Abstract
Background
Sunitinib, a multi-targeted receptor tyrosine kinase inhibitor, has demonstrated survival benefit in patients with metastatic renal cell carcinoma (mRCC); however, significant adverse events (AEs) have been associated with its use. The significant variation in the reported incidences of AEs has prompted this meta-analysis to quantify the risk and explore associated predictors.
Methods
According to predefined selection criteria, a literature search identified 12 studies that were included in the analyses.
Results
The meta-analysis included 5,658 patients; 66 % patients had prior systemic therapy whereas the remaining patients (34 %) received sunitinib in the first-line setting. For any grade toxicity, skin rash, fatigue, diarrhea, and mucositis were the most frequently encountered events (81, 52, 45, and 33 %, respectively). Anemia, neutropenia, or thrombocytopenia of any grade occurred in more than one-third of patients, although grades 3 or 4 were less common. Any grade raised by liver enzymes or serum creatinine occurred in 40 and 44 % of patients, respectively. Meta-regression analyses showed that study size was inversely related to the risk of experiencing fatigue, diarrhea, mucositis, anemia, and thrombocytopenia. In particular, the incidence of AEs was higher when sunitinib was used in pretreated versus naive patients; however, there was no significant difference between the two groups concerning the incidence of laboratory abnormalities. We addressed the limitations of reporting AEs in clinical studies.
Conclusions
The present meta-analysis quantified sunitinib-associated AEs. The derived estimates would be similar to that to be expected from the use of sunitinib in community practice in unselected patients with metastatic renal cell carcinoma (mRCC).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC), the most common form of kidney cancer, accounts for 2–3 % of all malignant diseases in the adult population [1]. Surgery is still the only known treatment with curative intent, with cytoreductive surgery and metastasectomy proved to provide a survival benefit as well [2]. Historically, there has been a lack in significant effective systemic therapeutic options for unresectable and metastatic RCC (mRCC). Cytokine therapy (interferon-alpha, interleukin II) previously was the only available treatment option; however, the benefit has been marginal and at the expense of serious toxicities [3, 4].
New novel therapies proved to be more effective than the old standards [5–8], and since 2005 up to early 2012, there have been seven FDA-approved molecular therapeutics for mRCC [9]. Sunitinib is a small-molecule, multiple receptor tyrosine kinase inhibitor that targets vascular endothelial growth factor receptors (VEGF-R types 1–3), platelet-derived growth factor receptors (PDGFR-α and -β), stem cell factor receptor (c-KIT), FMS-like tyrosine kinase (FLT-3), colony-stimulating factor (CSF-1R), and neurotrophic factor receptor (RET) [10, 11].
In a phase III randomized clinical trial first published in 2007 [5], sunitinib showed superiority over interferon-alpha as a first-line treatment in both objective response rates and progression-free survival (PFS) (11 vs. 5 months). Later, in 2009, updated survival data showed significant overall survival (OS) benefit (26.4 vs. 21.8 months) [12]. The authors reported a wide range of adverse events (AEs) in the sunitinib arm. Other studies reported widely variable rates and grades of sunitinib-associated AEs [13–18].
This variability has prompted the current meta-analysis to quantify sunitinib toxicity and to explore the reasons for such variability. To best of our knowledge, no such meta-analysis has been previously attempted.
Methods
Search strategy
Between January 1966 and September 2012, we identified studies of interest by first conducting an electronic literature search of the following databases: Medline via PubMed, EMBASE, OVID, Web of Science, evidence-based medicine (British Medical Journal), and the Cochrane Library. We also searched for relevant abstracts in annual conference proceedings between January 1984 and September 2012 for the American Society of Clinical Oncology and the European Society for Medical Oncology. RCC patients of any age were eligible for inclusion.
We used exploded Medical Subject Heading terms or keyword terms ‘renal,’ ‘kidney,’ ‘cell,’ and ‘clear.’ The terms were combined with ‘neoplasm, cancer, metastatic’ using the Boolean operator ‘and.’ Search results were also filtered against the terms (tyrosine kinase inhibitor, sunitinib). In the second step, these keywords were combined using the Boolean operator ‘and’ with ‘adverse events and/or side effects.’ In addition, we manually reviewed the reference lists of relevant studies to identify additional pertinent published articles.
