Abstract
Background
Preoperative serum systemic inflammatory response (SIR) in patients with colorectal cancer (CRC) has been reported to be a predictive biomarker of early recurrence. The molecular status of CRC, including microsatellite instability (MSI), BRAF and KRAS mutations, and tumor-infiltrating lymphocytes (TILs), has also been associated with recurrence in CRC patients treated with curative surgery.
Aim
We investigated the impacts of SIR status, TILs, and MSI on recurrence in curative CRC patients.
Methods
In this retrospective study, we enrolled 157 patients with stage I–III CRC undergoing curative surgery, for whom preoperative neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and C-reactive protein (CRP) data were available as indicators of SIR status. Molecular status was evaluated by counting TILs as the numbers of intratumoral Foxp3- and CD8-positive T cells by immunohistochemistry. MSI status was determined using five mononucleotide repeat microsatellite markers.
Results
Kaplan–Meier analysis of SIR indicators revealed that higher CRP, NLR, and PLR were associated with significantly poorer disease-free survival (DFS). Low levels of infiltrating CD8-positive T cells in CRC tissue was a significant predictor of poor DFS. Multivariate analysis showed that few infiltrating CD8-positive T cells and high serum CRP levels were independent predictive factors for recurrence. Furthermore, the combination of high CRP and few infiltrating CD8-positive T cells increased the predictive accuracy in these patients.
Conclusions
The results of this study suggest that both CRP levels in preoperative serum and CD8 T cells in CRC tissue are useful biomarkers for predicting early relapse in CRC patients treated with curative surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer and fourth leading cause of cancer-related deaths worldwide [1]. Despite improvements in surgical techniques, fatal disease recurrence occurs in 20–25 % of curatively treated patients [2]. Patients’ prognosis depends on the stage or anatomic extent of disease, based on the Union for International Cancer Control (UICC) tumor-node-metastasis (TNM) classification. The function of TNM staging has expanded from predicting prognosis to informing treatment choices [3, 4]. Several large randomized trials have suggested that all node-positive patients should receive adjuvant chemotherapy, while the value of adjuvant therapy for node-negative cases is controversial [3, 5]. The predictive value of TNM staging for identifying node-negative patients at risk of early recurrence in CRC is therefore limited. Accordingly, extensive research has been devoted to studying clinicopathological features and/or predictive molecular factors that may supplement TNM classification for predicting prognosis and recurrence in patients with CRC undergoing curative surgery.
The systemic inflammatory response (SIR) status is thought to be secondary to hypoxia or tumor necrosis and is associated with anti-apoptotic characteristics of cancer cells [6]. Preoperative serum SIR has been reported as a predictive biomarker of early recurrence in CRC patients treated with curative surgery [7], while neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and C-reactive protein (CRP) are considered to be indicators of SIR status.
The molecular status of CRC, including microsatellite instability (MSI) and tumor-infiltrating lymphocytes (TILs), is also associated with high risk of recurrence in CRC patients treated with curative surgery. TILs can act as an indicator of the host’s tumoral immune response and have been associated with recurrence and improved clinical outcome in CRC, as well as providing an attractive target for immunotherapy [8–12]. MSI represents an alternative pathway of colorectal carcinogenesis, in which tumors arise as a result of mutation or hypermethylation in the DNA mismatch repair system. MSI status has been reported to be an independent prognostic predictor of improved survival and time to recurrence [13].Therefore, both SIR-related host and local tumoral factors should be investigated to determine the factors predicting early recurrence in CRC patients treated with curative surgery.
This study therefore aimed to elucidate the clinical significance of both preoperative serum SIR and local tumor TILs and MSI status to identify biomarkers of recurrence risk in CRC patients treated with curative surgery.
Methods
Patients and Specimens
A total of 157 patients with stage I, II, or III primary CRC who underwent surgical resection at the Department of Gastrointestinal and Pediatric Surgery, Mie University, Mie, Japan, from 2007 to 2011 were analyzed. Patients with the following criteria were excluded: preoperative radiotherapy or chemotherapy, diagnosis of multiple CRCs, history of cancer in another organ, familial CRC, and inflammatory bowel disease. All patients were classified postoperatively based on the UICC TNM classification [14], using 10 % formalin-fixed, paraffin-embedded (FFPE) specimens. The histomorphology of the primary tumors and lymph nodes was confirmed by the Department of Pathology, Mie University, Mie, Japan. Written informed consent was obtained from all patients according to the guidelines approved by our institutional research board.
Laboratory Measurements of Neutrophils, Lymphocytes, CRP, and Carcinoembryonic Antigen
Neutrophils, lymphocytes, CRP, and carcinoembryonic antigen (CEA) were measured in routine blood samples obtained within 1 week before operation. Patients were divided into two groups using an NLR cutoff value of 3, based on a previous report [15]. Patients were also categorized according to PLR ≤150 or >150, as reported previously [16]. The cutoff value for CRP was 0.5 mg/dl (≤0.5 and >0.5 mg/dl) and for CEA was 5 ng/µl (≤5 and >5 ng/µl), according to the normal range at our institute.
Immunohistochemistry
FFPE specimens were sliced into 5-µm sections and subjected to immunohistochemical analysis to detect Foxp3 and CD8 expression. After deparaffinization and dehydration, the sections were placed in 10 mM sodium citrate buffer (pH 6.0) and autoclaved at 121 °C for 10 min for antigen retrieval. The sections were incubated in 3 % hydrogen peroxide for 10 min, blocked, and incubated in normal goat serum (Vector Laboratories Inc, Burlingame, CA, USA) overnight at 4 °C for Foxp3 and for 1 h at room temperature for CD8. The primary antibodies used were monoclonal mouse anti-human Foxp3 antibody (clone: 236A/E, Abcam, Cambridge, UK; dilution 1:100) for regulatory T cells and monoclonal rabbit anti-human CD8 (clone: EP1150, GeneTex, San Antonio, TX, USA; dilution 1:1000) for cytotoxic T cells. Antibody binding was visualized using Envision reagents (Dako REAL EnVision Detection System; peroxidase/DAB+, Dako Cytomation, Glostrup, Denmark). All the sections were counterstained with hematoxylin–eosin prior to dehydration and mounting.
Scoring Foxp3- and CD8-Positive T Cells
Foxp3- and CD8-positive T cells were counted using a scanner system under a Biorevo BZ-9000 microscope (Keyence, Osaka, Japan). Each slide was scanned microscopically, and intratumoral Foxp3- and CD8-positive T cells were photographed at a magnification of 400× in three representative high-power fields. Foxp3- and CD8-positive T cells with any detectable staining above background levels were considered positive.
KRAS/BRAF Mutation and MSI Analysis
FFPE sections (10 µm thick) from 157 surgical CRC patients were evaluated for mutations. Hematoxylin–eosin-stained FFPE sections were microdissected to extract DNA from the tumor cells. Genomic DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, Tokyo, Japan) according to the manufacturer’s protocol. DNA quantity and quality were assessed using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Houston, TX, USA). KRAS (exons 2 and 3) and BRAF (V600E) mutations were analyzed by pyrosequencing using the primers listed in Table 1. Reactions were run on a PyroMark Q96 ID system (Qiagen). MSI status was determined by polymerase chain reaction analyses of five mononucleotide repeat microsatellite markers (BAT-25, BAT-26, NR-21, NR-24, and NR-27), as recommended previously [17]. The primer sequences have been described previously [17]. Tumors with instability at more than three of these markers were classified as showing MSI, and those with instability at fewer than two markers as showing microsatellite stability (MSS).
Statistical Analysis
The associations between SIR status, TILs, MSI status, and clinicopathological features were analyzed using Chi-square tests. Disease-free survival (DFS) curves were analyzed using the Kaplan–Meier method, and differences were examined using log-rank tests. Cox’s proportional hazards regression tests were used to estimate univariate and multivariate hazard ratios for recurrence. Multivariate survival analyses were performed using factors identified as significant in univariate analyses. All P values were two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were carried out using JMP version 10 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
The study included a total of 90 men and 67 women with an average age of 66.9 years (range 35–89 years). The median follow-up time was 20.5 months (range 0.2–62.4 months). All patients were classified postoperatively based on the histopathological analysis using the UICC TNM classification criteria: stage I (n = 48), stage II (n = 55), and stage III (n = 54). Twenty-nine patients (18.4 %) developed recurrence.
Immunohistochemical Analysis of Tumor-Infiltrating Foxp3- and CD8-Positive T Cells
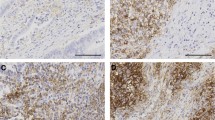
Intratumoral Foxp3- and CD8-positive T cells were detected (Fig. 1), with median numbers of 11 (range 0–99) and 15 (range 1–144), respectively. Patients were classified into high- and low-expressing groups for each antigen using the median values as cutoff points.
