Abstract
The ability of invasive mammals to adjust their diet in response to new or variable resources is often proposed to explain their invasion success on islands with differing environmental conditions, especially islands with strong spatiotemporal changes in the nature and abundance of their resources. In this study, we investigated how habitat heterogeneity and seasonal fluctuation in resource quality affect dietary breadth and plasticity in an island-invasive rodent, the black rat Rattus rattus, on a small Mediterranean island. We tested for dietary plasticity of rats at both the individual and population levels by using traditional dietary and stable isotope analyses at successively increasing time scales, coupled with a long-term study of individual rats in three habitats of close proximity. Dietary and movement analyses both indicated that R. rattus is able to exploit a wide range of resources and habitats. However, dietary plasticity and habitat breadth were far narrower at the individual level. Results revealed that rats exclusively used resources found in their local habitat, and very few individuals moved among adjacent habitats in pursuit of higher-quality resources, despite those resources being abundant in their immediate environment. This counterintuitive finding suggests that intraspecific interactions must restrict rat mobility. Our results suggest that even on small islands, accessibility of patchy and high-quality resources to individuals from the entire population is not systematic. This result has important implications when quantifying invasive rodent impacts on patchily distributed species, especially when studies use indirect methods such as dietary analyses as a substitute for direct observations of predatory behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a constantly changing world, species must continually adapt their behavior in order to succeed in their environment. Intrinsic attributes that may predispose a species to be successful are generally related to niche breadth (Ehrlich 1989; Williamson 1996; Vasquez 2005) and may include behavioral flexibility (Sol and Lefebvre 2000; Sol et al. 2002) and dietary or habitat breadth (Ehrlich 1989; Brousseau et al. 1996; Vasquez 2005; Jeschke and Trayer 2006; Blackburn et al. 2009). According to the optimal foraging theory, generalist foragers should exploit food that maximizes the net rate of energy intake by focusing on high-quality and abundant resources (MacArthur and Pianka 1966; Pyke et al. 1977). A nonspecialized forager can opportunistically switch to alternative resources as a result, for example, of seasonal resource fluctuations or of drastic environmental changes (Ben-David et al. 1997; Begg et al. 2003; Stapp and Polis 2003; Dell’Arte et al. 2007; Popa-Lisseanu et al. 2007). This is particularly true when resources periodically become available to species in their immediate environment (Pyke et al. 1977; Boutin 1990; Lin and Batzli 2001).
Invasive mammals have established on islands of various environmental conditions, and their impacts on native communities are often devastating (e.g., Courchamp et al. 2003). Island systems often undergo marked spatiotemporal variations in resource availability, often driven by the seasonal pulses of enriched resources. Examples include seasonal patterns of seabird or sea-turtle nesting (Polis et al. 1997a; Caut et al. 2008a) or seasonal flushes of plant productivity such as fruit and seed ripening (e.g., Polis et al. 1997b; Gregory and Macdonald 2009). Therefore, identifying specific foraging and habitat use strategies that support invasive mammals on islands and enable them to cope with sometimes extreme seasonal variation in trophic conditions is crucial to a better understanding of the invasive species’ impacts on native communities.
The three species of invasive rats, Rattus rattus (Linné 1758), R. norvegicus (Berkenhout 1769), and R. exulans (Peale 1968), are among the most successful vertebrate island invaders (Courchamp et al. 2003). Rats have invaded >80% of the world’s archipelagos (Atkinson 1985) and are identified as a leading cause of decline, extirpation, and extinction of island species (Towns et al. 2006; Jones et al. 2008). The ability of rats to establish on islands ranging from the wet tropics to subarctic tundra has been attributed to dietary and ecological flexibility (Courchamp et al. 2003; Caut et al. 2008a; Jones et al. 2008). Rats feed on plant leaves, stems, fruits, and roots (Daniel 1973; Clark 1981; Grant-Hoffman and Barboza 2010), macroinvertebrates (Palmer and Pons 1996; Rufaut and Gibbs 2003; Towns et al. 2009), reptiles (Towns et al. 2003, 2007), birds (Imber 1975; Blackburn et al. 2004; Jones et al. 2008), and some mammals (Harris 2009). Rats are also known to preferentially select food items with high energy and nutrient value, such as fruits, seeds, eggs, birds, and sea-turtle hatchlings (Imber 1975; Clark 1981; Caut et al. 2008a; Jones et al. 2008; Grant-Hoffman and Barboza 2010). Moreover, the spatiotemporal variability of these high-quality resources has often been hypothesized to induce dietary shifts in island-invasive rats toward alternative resources to compensate the temporary absence of primary resources (Stapp 2002; Stapp and Polis 2003; Major et al. 2006; Towns et al. 2006), yet this diet-switching ability has rarely been demonstrated.