Selection criteria
Studies were defined as eligible if they were (1) prospective or retrospective and published in the English language between January 1985 and September 2012; (2) included patients at any age or gender with mRCC; (3) reported on sunitinib AEs with or without reporting on efficacy either in the first- or subsequent-lines settings; (4) reported adequate AEs data or data allowing such outcomes to be computed; and (5) published as original articles (no case reports, case series less than 10 patients, reviews, comments, letters, or editorials). The decision to include or exclude studies was hierarchical, initially made based on the study title, followed by the abstract, and finally the complete body text. In the event of conflicting opinions, resolution was achieved though discussion.
When several articles reported on the same patient material, we only included in the analysis the most recent data, studies with the longer follow-up, or the most relevant studies. We excluded studies that only examined the effect of presurgical sunitinib. Also excluded were combined-modality designs, i.e., sunitinib combined with any standard or experimental agent.
Quality of included studies
The MINORS (Methodological Index for Non-Randomized Studies) tool was chosen for assessing the quality of the nonrandomized studies [19], whereas the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting criteria were used to assess the quality of randomized controlled trials [20]. The authors discussed any significant discrepancy in the quality scores assigned to reach a consensus.
Data extraction
All authors independently inspected each reference title identified by the search and applied the inclusion criteria. For possibly relevant articles and in cases of disagreement between reviewers, the full article was obtained and inspected independently by all authors. The data intended for extraction were discussed, and decisions were documented.
We used a standardized Microsoft Excel spreadsheet for data extraction. Two authors extracted the relevant data, and a third reviewer verified the abstraction. Extracted data included the followings: study characteristics (first author’s last name, publication year, country in which the study was carried out, and data source), study design, number of RCC patients, histology, sex distribution, mean/median age of patients, mean/median duration of follow-up, prior and study therapy details, efficacy, and AE data.
For each AE, we estimated the incidence rate (IR) according to the number of events reported during the observation period without taking into account the observation time length. Because there were a relatively small number of some events commonly for grade 3 or 4, the data were assumed to follow a Poisson distribution [21]. For each study, the IR of the AE and its 95 % confidence interval (CI) were calculated from Poisson models. Where not reported, we computed the CI for the risk assuming a Poisson distribution for the observed number of cases. Standard error (SE) for the natural logarithm of IR (ln IR) was derived from CI, applying the following equation: SE = ln (upper 95 % CI/lower 95 % CI)/(2 × z 1−a/2) [22]. Where appropriate, we also used the built-in calculator of the Review Manager Software (version 5.1.6 for Windows; The Cochrane Collaboration, Oxford, UK) to compute relevant data. When a zero rate of an AE was reported, meta-analysis was performed by using a value of one event in single-arm studies or one event in each arm of randomized studies (because mathematical difficulties arise with ln relative rate (RR) transformations involving zero (log of zero = minus infinity) [23].
Outcome measures
The primary outcome was the pooled IR for various sunitinib-associated AEs. The secondary outcome was the numbers-needed-to-harm (NNH) with sunitinib therapy to cause one AE, and a 95 % CI, were calculated as the reciprocal of the IR and its 95 % CI. Another secondary outcome was the difference in the incidence of AEs in the in first-line versus subsequent-lines setting.
Statistical analyses
We assessed heterogeneity of study results by inspecting graphical presentations and by calculating a χ 2 test of heterogeneity and the I 2 statistic of inconsistency [24, 25]. We defined statistically significant heterogeneity as a χ 2 P value less than 0.1 or an I 2 statistic greater than 50 %. The estimates of pooled IR, together with associated 95 % CI, were obtained using the DerSimonian and Laird random-effects model [26] using the Review Manager Software. We used random-effect models because of the variability of sample characteristics, interventions, and comparison conditions.
We performed meta-regression analysis to explore covariates that could explain heterogeneity using IBM SPSS statistical package ver. 19. The dependent variable was the lnIR weighted for the inverse of variance and using as predictors: source of data, median/mean age of included patients, gender, proportions of pretreated patients, performance status, median duration of therapy or median number of given cycles, efficacy data, or any additional relevant risk factors. We first conducted a univariate regression analysis for each predictor followed by a multivariate regression only including predictors found significant in the univariate analysis. Where appropriate, we assumed the data to be missing at random; therefore, observed study characteristics were used to impute missing data by means of multiple imputation [27].
A funnel plot estimating the precision of trials (plots of logarithm of the IR against the sample size) was examined for asymmetry to assess publication bias [28]. Publication bias was also quantified by the regression asymmetry test by Egger et al. [28]. In the test, we regressed IR or study size versus the inverse variance. The significance of the intercept was determined by the t test suggested by Egger (P < 0.05 was considered statistically significant publication bias). Any statistical tests were two sided. The methodology and reporting of this review conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [29].
Results
We identified 830 potentially relevant published articles. After exclusion of duplicate references, nonrelevant literature, and those articles that did not satisfy inclusion criteria, we included 12 candidate articles (Fig. 1). There were 9 single-arm [10, 13, 16–18, 30–33] and 3 randomized studies [12, 15, 34]. We also included an additional retrospective study (175 pretreated patients) that only reported on the incidence of hypertension and decreased ejection fraction (EF) [35]. The reported data of the latter study were included in the analysis of these AEs. Of the 12 included studies, there were several reports of overlapping and/or updated data with longer follow-up and more encountered events. For any analysis, we only used the updated results unless there were relevant data available in an earlier report and were not included in a publication that is more recent. Table 1 shows the clinical characteristics and the efficiency data of the included studies. In 2 of the 3 randomized studies, sunitinib was administered in the two study arms. Escudier et al. [15] compared morning versus evening sunitinib dosing; Motzer et al. [34] randomized patients between sunitinib 50 mg/day for 4 weeks followed by 2 weeks off treatment (4/2 schedule) versus continuous sunitinib 37.5 mg/day. In the third phase III randomized study, the authors compared the standard schedule of sunitinib 50 mg/day (4/2) against interferon-alpha in the first-line setting [12].
The funnel plot of 12 nonoverlapping studies showed mild asymmetry; however, the Egger linear regression tests were not significant (P = 0.059–0.90), indicating no evidence of significant publication bias.
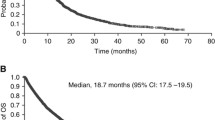
The meta-analysis included 5,658 patients: 3,176 (66 %) patients had prior systemic therapy whereas the remaining 1,942 (34 %) patients received sunitinib in the first-line setting. The median age was 60.5 years (95 % CI, 58.6–61.4 years). On average, 68 % of patients were male, and 89 % had performance status 0–1. Most patients (96 %) had a clear cell component. Objective response rate (ORR), PFS, and OS ranged from 17 to 54 %, from 7.1 to 12.6 months, and from 11 to 33.1 months, respectively.
Table 2 shows the pooled IRs of the AEs and NNH. For any grade toxicity, skin rash, fatigue, diarrhea, and mucositis were the most frequently encountered events (81, 52, 45, and 33 %, respectively). The most frequent grade 3 or 4 AE was fatigue (12 %). Hypertension of any grade occurred in 29 % of patients; 7 % experienced hypertension of higher grades. Anemia, neutropenia, or thrombocytopenia of any grade occurred in more than one-third of patients; grade 3 or 4 was less common. Pooled IR for abnormal liver enzymes and increased serum creatinine occurred in 40 and 44 % of patients, respectively. However, grades 3 or 4 hepatic or renal abnormalities were rare (3 and 2 %, respectively).
Analysis of the pooled IRs, however, showed significant heterogeneity that was more apparent in the analyses of any grade AEs (Table 2). To explore such heterogeneity, we performed a series of meta-regression analyses. The covariates explored were study size, single versus randomized studies, median age, proportion of male patients, proportion of patients with 0–1 performance status, median duration of therapy or median number of given cycles, proportion of patients who received prior systemic therapy, ORR, PFS, and OS.
Table 3 showed that study size was inversely related to the risk of experiencing fatigue, diarrhea, mucositis, anemia, and thrombocytopenia. Moreover, prior therapy increased the risk of mucositis; a higher proportion of male patients was associated with anemia events; and patients who lived longer experienced a greater incidence of thrombocytopenia. The meta-regression failed to explain heterogeneity in the incidence of skin rash, nausea, vomiting, hypertension, neutropenia, or impaired hepatic or renal function.
To compare the incidence of AEs among those who received sunitinib in the first-line setting versus that reported for pretreated patients, we performed a series of meta-analyses where such data were available. Table 4 shows that several clinical AEs occurred more frequently in pretreated patients [fatigue (any grade), diarrhea (grades 3 and 4), nausea (any grade), vomiting (grades 3 and 4), epistaxis (any grades), and limb pain (any grade)]. On the other hand, there was no statistically significant difference between the two groups concerning the incidence of laboratory abnormalities.
Discussion
This meta-analysis showed the toxicity profile of sunitinib in a broad mRCC population. The meta-analysis included 5,658 patients (34 % received sunitinib in the first-line setting). The clinical characteristics of patients in the current meta-analysis [median age of 60.5 years, male preponderance (68 %), and predominantly harboring clear cell component (96 %)] were comparable to the known clinical features in an unselected mRCC population [36, 37]. Therefore, it would be expected that the incidence of sunitinib-induced AEs to be encountered in community practice would be similar to that shown in the current analysis.
Any grade skin rash was the most frequently encountered AE occurred with a pooled IR of 81 %. On the other hand, hand–foot syndrome (HFS) of any grade occurred in 30 % of patients, with 8 % experiencing grade 3 or 4. The exact pathogenesis of HFS associated with sunitinib use is still unknown. Although there is no specific remedy for HFS, topical application of moisturizers, pain management, and dose reduction or interruptions may ameliorate symptoms.
The pooled IR of any type of fatigue was 52 %. To date, the mechanisms for both types of fatigue (cancer-related and sunitinib-induced) are still poorly understood. Fatigue may be caused or exacerbated by underlying dehydration, hypothyroidism, hypercalcemia, anemia, heart failure, or depression. Currently, there are very few evidence-based interventions to treat fatigue. The National Comprehensive Cancer Center fatigue guidelines recommend screening of cancer patients at the initial visit [38]. The guidelines recommend a qualitative or quantitative scale to assess fatigue intensity. Kollmannsberger et al. [39] provided general recommendations for the management of sunitinib-induced fatigue, suggesting several behavioral modifications, e.g., taking short naps, drinking plenty of fluids, light exercise, etc. They also recommended sunitinib dose reduction and/or brief treatment interruption if appropriate.
The IRs of any grade of diarrhea, nausea, vomiting, and mucositis occurred in 45, 36, 21, and 33 %, respectively. On the other hand, grades 3 and 4 were rare. The underlying pathogenesis for sunitinib-induced diarrhea is not known, and it is usually distinctive from chemotherapy-induced diarrhea. Sunitinib-induced diarrhea can occur with days of diarrhea mixed with days of normal bowel movements. In the management of sunitinib-induced emesis, caution should be exercised when combining sunitinib with antidopaminergic agents, as these have been associated with prolonged QT/QTc intervals [40].
Although IR of any grade mucositis was 33 %, higher grades were rare (3 %). Patients with sunitinib-induced mucositis usually report a general sensitivity in the mouth, which feels sore, or they have alterations in taste, but clinical findings are largely normal without the typical physical signs of a mucositis/stomatitis caused by chemotherapy.
The pooled analysis of IRs of cardiovascular toxicity was comparable to that reported from studies that primarily intended to examine such complications [35, 41]. The pooled IR of hypertension of any grade was 29 % (grade 3 or 4, 7 %), whereas pooled IR of decreased EF was rare (2 %). The latter, however, was estimated from three studies [10, 12, 13]. Kappers et al. [41] investigated the effects of sunitinib on blood pressure, its circadian rhythm, and potential mechanisms, in 15 patients with mRCC or gastrointestinal stromal tumors. The authors concluded that sunitinib induces a reversible rise in blood pressure (BP) associated with activation of the endothelin-1 system and suppression of the renin-angiotensin system. It another study, endomyocardial biopsy samples of patients during sunitinib treatment showed changes in mitochondrial structure [42]. Several reports suggested that the impaired ATP generation secondary to mitochondrial dysfunction is the underlying mechanism for the development of cardiac dysfunction [43, 44].
Among 175 patients with mRCC, grade 3 hypertension was seen in 10 % of patients, and of those, 71 % experienced left ventricular systolic dysfunction [35]. Of all patients in this series, 18.9 % developed some degree of cardiac abnormality, and 7 % developed congestive heart failure (CHF). History of coronary artery and hypertension history were the only significant independent predictors of CHF.
The IR of hypothyroidism was 9 %. The literature showed a discrepancy between IR reported in prospective trials and retrospective series, most likely caused by infrequent testing for hypothyroidism, particularly in early studies, before hypothyroidism was recognized as a common side effect [39]. In a recently published Japanese study of 17 patients with mRCC receiving sunitinib, the investigators prospectively evaluated the thyroid volume serially using CT volumetry on a cervical-pelvic CT scan [45]. Interestingly, hypothyroidism during sunitinib treatment occurred in 8 of 8 patients who experienced more than 50 % reduction in the thyroid volume (one patient was hypothyroid at baseline). In this study, histological changes in the thyroid gland in the 4 autopsied patients and all patients showed atrophy of thyroid follicles and degeneration of follicular epithelial cells.
Thyroid dysfunction while receiving sunitinib can present as thyroid-stimulating hormone (TSH) elevation only with normal T4 levels (subclinical hypothyroidism) or TSH elevation and low T4 (overt hypothyroidism). Although the exact mechanism by which this complication occurs remains unknown, it has been suggested that sunitinib may induce a destructive thyroiditis through follicular cell apoptosis [46], or it may include endothelial dysfunction, regression of fenestrated capillaries, inhibition of iodine uptake, and reduced synthesis of thyroid hormone [47, 48]. The relationship between sunitinib-induced hypothyroidism and the drug effect on gland vascularity has been controversial [48, 49]. Also controversial is the relationship between the development of hypothyroidism and sunitinib clinical benefit [50, 51].
The meta-regression analyses showed that study size explained the heterogeneity in the IRs for several AEs (fatigue, diarrhea, mucositis, anemia, and thrombocytopenia), whereas larger studies reported lower IRs. In a recently published systemic review of 156 studies reporting on AEs [52], the authors concluded that smaller studies reported higher AE rates and more significant variation. The authors suggested that large studies almost exclusively use the International Statistical Classification of Diseases and Related Health Problems (ICD) for coding events, whereas small trials look at cases more carefully, commonly using chart review. ICD coding is able to display only a fraction of events, which might explain the comparatively low estimates [53].
The meta-analysis also showed that sunitinib-associated AEs were more prevalent in pretreated patients compared with those who received sunitinib in the first-line setting. The study of Tomita et al. [32] was the only study that directly compared the IR of AEs in those two groups.
Although the current meta-analysis is the only known attempt to quantify sunitinib-associated AEs in mRCC, the analysis has several limitations. Of all the included studies, three were randomized and in two of these sunitinib was given to patients in the two comparators [15, 34]. Moreover, there were several limitations that are inherent to studies which report on AEs. First, in many occasions it is almost impossible to distinguish drug- versus disease-related AEs, e.g., fatigue. Second, it has been shown that the IR of reported AEs could be affected by the methods used in reporting [54, 55]. In 214 men with benign prostatic hyperplasia, patients assigned to the checklist group reported a total of 238 adverse events; in comparison, patients who were asked an open-ended question or an open-ended, defined question reported 11 and 14 adverse events, respectively [54]. Third, there is also a limitation attributable to the nocebo phenomenon, which refers to the AEs reported on a placebo arm. Rosenzweig et al. [56] reported a 19 % incidence of AEs in healthy volunteers during placebo administration using data from 1,228 volunteers from 109 double-blind, placebo-controlled pharmacology trials. Also, Hillman et al. [57] reported that nearly 50 % of AEs were reported as attributed to the study drug on the placebo arm of two large phase III randomized clinical trials.
We conclude that the present meta-analysis provided an adequate estimate of sunitinib-associated AEs. The pattern derived from the included studies would be similar to that to be expected from the use of sunitinib in community practice in unselected patients with mRCC.
References
Rini BI, Campbell SC, Escudier B (2009) Renal cell carcinoma. Lancet 373(9669):1119–1132. doi:10.1016/S0140-6736(09)60229-4
Flanigan RC, Mickisch G, Sylvester R et al (2004) Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 171(3):1071–1076
Fyfe G, Fisher RI, Rosenberg SA et al (1995) Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13(3):688–696
McDermott DF, Regan MM, Clark JI et al (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 23(1):133–141. doi:10.1200/jco.2005.03.206
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2):115–124. doi:10.1056/NEJMoa065044
Hudes G, Carducci M, Tomczak P et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356(22):2271–2281. doi:10.1056/NEJMoa066838
Escudier B, Szczylik C, Hutson TE et al (2009) Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27(8):1280–1289. doi:10.1200/jco.2008.19.3342
Sun M, Lughezzani G, Perrotte P et al (2010) Treatment of metastatic renal cell carcinoma. Nat Rev Urol 7(6):327–338. doi:10.1038/nrurol.2010.57
http://www.cancer.gov/cancertopics/druginfo/kidneycancer. Accessed 10 Sep 2012
Motzer RJ, Michaelson MD, Redman BG et al (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24(1):16–24. doi:10.1200/JCO.2005.02.2574
Mendel DB, Laird AD, Xin X et al (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9(1):327–337
Motzer RJ, Hutson TE, Tomczak P et al (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27(22):3584–3590. doi:10.1200/JCO.2008.20.1293
Gore ME, Szczylik C, Porta C et al (2009) Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 10(8):757–763. doi:10.1016/s1470-2045(09)70162-7
Porta C, Paglino C, Imarisio I et al (2011) Safety and treatment patterns of multikinase inhibitors in patients with metastatic renal cell carcinoma at a tertiary oncology center in Italy. BMC Cancer 11:105. doi:10.1186/1471-2407-11-105
Escudier B, Roigas J, Gillessen S et al (2009) Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol 27(25):4068–4075. doi:10.1200/jco.2008.20.5476
Rini BI, Michaelson MD, Rosenberg JE et al (2008) Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 26(22):3743–3748. doi:10.1200/jco.2007.15.5416
Barrios CH, Hernandez-Barajas D, Brown MP et al (2012) Phase II trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma. Cancer (Phila) 118(5):1252–1259. doi:10.1002/cncr.26440
Kontovinis LF, Papazisis KT, Touplikioti P et al (2009) Sunitinib treatment for patients with clear-cell metastatic renal cell carcinoma: clinical outcomes and plasma angiogenesis markers. BMC Cancer 9:82. doi:10.1186/1471-2407-9-82
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. A N Z J Surg 73(9):712–716 (pii: 2748)
Gallo V, Egger M, McCormack V et al (2011) STrengthening the Reporting of OBservational studies in Epidemiology—Molecular Epidemiology (STROBE-ME): an extension of the STROBE Statement. PLoS Med 8(10):e1001117. doi:10.1371/journal.pmed.1001117
Clayton D, Hills M (1993) Statistical models in epidemiology. Oxford University Press, Oxford
Pirani M, Marcheselli R, Marcheselli L et al (2011) Risk for second malignancies in non-Hodgkin's lymphoma survivors: a meta-analysis. Ann Oncol 22(8):1845–1858. doi:10.1093/annonc/mdq697
Alder N, Fenty J, Warren F et al (2006) Meta-analysis of mortality and cancer incidence among workers in the synthetic rubber-producing industry. Am J Epidemiol 164(5):405–420. doi:10.1093/aje/kwj252
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. doi:10.1136/bmj.327.7414.557
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. doi:10.1016/0197-2456(86)90046-2
Donders AR, van der Heijden GJ, Stijnen T et al (2006) Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 59(10):1087–1091. doi:10.1016/j.jclinepi.2006.01.014
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Moher D, Liberati A, Tetzlaff J et al (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341. doi:10.1016/j.ijsu.2010.02.007
Motzer RJ, Rini BI, Bukowski RM et al (2006) Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295(21):2516–2524. doi:10.1001/jama.295.21.2516
Ansari J, Fatima A, Fernando K et al (2010) Sunitinib in patients with metastatic renal cell carcinoma: Birmingham experience. Oncol Rep 24(2):507–510
Tomita Y, Shinohara N, Yuasa T et al (2010) Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 40(12):1166–1172. doi:10.1093/jjco/hyq146
Josephs D, Hutson TE, Cowey CL et al (2011) Efficacy and toxicity of sunitinib in patients with metastatic renal cell carcinoma with severe renal impairment or on haemodialysis. BJU Int 108(8):1279–1283. doi:10.1111/j.1464-410X.2010.09990.x
Motzer RJ, Hutson TE, Olsen MR et al (2012) Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol 30(12):1371–1377. doi:10.1200/jco.2011.36.4133
Di Lorenzo G, Autorino R, Bruni G et al (2009) Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol 20(9):1535–1542. doi:10.1093/annonc/mdp025
Cairns P (2010) Renal cell carcinoma. Cancer Biomark 9(1–6):461–473. doi:10.3233/CBM-2011-0176
Ng CS, Wood CG, Silverman PM et al (2008) Renal cell carcinoma: diagnosis, staging, and surveillance. AJR Am J Roentgenol 191(4):1220–1232. doi:10.2214/AJR.07.3568
National Comprehensive Cancer Network: cancer-related fatigue. (2012). http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf
Kollmannsberger C, Bjarnason G, Burnett P et al (2011) Sunitinib in metastatic renal cell carcinoma: recommendations for management of noncardiovascular toxicities. Oncologist 16(5):543–553. doi:10.1634/theoncologist.2010-0263
Navari RM, Koeller JM (2003) Electrocardiographic and cardiovascular effects of the 5-hydroxytryptamine3 receptor antagonists. Ann Pharmacother 37(9):1276–1286. doi:10.1345/aph.1C510
Kappers MH, van Esch JH, Sluiter W et al (2010) Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 56(4):675–681. doi:10.1161/HYPERTENSIONAHA.109.149690
Chu TF, Rupnick MA, Kerkela R et al (2007) Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370(9604):2011–2019. doi:10.1016/S0140-6736(07)61865-0
Force T, Krause DS, Van Etten RA (2007) Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 7(5):332–344. doi:10.1038/nrc2106
Cheng H, Force T (2010) Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res 106(1):21–34. doi:10.1161/CIRCRESAHA.109.206920
Shinohara N, Takahashi M, Kamishima T et al (2011) The incidence and mechanism of sunitinib-induced thyroid atrophy in patients with metastatic renal cell carcinoma. Br J Cancer 104(2):241–247. doi:10.1038/sj.bjc.6606029
Desai J, Yassa L, Marqusee E et al (2006) Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med 145(9):660–664 (pii: 145/9/660)
Wong E, Rosen LS, Mulay M et al (2007) Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid 17(4):351–355. doi:10.1089/thy.2006.0308
Mannavola D, Coco P, Vannucchi G et al (2007) A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab 92(9):3531–3534. doi:10.1210/jc.2007-0586
Makita N, Miyakawa M, Fujita T et al (2010) Sunitinib induces hypothyroidism with a markedly reduced vascularity. Thyroid 20(3):323–326. doi:10.1089/thy.2009.0414
Wolter P, Stefan C, Decallonne B et al (2008) The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br J Cancer 99(3):448–454. doi:10.1038/sj.bjc.6604497
Schmidinger M, Vogl UM, Bojic M et al (2011) Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer (Phila) 117(3):534–544. doi:10.1002/cncr.25422
Lessing C, Schmitz A, Albers B et al (2010) Impact of sample size on variation of adverse events and preventable adverse events: systematic review on epidemiology and contributing factors. Qual Saf Health Care 19(6):e24. doi:10.1136/qshc.2008.031435
Romano PS, Chan BK, Schembri ME et al (2002) Can administrative data be used to compare postoperative complication rates across hospitals? Med Care 40(10):856–867. doi:10.1097/01.MLR.0000027452.96163.A4
Bent S, Padula A, Avins AL (2006) Brief communication: better ways to question patients about adverse medical events: a randomized, controlled trial. Ann Intern Med 144(4):257–261
Ioannidis JP, Mulrow CD, Goodman SN (2006) Adverse events: the more you search, the more you find. Ann Intern Med 144(4):298–300
Rosenzweig P, Brohier S, Zipfel A (1993) The placebo effect in healthy volunteers: influence of experimental conditions on the adverse events profile during phase I studies. Clin Pharmacol Ther 54(5):578–583
Hillman SL, Mandrekar SJ, Bot B et al (2010) Evaluation of the value of attribution in the interpretation of adverse event data: a North Central Cancer Treatment Group and American College of Surgeons Oncology Group investigation. J Clin Oncol 28(18):3002–3007. doi:10.1200/JCO.2009.27.4282
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors contributed equally to this work.
About this article
Cite this article
Ibrahim, E.M., Kazkaz, G.A., Abouelkhair, K.M. et al. Sunitinib adverse events in metastatic renal cell carcinoma: a meta-analysis. Int J Clin Oncol 18, 1060–1069 (2013). https://doi.org/10.1007/s10147-012-0497-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0497-2