Immunohistochemical staining of Foxp3- and CD8-positive TILs. a Foxp3-positive TILs/low density (original magnification ×400). b CD8-positive TILs/low density (original magnification ×400). c Foxp3-positive TILs/high density (original magnification ×400). d CD8-positive TILs/high density (original magnification ×400). e, f Intratumoral densities of positively stained cells were measured using an automatic image analysis system
KRAS/BRAF Mutation and Tumor MSI Analysis
KRAS and BRAF mutations were analyzed in CRCs from 152 patients. Fifty patients (32.9 %) had KRAS codon mutations, and five (0.03 %) harbored BRAF mutations. A total of 98 patients (64.5 %) had no mutations in either the BRAF or KRAS gene (wild type). MSI was analyzed in 151 patients, of whom 142 showed MSS and nine showed MSI.
Associations Between SIR Status as Host Factor and Clinicopathological Features
We analyzed the association between preoperative SIR status and clinicopathological features (Table 2). High NLR was significantly associated with lymphatic invasion (P = 0.0371) and recurrence (P = 0.0062), while high PLR was also associated with recurrence (P = 0.006). In addition, high CRP level was significantly associated with T classification (P = 0.0004), recurrence (P = 0.0016), and TNM stage (P = 0.0091).
Associations Between TILs, MSI, and CEA as Tumor Factors and Clinicopathological Features
We analyzed the correlations between TILs, MSI, and CEA as tumor factors and clinicopathological features (Table 3). A low CD8-positive T cell count was significantly associated with venous invasion (P = 0.0185) and recurrence (P = 0.0084). However, Foxp3-positive T cell was not associated with clinicopathological features. MSI was significantly associated with undifferentiated histology (P = 0.022). High CEA levels were significantly associated with age (P = 0.0488), T classification (P < 0.0001), lymphatic invasion (P = 0.0133), venous invasion (P = 0.0234), recurrence (P = 0.0015), and TNM stage (P = 0.0006).
Associations Between MSI and TILs
We also investigated the associations between MSI and TILs. There was a trend toward more infiltrating Foxp3-positive T cells in patients with MSI, though the association was not significant (103 cells in MSI vs. 74 cells in MSS; P = 0.0585) (Fig. 2a), and significantly more infiltrating CD8-positive T cells were detected in patients with MSI (130 cells in MSI vs. 73 cells in MSS; P = 0.0001) (Fig. 2b).
Few Infiltrating CD8-Positive T Cells and High CRP Levels Are Independent Predictors of Recurrence in CRC Patients Treated with Curative Surgery
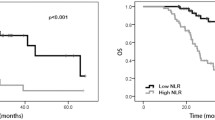
We analyzed DFS according to SIR status. Kaplan–Meier analysis showed that patients with high NLR, PLR, and CRP as host factors had significantly poorer DFS than patients with lower levels (P = 0.0232, P = 0.0233, P = 0.0017, respectively) (Fig. 3a–c). We also investigated DFS according to tumor status, including TILs, MSI, and preoperative CEA. Patients with fewer infiltrating Foxp3-positive T cells had poorer DFS than those with more infiltration, though the difference was not significant (P = 0.2568) (Fig. 3d), while patients with fewer infiltrating CD8-positive T cells had significantly poorer DFS than those with more infiltration (P = 0.0028) (Fig. 3e). In contrast, there was no association between MSI and DFS (Fig. 3f). High preoperative CEA levels were significantly associated with early recurrence (P = 0.0010) (Fig. 3g).
Kaplan–Meier curves for DFS classified according to SIR status, TILs, MSI status, and CEA levels in CRC patients treated with curative surgery. a DFS according to NLR. b DFS according to PLR. c DFS according to CRP levels. d DFS according to density of Foxp3-positive T cells. e DFS according to density of CD8-positive T cells. f DFS according to MSI status. g DFS according to CEA levels. h DFS according to combined score of few infiltrating CD8-positive T cells and high CRP levels
We also performed univariate analysis to detect important factors associated with DFS. T3/4 classification (P < 0.0001), lymphatic node metastasis (P = 0.0028), poor differentiation/mucinous (P = 0.0120), lymphatic invasion (P = 0.0307), venous invasion (P = 0.0002), high CEA levels (P = 0.0010), high CRP levels (P = 0.0044), high NLR (P = 0.0266), high PLR (P = 0.0182), and few infiltrating CD8-positive T cells (P = 0.0026) were all significant predictive factors for poor DFS (Table 4). Multivariate analysis of these factors identified few infiltrating CD8-positive T cells (hazard ratio (HR) = 4.3, P = 0.0059) as a tumor factor and high CRP (HR = 3.07, P = 0.0145) as a host factor, as independent predictive markers for recurrence in CRC patients treated with curative surgery (Table 4).
Combination of Few Infiltrating CD8-Positive T Cells and High CRP Levels Increase the Predictive Accuracy for Early Recurrence in CRC Patients Treated with Curative Surgery
We assessed the effect of combining independent predictive factors (preoperative CRP and intratumoral CD8 T cells) on the predictive accuracy for early recurrence in CRC patients treated with curative surgery. We divided the patients into three groups based on the following scores: high CRP, +1; low CRP, 0; few infiltrating CD8-positive T cells, +1; more infiltrating CD8-positive T cells, 0. Sixteen patients had a score of 2, 77 had a score of 1, and 59 had a score of 0. Kaplan–Meier analysis showed that patients with a score of 2 had significantly poorer DFS than the other two groups (P < 0.0001) (Fig. 3h) and that the risk of recurrence was increased when both independent factors were combined (HR = 5.26).
Discussion
The UICC staging system provides the most reliable indication of prognosis and is useful for discriminating between patients with early-stage disease and those with advanced disease. However, its ability to predict prognosis in patients with intermediate levels of tumor invasion (stage II or stage III) is less accurate. Sensitive biomarkers are therefore needed to allow the targeting of postoperative adjuvant chemotherapy to those patients at highest risk of early relapse, with a resultant improvement in survival. This study provides the first comprehensive analysis for identifying predictive biomarkers based on both the host immune response and the local tumor molecular status. We showed that high SIR status, including NLR, PLR, and CRP levels as host factors, and few intratumoral CD8-positive T cells and high CEA levels as tumor factors, was significantly associated with early recurrence in patients with surgically resected CRC. In addition, multivariate analysis revealed that high CRP levels and few intratumoral CD8-positive T cells were independent predictors of recurrence. Furthermore, the combination of both host and tumor factors increased the predictive accuracy for determining recurrence risk in CRC patients treated with curative surgery. This new predictive test, which is based on the widely available results of CRP assays, combined with CD8 immunohistochemistry that is feasible in most laboratories, represents a step forward for accurately identifying curatively treated CRC patients at risk of recurrence in the clinical setting.
Cancer progression depends on complex interactions between the tumor, its microenvironment, and the host immune response. Inflammation has been implicated in the pathogenesis of many adult malignancies and is now recognized as a hallmark of tumorigenesis [18]. Park et al. [19] showed that the host inflammatory response to CRC influenced disease recurrence and survival via the SIR. However, the tumor may also encourage the inflow of inflammatory lymphocytes, resulting in cell destruction within the surrounding tissue, generating a more widespread, nonspecific inflammatory response [20]. Several studies have shown an association between the local inflammatory response, indicated by increased T cell tumor infiltration, and improved prognosis and recurrence in CRC [8, 21, 22]. It is therefore important to carry out a comprehensive analysis of biomarkers of early recurrence based on both systemic and local inflammatory factors in patients with CRC treated with curative intent.
SIR status, reflected by CRP, NLR, and PLR, is thought to be a surrogate indicator of the host immune response to tumors and has been shown to act as a biomarker of outcome in a variety of malignancies [23–25].
CRP is an essential acute-phase reactant that acts as a surveillance molecule for activation of the adaptive immune system. It is synthesized in hepatocytes and is upregulated by cytokines such as interleukin-6 and tumor necrosis factor-α [26]. Several studies have demonstrated associations between high CRP levels and increased risk of early recurrence and poor outcome in CRC patients treated with curative surgery [27–29]. However, although CRP represents an excellent biomarker for oncological outcome, we should be aware of its limitations; CRP levels cannot discriminate between patients with several cancers, including CRC, and those with inflammatory conditions such as inflammatory bowel disease, collagen disease, rheumatic disease, and cardiovascular disease [30].
Patients with high NLRs also have the balance tipped in favor of pro-tumor inflammatory status, meaning that a high NLR is a predictor of recurrence in CRC patients treated with curative surgery [31, 32]. Both thrombocytosis and lymphocytopenia have also been shown to correlate with the degree of host systemic inflammation, and the ratio of these factors (PLR) might reflect a novel inflammatory factor incorporating these two individual hematologic factors [33]. Szkandera et al. [34] found that PLR was a predictive marker of recurrence in CRC patients treated with curative surgery. Collectively, SIR status thus comprises potential biomarkers for predicting early recurrence in CRC, though no previous studies have compared the predictive values of CRP level, NLR, and PLR in terms of CRC recurrence. However, the current multivariate analysis demonstrated that high CRP level was the best component of SIR for predicting recurrence in CRC patients treated with curative surgery.
CRCs are generally immunogenic and often infiltrated by T cells [35], suggesting that clinical outcome and recurrence may depend on the status of the local adaptive immune response. Upon antigenic stimulation, CD8-positive T cells differentiate into effector cells that kill tumor cells by releasing toxic granules such as granzyme B and perforins [36, 37]. A high density of TILs in CRC tissue is associated with increased tumor cell apoptosis [38]. However, regulatory T cells expressing the Foxp3 transcription factor may block the adaptive immune response [39]. Foxp3-positive T cells can suppress host-mediated antitumor immunity and tumor-specific cytotoxicity, suggesting regulatory T cell deletion as a potential therapeutic strategy [40]. Recent evidence has also indicated that signaling by the T cell chemoattractant CCL5 can recruit Foxp3-positive T cells to tumors and enhance their ability to kill CD8-positive T cells, thereby providing a mechanism of immune escape [41]. A high density of infiltrating Foxp3-positive T cells has thus been shown to be associated with an adverse prognosis in some tumor types [39, 42]. However, several reports have indicated favorable impact of infiltrating Foxp-3 T cells in other tumors, including CRC [43–47], and the role of Foxp3-positive T cells thus remains controversial. The results of the present study revealed that, as components of the local inflammatory response, infiltrating CD8-negative T lymphocytes, but not Foxp3-positive T lymphocytes, were significantly associated with poor DFS and were an independent predictive factor for early recurrence in CRC patients treated with curative surgery.
The majority of hereditary nonpolyposis CRC tumors are characterized by MSI, while only 10–15 % of sporadic CRC cases display MSI, predominantly caused by epigenetic hypermethylation of the MLH1 mismatch repair gene. A previous systematic review of the prognostic and predictive values of MSI status found that MSI was associated with better overall survival and DFS [48]. Closer analysis of the clinical data suggested no benefit from 5-fluorouracil treatment in patients with MSI CRC, supported by the fact that patients with MSI tumors have a better prognosis than those with MSS [49]. The better outcome in patients with MSI may be partly attributable to the generation of neoantigens as a result of mutational frameshifts within the coding regions of specific genes, as a consequence of inactivation of DNA mismatch repair in CRC epithelial cells. This attracts specific immune cells that help to contain the tumor and limit metastasis [50, 51]. Several previous studies in CRC patients have demonstrated an association between MSI and increased intraepithelial CD8-positive T cells compared with patients with MSS [51–53]. In addition, the density of intratumoral Foxp3-positive T cells is significantly higher in MSI compared with MSS CRC, paralleling the enhanced number of CD8-positive cells [54]. Our current study found no significant relationship between MSI status and recurrence, possibly because of the small sample size; however, we did demonstrate a significant association between TILs and MSI status in CRC.
In conclusion, this study demonstrates that recurrence in CRC patients treated with curative surgery is affected by both systemic and local inflammatory statuses. Host inflammatory status, represented by preoperative serum CRP levels, and local tumor inflammatory status, represented by CD8-positive infiltrating T cells, were independent predictive factors for recurrence in curatively resected CRC patients. The combination of CRP level and intratumoral CD8-positive T cells could thus be used to identify CRC patients who require adjuvant chemotherapy after curative surgery.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917.
Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC Cancer Staging Manual 7th ed. New York, NY: Springer; 2010:237–246.
Kornmann M, Formentini A, Ette C, et al. Prognostic factors influencing the survival of patients with colon cancer receiving adjuvant 5-FU treatment. Eur J Surg Oncol. 2008;34:1316–1321.
Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008;61:561–569.
Benson AB 3rd. New approaches to the adjuvant therapy of colon cancer. Oncologist. 2006;11:973–980.
Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163.
McMillan DC, Wotherspoon HA, Fearon KC, Sturgeon C, Cooke TG, McArdle CS. A prospective study of tumor recurrence and the acute-phase response after apparently curative colorectal cancer surgery. Am J Surg. 1995;170:319–322.
Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964.
Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618.
Ali AA, McMillan DC, Matalka II, McNicol AM, McArdle CS. Tumour T-lymphocyte subset infiltration and tumour recurrence following curative resection for colorectal cancer. Eur J Surg Oncol. 2004;30:292–295.
Zlobec I, Terracciano LM, Lugli A. Local recurrence in mismatch repair-proficient colon cancer predicted by an infiltrative tumor border and lack of CD8+ tumor-infiltrating lymphocytes. Clin Cancer Res. 2008;14:3792–3797.
Camus M, Tosolini M, Bet Mlecnik, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–2693.
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. City: John Wiley & Sons; 2011.
Chiang SF, Hung HY, Tang R, et al. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012;27:1347–1357.
Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726–734.
Goel A, Nagasaka T, Hamelin R, Boland CR. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS ONE. 2010;5:e9393.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer–related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081.
Park JH, McMillan DC, Horgan PG, Roxburgh CS. The impact of anti-inflammatory agents on the outcome of patients with colorectal cancer. Cancer Treat Rev.. 2014;40:68–77.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867.
Dahlin AM, Henriksson ML, Van Guelpen B, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671–682.
Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012;38:451–466.
McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540.
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230.
Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110:2524–2530.
Nakazaki H. Preoperative and postoperative cytokines in patients with cancer. Cancer. 1992;70:709–713.
Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176:335–338.
Nozoe T, Matsumata T, Sugimachi K. Preoperative elevation of serum C-reactive protein is related to impaired immunity in patients with colorectal cancer. Am J Clin Oncol. 2000;23:263–266.
Gunter MJ, Stolzenberg-Solomon R, Cross AJ, et al. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483–2487.
Vermeire S, Van Assche G, Rutgeerts P. The role of C-reactive protein as an inflammatory marker in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol.. 2005;2:580–586.
Ding PR, An X, Zhang RX, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427–1433.
Farid SG, Iqbal A, Khan S, Morris-Stiff G, Comment on Mallappa, et al. preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:909–910.
Smith RA, Bosonnet L, Ghaneh P, et al. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery. 2008;143:658–666.
Szkandera J, Pichler M, Absenger G, et al. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. Am J Surg. 2014;208:210–214.
Sherwood AM, Emerson RO, Scherer D, et al. Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunol Immunother. 2013;62:1453–1461.
Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–1693.
Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8 + T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043.
Sinicrope FA, Rego RL, Garrity-Park MM, et al. Alterations in cell proliferation and apoptosis in colon cancers with microsatellite instability. Int J Cancer. 2007;120:1232–1238.
Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–918.
Phan V, Disis ML. Tumor stromal barriers to the success of adoptive T cell therapy. Cancer Immunol Immunother. 2008;57:281–283.
Chang LY, Lin YC, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72:1092–1102.
Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593.
Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192.
Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair–proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643.
Correale P, Rotundo MS, Del Vecchio MT, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435–441.
Lee AM, Clear AJ, Calaminici M, et al. Number of CD4 + cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J Clin Oncol. 2006;24:5052–5059.
Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472.
Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46:2788–2798.
Warusavitarne J, Schnitzler M. The role of chemotherapy in microsatellite unstable (MSI-H) colorectal cancer. Int J Colorectal Dis. 2007;22:739–748.
Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099.
Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997.
Baker K, Foulkes WD, Jass JR. MSI-H colorectal cancers preferentially retain and expand intraepithelial lymphocytes rather than peripherally derived CD8+ T cells. Cancer Immunol Immunother. 2009;58:135–144.
Lee SY, Miyai K, Han HS, et al. Microsatellite instability, EMAST, and morphology associations with T cell infiltration in colorectal neoplasia. Dig Dis Sci. 2012;57:72–78.
Michel S, Benner A, Tariverdian M, et al. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. 2008;99:1867–1873.
Acknowledgments
This work was supported in part by a Grant in Aid for Scientific Research (21791280) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Conflict of interest
The authors have no conflicts of interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mori, K., Toiyama, Y., Saigusa, S. et al. Systemic Analysis of Predictive Biomarkers for Recurrence in Colorectal Cancer Patients Treated with Curative Surgery. Dig Dis Sci 60, 2477–2487 (2015). https://doi.org/10.1007/s10620-015-3648-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3648-2