Comparative analyses of naturally occurring stable isotope ratios of carbon (13C/14C, expressed as δ13C) and nitrogen (15N/14N, expressed as δ15N) between consumers’ tissues of different turnover rates (e.g., liver vs. muscle) are a powerful tool for tracking dietary and habitat use through time and to determine seasonal dietary and habitat shifts (Phillips and Eldridge 2006; Crawford et al. 2008), especially when resources differ in their carbon origin (e.g., marine vs. terrestrial or C3 plants vs. C4 plants) or trophic levels (e.g., N-enriched habitats within seabird colonies on shore vs. oligotrophic inland; Caut et al. 2008a). Caut et al. (2008a) recently demonstrated a shift in black rat (R. rattus) isotopic signatures on a dry tropical island, reflecting a dietary shift between seasons varying in resource availability. However, whether dietary plasticity was attributable to marked shifts in individual diet or, alternatively, to differences in diet between different sampled subpopulations remains unclear, as the study did not track individual rats. The ability to track seasonal dietary or habitat shifts in individual rats would be improved by combining resource use analyses at successively larger time scales with individual-based movement analyses over several periods of resource availability. However, this multisource approach has never been used for invasive rodents on islands.
In this study, we investigated how habitat heterogeneity and seasonal fluctuation in resource quality within adjacent habitats affect dietary breadth and plasticity in an invasive rodent, the black rat, on a small Mediterranean island. We selected three small habitat patches in very close proximity to ensure that resources would be successively available to rats in their immediate environment. We investigated the capacity of rats to opportunistically switch diet when higher-quality resources became available. More specifically, we tested for the level at which plasticity became apparent by analyzing resource use by individuals at various time scales (i.e., individual-level plasticity) and comparing diet among individuals (i.e., population-level plasticity) from different habitats and during different seasons over 3 years. To investigate spatiotemporal patterns of resource and habitat use by rats, analyses of fecal contents (i.e., reflecting the daily diet) and stable isotopes of liver (i.e., reflecting the diet of the previous week) and muscle (i.e., reflecting the diet of the previous month) were combined with a 2-year capture–mark–recapture survey of rats and a 1-month radio-tracking study of rat movements within and among adjacent habitats. As rats are opportunistic generalist foragers, we hypothesized they would successively use the different resources according to their availability and quality in order to maximize energy intake, especially during drastic seasons. We expected that dietary breadth and plasticity would vary at the individual level and that rats would move among adjacent habitats through seasons.
Methods
Study site
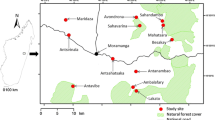
This study was conducted on Bagaud Island (58 ha; 1.48 km long; 0.59 km wide), Port-Cros National Park, 7.5 km from the southeast coast of France, in the Mediterranean Sea (Fig. 1). The island is mainly composed of acid rock substrate and reaches 57 m above sea level. Mean monthly temperatures range from 9.5 to 24.7°C. Annual precipitation varies from 1 to 151.6 mm (Levant Island Meteorological Office 1998–2008). The black rat was probably introduced during Roman times (Ruffino et al. 2009) but persists, despite no permanent fresh water, as the only nonflying mammal on the island.
For this study, we identified three contrasted habitats in close proximity and without geographical barriers (Fig. 1), but with resources that vary in quality, availability, and by season: (1) Gull habitat (hereafter GU) comprises a yellow-legged gull (Larus michahellis) colony with a ruderal grassland mainly composed of Fabaceae, Poaceae, and Juncaceae. In this habitat, plant and arthropod communities were expected to be substantially enriched in nitrogen (especially 15N) due to high guano deposition (Vidal et al. 1998; Orgeas et al. 2003). March through May is the gull breeding period and thus are the 3 months of the year when gull influence is maximal (e.g., high input of marine-derived nutrients, adults feeding chicks, egg/chick carcasses); (2) iceplant habitat (hereafter IC) is as a 1,500-m² patch of the mat-forming Carpobrotus spp. (Aizoaceae). This invasive plant abundantly produces large, fleshy, fig-like fruits enriched in 13C, energy (310 kJ/100 g dry mass−1) and water (80% water; Vilà and D’Antonio 1998) that mature during the dry Mediterranean summer; (3) scrubland habitat (hereafter SC), a native dry Mediterranean mattoral, is as a lower-quality habitat compared with GU and IC. This habitat is composed of a dense, high mattoral (2–3 m) dominated by Pinus halepensis, Erica arborea, Myrtus communis, Arbutus unedo, and Phyllirea spp. (Médail 1998) and a much less dense low-growing scrubland (50 cm) dominated by Pistacia lentiscus and Juniperus phoenicea (Fig. 1). In each habitat, resources are seasonal, unavailable at the same time, but differentially enriched in nutrients (e.g., marine-derived nutrients in GU; fresh water and carbohydrates in IC). On Bagaud Island, black rat density, reproductive output, and individual growth are substantially enhanced by the enriched resources found in GU and IC (Ruffino 2010), indicating that those resources are of high quality for the rats.
Dietary analyses
Rat trapping
In 2006–2007, resources use by rats were assessed by trapping animals within the three study habitats (GU, IC, SC) over three seasons: spring (early May 2006, hereafter MAY), summer (early September 2006, hereafter SEP), and winter (early February 2007, hereafter FEB). Twenty-five live traps (BTS-Mécanique, Manufrance, Saint-Etienne, France) were set in each habitat over two or three consecutive nights. Traps were baited with peanut butter before dusk, checked twice a night to avoid rapid digestion of gut contents by trapped rats through the night, and closed each morning. Rats were euthanized, weighed, and sexed. Fecal contents and tissue samples were collected for, respectively, dietary and stable isotope analyses.
Fecal-content analysis
Rat fecal contents were analyzed using a compound microscope for the three habitats during the three sampling seasons in 2006–2007. The three terminal feces of each rat were removed from the gut and thoroughly rinsed with water through a 250-μm mesh sieve to remove the smallest fragments. For each rat, three microscope slides were analyzed providing a mean of 259 ± 96 standard deviation (SD) items analyzed per rat. Plant items were identified to the lowest systematic level possible with the use of a reference collection of the epidermal parts of most plants found on the island. A relative abundance index for each consumed item was calculated as the mean individual abundance of each item for each habitat–season combination.
Stable isotope analysis
All available potential food items for rats were sampled during each season and for each habitat. Samples of rat liver and muscle were preserved in 70% alcohol before being processed. All samples were dried and ground to fine powder. Specific parts of plants identified to be consumed by rats were treated separately. Isotopic analyses were performed by a spectrometer Delta V Plus (Service Central d’Analyses, CNRS Solaize, France). Stable carbon (C) and nitrogen (N) isotope ratios were expressed as:
where R = 13C/12C or 15N/14N for δ13C or δ15N, respectively. The standard for C is the International Atomic Energy Agency–National Bureau of Standards (IAEA-NBS) 21 (graphite −28.13‰) and for N the IAEA-N1 (+0.4‰) and IAEA-N2 (+20.3‰). Ten replicate assays of internal laboratory standards indicated measurement maximum errors (SD) of ±0.15 and ±0.2‰ for stable C and N isotope measurements, respectively.
Iceplant fruits have a distinct isotopic signature compared with C3 plants and other resources on Bagaud Island, and we used multisource mixing models implemented in the Stable Isotope Analysis in R (SIAR) package (Parnell et al. 2008) to assess the relative contributions of iceplant fruits in the diet of rats from IC and SC across seasons. By quantifying the assimilated proportion of iceplant fruits in the consumer’s diet, isotopic mixing models overcome the possible biases related to fecal content analysis, which can only quantify excreted proportions of seeds or pulp in the diet. No attempt was made to run isotopic models for rats trapped in GU, as no evidence of iceplant fruit consumption was recorded in this habitat with traditional dietary analysis (see “Results”). Compared with traditional multisource mixing models, SIAR has provided results that are markedly more robust when it comes to quantifying feeding preferences for generalist consumers (Moreno et al. 2010; Parnell et al. 2010). By using Bayesian inference, SIAR allows all sources of variation and uncertainty (i.e., SDs in consumer and source signatures) to be propagated through the model to return a true probability distribution of estimated dietary proportions (Jackson et al. 2009; Parnell et al. 2010). Liver tissue was preferentially used to reconstruct resource use by rats, as the turnover rates of stable isotopes are high in liver and reflect the diet of the previous week, whereas turnover rates in muscle are around 1 month (Kurle 2009). As discrimination factors depend on several sources of variation (e.g., taxon, environment, tissue; Caut et al. 2008b), discrimination factors (Δ13C and Δ15N) for rat liver were calculated using specific regression equations between rat liver Δ13C and Δ15N and their corresponding dietary isotopic ratios (Caut et al. 2008b).
Movement analyses
Capture–mark–recapture
In 2007–2009, we investigated whether rats would move among adjacent habitats in response to seasonal availability of resources. From April 2007 to April 2009, 19 capture–mark–recapture sessions were conducted at intervals of 1–3 months. During the 2 years of study, 117 trap stations (BTS-Mécanique, Manufrance, Saint-Etienne, France) were set permanently to cover the three study habitats (SC, GU, IC; 4.25 ha) and record rat movements. Geolocalized traps were set every 20–25 m, depending on vegetation cover. Traps were baited with peanut butter before dusk, set between 3 and 8 consecutive nights depending on weather conditions, and all checked before 09:00. During cold and humid seasons, bedding material was provided inside traps. Rats were individually marked using subcutaneous PIT tags (type FDX-B, IER Paris, France), sexed, weighed to the nearest 2.5 g, checked for sexual maturity, and released after capture. Software Ranges7 (Kenward et al. 2006) was used to calculate distances moved between subsequent captures.
Radio tracking
In August 2008, radio tracking was used to provide a fine-scale measure of foraging behavior and habitat use over days and weeks for comparison with the dietary analyses and capture–recapture data. Nightly movements of a subset of PIT-tagged individuals were monitored around IC during summer, when iceplant fruits were at maximum attractiveness. We specifically focused on PIT-tagged adult rats that had a long-time history of capture (several recaptures over multiple trapping sessions). These criteria restricted the number of potential candidates for radio tracking. Over 12 extensive trapping nights at the end of July, only 13 rats fitted our criteria. We lost two signals during the first days of the experiment, and radio-tracking data were gathered for 11 rats. Of 11 radio-tracked individuals, seven were collared within the 100- to 200-m boundary strip around IC. Cable-tie radio collars (Biotrack Ltd, Dorset, UK) weighing <5% of individual rats’ body weight (Kenward 2007) were fitted. Individuals were tracked on foot using a hand-held TR4 receiver and a flexible three-element Yagi antenna (Telonics Inc., AZ, USA) for 21 nights to provide two to five fixes per night and a mean of 40 (20–54) fixes per rat. The 50% home-range Fixed Kernels (FK) were estimated to locate the core areas of each animal and check whether they would overlap IC. Maximum home-range widths were obtained from 100% minimum convex polygons (MCPs). Both 50% FK and 100% MCP were estimated with Ranges7.
Statistical analysis
Food consumed by rats and identified by fecal analysis was assigned to the following groups: C3, plants use enzyme Rubisco to fix CO2; C4, plants use enzyme phosphoenolpyruvate carboxylate to fix CO2 (only formed by Carpobrotus spp. on Bagaud); Arthropods 1, low-order consumers such as herbivores and detritivores; Arthropods 2, high-order consumers such as predators; Gulls. For each season, the effect of habitat on δ13C and δ15N of consumed food (all groups combined per season), and on both rat liver and muscle were tested with analysis of variance (ANOVA). Dependent variables were tested for normality of distribution before conducting parametric tests. Significant interactions between habitats and seasons were detected by post hoc Scheffe tests. To identify whether rats showed dietary shift toward GU and IC resources during the periods of maximum availability and attractiveness (i.e., spring: peak of marine-derived resources; summer: peak of iceplant fruit ripening), we analyzed data as follows: First, centroid hierarchical cluster analyses (Euclidian distances) were performed on rat liver and muscle isotopic ratios for each habitat–season combination to: (1) identify groups of individuals that could be clustered into three isotopic “niches,” related to the three study habitats; then (2) identify marginal individuals that may have switched habitats in 1-week or 1-month time scales. Before clustering, response variables were standardized to a mean of 0 and an SD of 1. Second, we compared the magnitude of variation in both isotopic ratios of the same rats over a 1-month scale among habitat and seasons to pinpoint any shift of isotopic signatures. Depletion or enrichment of isotopic ratios between tissues were calculated as follows: δxY(liver) − δxY(muscle). Dδ15N and Dδ13C mean values were compared among habitats and seasons with nonparametric bootstrap estimates of confidence intervals (CI). One thousand values were randomly sampled with replacement for each habitat–season combination from our data and 95% CI were calculated from these bootstrap distributions. To test whether the occurrence of isotopic shift is related to body mass or sex, we performed the following analyses: Spearman’s rank correlations between empty body mass (i.e., digestive tract removed) of individuals and both Dδ15N and Dδ13C were applied to test for a relationship between isotopic ratio variation and body mass. Nonparametric Mann–Whitney tests were performed to test whether Dδ13C or Dδ15N may differ by sex. Mann–Whitney tests were performed to test differences in: (1) mean distances moved by animals between subsequent captures between sexes; (2) mean distances moved by animals between subsequent captures within the same trapping session and between different sessions. Spearman’s rank correlations were used to test for a relationship between distances moved between different sessions and time between subsequent captures, with the expectation that large distances would indicate range shifts by animals. Individual movement probabilities (ψ) between habitats were estimated by calculating a state-transition matrix based upon records of rat captures by habitat. This matrix is only partially observed (the habitat of an uncaptured rat during a session is unknown), and so we imputed missing values using a Bayesian approach with a Dirichlet (1,1,1) prior on our state-transition matrix ψ gh for probabilities of movement between sessions from habitat g to h (where \( \sum\nolimits_{h = 1}^{3} {\psi_{gh} = 1} \) for g = 1, 2, 3) (see Schofield et al. 2009). We estimated ψ gh in WinBUGS 1.4 with the first 1,000 iterations discarded as burn-in, and a further 5,000 iterations for statistical summary of the stationary posterior distribution. This method assumes survival is consistent across habitats, which appeared to be true (Ruffino 2010).
Results
Resource use
The fecal content of 87 rats was obtained from 30 rats in May 2006, 29 in September 2006, and 28 in February 2007. Analyses of diet revealed that, across the population, rats consumed a wide range of food throughout habitats and seasons (Table 1). The animal part in the diet was low, mostly represented by terrestrial arthropod remains, with relative abundance ranges from 3–6% to 17–20% according to season and habitat. In May 2006, bird feathers were only recorded in GU. Plant material dominated in the diet, with an overall mean number of different plant taxa consumed ranging from 7 (SD = 3) to 9 (SD = 4). The main species consumed varied with seasons, were characteristic of the habitat where rats were trapped, and mainly comprised Fabaceae, Poaceae, and Juncaceae in GU; Cyperaceae, Rubiaceae, and Smilaceae in SC; and Aizoaceae in IC.
For each season, isotopic ratios of food consumed by rats significantly differed among habitats for both δ13C (F 2,82 = 4.06, P < 0.05) and δ15N (F 2,82 = 31.83, P < 0.001; Table 2). Isotopic ratios of rat liver and muscle for each season all showed a significant effect of habitat on both δ13C and δ15N (all P values < 0.001; Fig. 2). Overall, Scheffe tests showed significant interactions between habitats for both ratios and all seasons, except for differences in δ13C between SC and GU for liver in MAY and for muscle in FEB and MAY. For each season, both rat tissues were significantly enriched in δ15N in the GU-influenced habitat (compared with IC and SC (Fig. 2). In SEP, during the maximum availability of iceplant fleshy fruits, both rat tissues were significantly enriched in δ13C in IC compared with SC and GU (Fig. 2).
a–f Centroid distance clustering analyses on stable isotope signatures of liver (a, c, e) and muscle (b, d, f) of rats trapped in the gull (GU) (open triangles), iceplant (IC) (open circles), and scrubland (SC) (open squares) habitats during three seasons (May 2006: a, b; September 2006: c, d; February 2007: e, f). Dashed circles clusters, filled symbols mean isotopic signatures of rats trapped in the gull (filled triangles), iceplant (filled circles), and scrubland (filled squares) habitats [with standard deviation (SD) shown by bars] for each tissue–season combination. Similarity between each pair of plots indicates little change in individual diet on a 1-month scale. Black arrows rats included in a different cluster from the habitat in which they were captured. For each habitat–season combination, some individuals trapped in IC (referred as IC-2 in the “Results” section) were clustered with individuals trapped in SC (these cases are not indicated with arrows to maximize clarity). Analyses of diet indicate that these individuals referred to rats dwelling in the immediate interface between SC and the IC patches yet rarely fed on IC fruits (see “Discussion”). Dashed arrows rats that shifted habitats on a 1-month scale (individuals identified by their number)
Centroid hierarchical cluster analyses revealed the same three main clusters for each season (Fig. 2). Each cluster was mainly composed of individuals captured in one of the three study habitats. Overall, very few marginal individuals were identified to switch habitats in 1-week or 1-month time scales; the isotopic signatures of only two rats shifted from one habitat to another between tissues, and six rats were clustered for their liver and muscle isotopic signatures in a different habitat from where they were captured (Fig. 2). For each season, some individuals trapped in IC were clustered with some individuals trapped in SC.
To run SIAR models, IC was split in two groups (Table 3) according to the hierarchical cluster analysis results (see above). IC-1 was related to individuals trapped in IC whose isotopic signatures were clustered in an independent group (circles in Fig. 2), whereas IC-2 was related to individuals trapped in IC but whose isotopic signatures were clustered with those of individuals trapped in SC (circles clustered with rectangles in Fig. 2). SIAR estimates showed that rats from adjacent IC and SC significantly diverged in the assimilated proportions of iceplant fruits across seasons. In particular, the model estimated a large relative contribution of iceplant fruits in rat diet for IC-1 for all seasons, but more specifically for SEP, and a very low proportion of iceplant fruits in the diet of rats from IC-2 and adjacent SC (Table 3).
Variation in isotopic ratio values between rat tissues (i.e., variation through the previous 4 weeks before sampling: Dδ13C and Dδ15N) was <1‰ for all habitat–season combinations, except in GU for Dδ15N in MAY and SEP and for Dδ13C in FEB (Fig. 3a, b). In spring (i.e., MAY), during the middle of the gull nesting period, the enrichment in mean δ15N of rats trapped in GU was significantly higher compared with the two other habitats during the same season and the two other seasons for GU (Fig. 3b). In summer (i.e., SEP), during the peak of iceplant fruit ripening, the depletion in mean δ13C of rats trapped in IC was significantly higher than the two other habitats, especially SC (Fig. 3a). No correlation was found between empty body mass of individuals and both Dδ13C (Spearman r s = 0.14, n = 138) and Dδ15N (Spearman r s = −0.01, n = 138). Neither Dδ13C nor Dδ15N were significantly different between sexes (Mann–Whitney tests, P > 0.05).
Habitat use
From April 2007 to April 2009, there were 2,236 captures of 610 rats marked over 19 capture–recapture sessions. Nearly half the marked rats were recaptured in at least two different trapping sessions. Mean and maximal distances moved by rats between subsequent captures were, respectively, 30 m (SD = 37) and 451 m. Mean distances moved between subsequent captures were significantly higher for males than for females (mean distMale = 36 m ± 42 SD, n M = 210; mean distFemale = 23 m ± 30 SD; n F = 196, Z = −3.9, P < 0.001). Mean distances moved between subsequent captures were significantly higher (Z = 9.4, P > 0.001) for animals trapped during different sessions (33 m ± 40 SD, n = 290) than those trapped within the same session (14 m ± 15 SD, n = 402). Spearman rank correlations showed a slight correlation between distances moved between sessions and time between subsequent captures (n = 596, Spearman r s = 0.22, P < 0.001).
The habitat transition matrix revealed low probabilities of movement between habitats (Table 4). In contrast, the probability of rats staying within the same habitat between subsequent captures was >0.87 in each habitat. The 50% FK core areas of all radio-tracked individuals remained in the same habitat in which they were collared and trapped by capture–recapture. Estimated 100% MCPs showed a mean home-range width of 133 m ± 51 SD. Males had slightly larger mean home ranges than females (males 100% MCP = 0.95 ha ± 0.63 SD, 95% Kernel = 0.79 ha ± 0.62 SD, n = 8; females 100% MCP = 0.63 ha ± 0.25 SD, 95% Kernel = 0.62 ha ± 0.24 SD, n = 3). The seven rats collared >100 m from the iceplant patch were never recorded within the patch during the iceplant fruit-ripening season (August).
Discussion
At a small spatial scale, the isotopic signatures of resources varied locally among habitats on Bagaud Island. Gulls substantially enriched local trophic webs in 15N during their breeding season in spring, and iceplant produced fruits enriched in 13C during summer. These nutrient enrichments seemed to persist in the local food webs even when gull and iceplant fruit resources were reduced and no longer available. For each season, rat isotopic signatures were clustered by habitats, and the high 15N and 13C enrichment in their tissues indicated that these high-order consumers were locally subsidized by gulls and iceplant fruits, respectively.
Fecal content and stable isotope analyses confirmed previous suggestions on the generalist foraging behavior (dietary breadth) of introduced black rats and the ability of the species, as a whole, to exploit a wide range of resources and establish in habitats of variable quality (Clark 1981; Harper et al. 2005; Towns et al. 2006; Caut et al. 2008a). On Bagaud Island, within each habitat, rats selected different food items across seasons, probably in relation to their seasonal phenology, abundance, nutritional value, and palatability (Clark 1981; Grant-Hoffman and Barboza 2010). However, dietary plasticity of individual rats was far narrower. Surprisingly, even for habitats only tens of meters apart, the diet of rats was substantially distinct among adjacent habitats for each season. Moreover, site fidelity remained high despite the marked influences of gull-derived resources and, to a lesser extent, iceplant fruits, on rat population dynamics (e.g., higher individual growth rates, higher reproductive output and density; Ruffino 2010), especially during extreme climatic conditions such as dry years with low terrestrial productivity. This specific pattern of resource use was found consistently over successively larger time scales (i.e., days, weeks, and months) and revealed a specific or even exclusive use of resources by rats in their local habitat. Gull and iceplant fruit resources, in particular, were extensively used by rats when they became available, but only to benefit resident individuals.
The narrow feeding range of individual rats was confirmed by our live trapping studies. However, such studies are prone to biases when habitat use and the home range size of animals are estimated from successive records of trapping events. Possible biases were overcome by combining a long-term capture–recapture survey with radio tracking of the movements of a subsample of individuals at a finer spatiotemporal scale. Our radio-tracking results reinforced the apparent high residency rate of black rats. Although rats showed a home-range span large enough to reach alternative habitats during the dry summer, they remained in the surrounding scrubland. Even though our capture–recapture survey indicated higher mean travel distances by rats with time between captures, distances moved remained low (<40 m), suggesting no apparent range shift with time. Unfortunately, there are few individual-based movement studies on rodent populations covering a long period of time where seasonal resources fluctuate (but see Moller and Craig 1987), even though these data are very useful for highlighting habitat or dietary shifts over time. The apparent short distances moved by black rats between captures and low individual dispersal toward unfamiliar areas (i.e., outside their home range area) were consistent with some other capture–recapture studies on rodents (e.g., Moller and Craig 1987; Jones 1989) but would be worthy of more investigation. Indeed, the capacity of small mammals to move among habitats and disperse long distances may depend on the spatial distribution (i.e., patchiness) and temporal availability of resources (Dowding and Murphy 1994; Tobin et al. 1996; Gauffre et al. 2008), the presence of geographical corridors or barriers (Krohne and Hoch 1999), or metapopulation dynamic processes (Krohne 1997; Lin and Batzli 2001).
The variation in isotopic ratios of the same rats over a 1-month scale (i.e., variation between tissues) was low, and only a few individuals switched habitats when the availability of high-quality resources was the greatest. This switching by some individuals was not linked to either body mass or sex and may be an outcome of other population processes such as being forced out of territories. Even rats dwelling in the immediate interface between SC and the IC patches rarely used iceplant fruits during the dry summer, whereas rats resident in the IC strongly relied on fruits at the time (Fig. 2; Table 3). Food supplementation experiments and manipulations of habitat quality in the field often encourage immigration toward food-supplemented areas or higher-quality habitats and increase residential times of newly established individuals (Boutin 1990), especially omnivorous rodents (Banks and Dickman 2000; Lin and Batzli 2001; Van Aarde and Jackson 2007). Therefore, our finding is counterintuitive for a generalist invasive forager that may have had access to higher-quality resources in close proximity throughout the year. These unexpected findings are likely to be related to other population processes, such as intraspecific territorial interactions, that may limit access to high-quality resources by subordinates.
There is extensive evidence that some species of ecological generalists, which use diverse resources at the population level, are in fact individuals with narrower ranges of resource use (Bolnick et al. 2003, 2007; Brooke McEachern et al. 2006; Quevedo et al. 2009). However, studies demonstrating distinct foraging strategies in individuals of generalist species occupying small habitat patches in close proximity are rare (but see Brooke McEachern et al. 2006). Reduced niche breadth in individuals of generalist species may minimize intraspecific competition by reducing resource use overlap (Bolnick et al. 2007) and is observed in heterogeneous landscapes or in individuals exhibiting a strong territoriality (Angerbjorn et al. 1994; Urton and Hobson 2005). Territory defense through scent marks and/or agonistic behavior toward unfamiliar conspecifics may provide a plausible explanation for the low mobility of rats and the privileged access to enriched resources by dominants and residents, as predicted by the ideal despotic distribution (Fretwell and Lucas 1970) and already demonstrated for other rodents (Spencer and Cameron 1983; Gray et al. 2002; Jensen et al. 2005). Moreover, when the high-quality food source is clumped in space to form patchy habitats, dominant individuals may tend to monopolize food sources (Boutin 1990). In this sense, compared with rats that did not have access to these enriched resources (Ruffino 2010), the larger body mass of rats foraging on gull-subsidized resources on Bagaud Island could favor social dominance and aggression (i.e., Spencer and Cameron 1983).
By combining dietary and trophic analyses at various time scales with intensive individual-based movement monitoring over several rat generations, we were able to examine how habitat heterogeneity and seasonal fluctuation of resource quality within adjacent habitats affect dietary breadth and plasticity at the individual and population levels in an invasive generalist rodent. The diet-switching ability of introduced rats to benefit from seasonal high-quality resources is often proposed to explain their wide invasion success and diverse impact from arctic to tropical islands (Stapp 2002; Stapp and Polis 2003; Major et al. 2006; Towns et al. 2006). Caut et al. (2008a) recently demonstrated a dietary shift in R. rattus on a dry tropical island between seasons but did not track individual rats. Conversely, on Bagaud Island, we found that individual rat diet was relatively consistent with the resources available in their immediate habitat across seasons, despite the very close proximity of fluctuating resources of differential quality in the wider environment. The different feeding patterns observed between the two systems may be related to multiple intrinsic and extrinsic factors, such as local population density, intraspecific interactions, or attractiveness of resources. Climate is also likely to influence resource use by invasive rats on islands. Tropical islands tend to have much higher variation in resource availability, implying that rats would have to move longer distances to meet their dietary requirements as resources seasonally switch, compared with Mediterranean islands where rats would be less limited by resources.
Conclusions and implications
The pattern of resource use observed in our study seems surprising for such a successful invader, renowned for its ecological flexibility and dietary plasticity. Although our results support high species- and population-level dietary breadth and plasticity, they show a much narrower individual dietary and habitat breadth, constrained by complex mechanisms probably related to social organization and territory defense behavior. Restricted dietary breadth of individual black rats along with limited movements between habitats may promote differentiation among subpopulations in dynamics and structure, reinforcing the benefits of enriched resources (Ruffino 2010). On the other hand, our results add to growing evidence that invasive black rats can occupy various habitats, even those of low quality, which should explain their wide invasion success on islands, especially those with extreme environments. Our results also suggest that even on a small island, the accessibility of patchy and high-quality resources to rats from the entire population is not systematic. This result has important implications when quantifying invasive rat impact on patchily distributed species, especially when studies use indirect methods, such as dietary analyses, as a substitute for direct observations of feeding habits. To conclude, our study emphasizes the value of integrating methods and the need for more long-term individual-based monitoring studies to assess both resource and habitat use of invasive species at different spatiotemporal scales in changing environments.
References
Angerbjorn A, Hersteinsson P, Liden K, Nelson E (1994) Dietary variation in arctic foxes (Alopex lagopus)—an analysis of stable isotopes. Oecologia 99:226–232
Atkinson IAE (1985) The spread of commensal species of Rattus to oceanic islands and their effects on island avifaunas. ICPB Tech Publ 3:35–81
Banks PB, Dickman CR (2000) Effects of winter food supplementation on reproduction, body mass, and numbers of small mammals in montane Australia. Can J Zool 78:1775–1783
Begg CM, Begg KS, Du Toit JT, Mills MG (2003) Sexual and seasonal variation in the diet and foraging behaviour of a sexually dimorphic carnivore, the honey badger (Mellivora capensis). J Zool 260:301–316
Ben-David M, Flynn RW, Schell DM (1997) Annual and seasonal changes in diets of martens: evidence from stable isotope analysis. Oecologia 111:280–291
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on oceanic islands. Science 305:1955–1958
Blackburn T, Cassey P, Lockwood J (2009) The role of species traits in the establishment success of exotic birds. Glob Chang Biol 15:2852–2860
Bolnick DL, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Bolnick DL, Svanbäck R, Araujo MS, Persson L (2007) Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc Natl Acad Sci USA 104:10075–10079
Boutin S (1990) Food supplementation experiments with terrestrial vertebrates: patterns, problems and the future. Can J Zool 68:203–220
Brooke McEachern M, Eagles-Smith CA, Efferson CM, Van Vuren DH (2006) Evidence for local specialization in a generalist mammalian herbivore, Neotoma fuscipes. Oikos 113:440–448
Brousseau P, Lefebvre J, Giroux JF (1996) Diet of ringbilled gull chicks in urban and non-urban colonies in Quebec. Colon Waterbird 19:22–30
Caut S, Angulo E, Courchamp F (2008a) Diet shift of an invasive predator: rats, seabirds and sea turtles. J Appl Ecol 45:428–437
Caut S, Angulo E, Courchamp F (2008b) Discrimination factors (Δ15N and Δ13C) in an omnivorous consumers; effect of diet isotopic ratios. Funct Ecol 22:255–263
Clark DA (1981) Foraging patterns of black rats across a desert-montane forest gradient in the Galapagos islands. Biotropica 13:182–184
Courchamp F, Chapuis J-L, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383
Crawford K, McDonald RA, Bearhop S (2008) Applications of stable isotope techniques to the ecology of mammals. Mammal Rev 38:87–107
Daniel MJ (1973) Seasonal diet of the ship rat (Rattus r. rattus) in lowland forest in New Zealand. Proc NZ Ecol Soc 20:21–30
Dell’Arte GL, Laaksonen T, Norrdahl K, Korpimäki E (2007) Variation in the diet composition of a generalist predator, the red fox, in relation to season and density of main prey. Acta Oecol 31:276–281
Dowding JE, Murphy EC (1994) Ecology of ship rats (Rattus rattus) in a Kauri forest (Agathis australis) in Northland, New Zealand. NZ J Ecol 18:19–28
Ehrlich P (1989) Attributes of invaders and the invading processes: vertebrates. In: Drake JA, Mooney HA, di Castri F, Groves RH, Kruger FJ, Rejmanek M, Williamson M (eds) Biological invasions: a global perspective. Wiley, Chichester, pp 315–328
Fretwell SD, Lucas HL (1970) On territorial behavior and other factors influencing habitat destruction in birds. I. Theoritical development. Acta Biotheor 19:16–36
Gauffre B, Estoup A, Bretagnolle V, Cosson JF (2008) Spatial genetic structure of a small rodent in a heterogeneous landscape. Mol Ecol 17:4619–4629
Grant-Hoffman MN, Barboza PS (2010) Herbivory in invasive rats: criteria for food selection. Biol Invasions 12:805–825
Gray SJ, Jensen SP, Hurst JL (2002) Effects of resource distribution on activity and territory defence in house mice, Mus domesticus. Anim Behav 63:531–539
Gregory SD, Macdonald DW (2009) Prickly coexistence or blunt competition? Opuntia refugia in an invaded rodent community. Oecologia 159:225–236
Harper GA, Dickinson KJM, Seddon PJ (2005) Habitat use by three rat species (Rattus spp.) on Stewart Island/Rakiura, New Zealand. NZ J Ecol 29:251–260
Harris DB (2009) Review of negative effects of introduced rodents on small mammals on islands. Biol Invasions 11:1611–1630
Imber FI (1975) The murine rodents Rattus rattus, exulans and norvegicus as avian predators. Atoll Res Bull 182:1–13
Jackson AL, Inger R, Bearhop S, Parnell A (2009) Erroneous behaviour of MixSIR, a recently published Bayesian isotope mixing model: a discussion of Moore & Semmens (2008). Ecol Lett 12:E1–E5
Jensen SP, Gray SJ, Hurst JL (2005) Excluding neighbours from territories: effects of habitat structure and resource distribution. Anim Behav 79:785–795
Jeschke JM, Trayer DL (2006) Determinants of vertebrate invasion success in Europe and North America. Glob Chang Biol 12:1608–1619
Jones TW (1989) Dispersal distance and the range of nightly movements in Merriam’s Kangaroo rats. J Mammal 70:27–34
Jones HP, Tershy BR, Zavaleta ES, Croll DA, Keitt BS, Finkelstein ME, Howald GR (2008) Severity of the effects of invasive rats on seabirds: a global review. Conserv Biol 22:16–26
Kenward RE (2007) A manual for wildlife radio tagging. Academic Press, London
Kenward RE, South AB, Walls SS (2006) Ranges7. Online manual. Anatrack Ltd, Wareham
Krohne DT (1997) Dynamics of metapopulations of small mammals. J Mammal 78:1014–1026
Krohne DT, Hoch GA (1999) Demography of Peromyscus leucopus populations on habitat patches: the role of dispersal. Can J Zool 77:1247–1253
Kurle CM (2009) Interpreting temporal variation in omnivore foraging ecology via stable isotope modeling. Funct Ecol 23:733–744
Lin Y-TK, Batzli GO (2001) The influence of habitat quality on dispersal, demography, and population dynamics of voles. Ecol Monogr 71:245–275
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:377–385
Major HL, Jones IL, Charette MR, