated with a favorable prognosis in rectal cancer [28]. On the other hand, in esophageal cancer, Tsuchikawa et al. reported that the numbers of CD4-positive and CD8-positive T cells were increased by neoadjuvant chemotherapy, and concluded that their immunological modification might exert direct cytotoxicity on tumor cells [29]. We investigated the change of PD-L1 gene expression between pre- and post-irradiation in four colon cancer cell lines (DLD1, SW480, HT29, and Lovo) using quantitative real-time polymerase chain reaction (qRT-PCR) because the comparison of PD-L1 expression between pretreatment biopsy samples and postoperative specimens was difficult. An irradiation dose of 2.5 and 5 Gy was used for each colon cancer cell line, respectively. Total RNA was extracted at the indicated time points after irradiation. qRT-PCR was performed for expression analysis of PD-L1 gene expression and the serial changes of the gene expressions at pretreatment, and at 1, 3, and 5 day after irradiation were examined. We observed that PD-L1 gene expression was decreased by irradiation in each cell line (data not shown). These results suggest that radiotherapy may reduce PD-L1 expression and result in promoting host immune response to cancer cells. Since PD-L1 can suppress the cytotoxic T-cell-mediated immune response, the combination of neoadjuvant therapies and inhibition of PD-L1 expression may synergistically enhance tumor shrinkage and reduce tumor recurrence in rectal cancer.
In conclusion, PD-L1 expression was significantly associated with tumor relapse and poor prognosis. Our data suggest that inhibition of PD-L1 may be a new immunotherapeutic strategy to reduce tumor recurrence and improve prognosis in patients with rectal cancer after neoadjuvant CRT. However, the data in this study should be interpreted with caution. Significant limitations of this study were the small number of patients evaluated and the retrospective nature. This study also included two preoperative radiation regimens with different time intervals between pretreatment and surgery. Furthermore, our short-course regimen deviated from standard methods. Therefore, a larger study population, long-term follow-up, and standardization of the pretreatments are required to validate the present results.
References
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
van den Brink M, Stiggelbout AM, van den Hout WB et al (2004) Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol 22:3958–3964
Guillem JG, Chessin DB, Cohen AM et al (2005) Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 241:829–836 discussion 836–828
Bosset JF, Collette L, Calais G et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123
Peeters KC, Marijnen CA, Nagtegaal ID et al (2007) The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 246:693–701
Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A (2014) Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol 153:145–152
Dolan DE, Gupta S (2014) PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 21:231–237
Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–242
Keir ME, Liang SC, Guleria I et al (2006) Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203:883–895
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Dong H, Strome SE, Salomao DR et al (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Sabatier R, Finetti P, Mamessier E et al (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6:5449–5464
Shen JK, Cote GM, Choy E et al (2014) Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res 2:690–698
Curiel TJ, Wei S, Dong H et al (2003) Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9:562–567
Zhang L, Gajewski TF, Kline J (2009) PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 114:1545–1552
Iwai Y, Ishida M, Tanaka Y et al (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293–12297
Hirano F, Kaneko K, Tamura H et al (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65:1089–1096
Yang W, Chen PW, Li H, Alizadeh H, Niederkorn JY (2008) PD-L1: PD-1 interaction contributes to the functional suppression of T-cell responses to human uveal melanoma cells in vitro. Invest Ophthalmol Vis Sci 49:2518–2525
Saigusa S, Inoue Y, Tanaka K et al (2013) Lack of M30 expression correlates with factors reflecting tumor progression in rectal cancer with preoperative chemoradiotherapy. Mol Clin Oncol 2:99–104
Ryan R, Gibbons D, Hyland JM et al (2005) Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 47:141–146
Pages F, Berger A, Camus M et al (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Mori K, Toiyama Y, Saigusa S et al (2015) Systemic analysis of predictive biomarkers for recurrence in colorectal cancer patients treated with curative surgery. Dig Dis Sci 60:2477–2487
Amarnath S, Mangus CW, Wang JC et al (2011) The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med 3:111ra120
Salama P, Phillips M, Grieu F et al (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192
Yoon HH, Orrock JM, Foster NR et al (2012) Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One 7:e42274
Huang Y, Liao H, Zhang Y et al (2014) Prognostic value of tumor-infiltrating FoxP3+ T cells in gastrointestinal cancers: a meta analysis. PLoS One 9:e94376
Shinto E, Hase K, Hashiguchi Y et al (2014) CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol 21(Suppl 3):S414–S421
Tsuchikawa T, Md MM, Yamamura Y et al (2012) The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol 19:1713–1719
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Saigusa, S., Toiyama, Y., Tanaka, K. et al. Implication of programmed cell death ligand 1 expression in tumor recurrence and prognosis in rectal cancer with neoadjuvant chemoradiotherapy. Int J Clin Oncol 21, 946–952 (2016). https://doi.org/10.1007/s10147-016-0962-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-0962-4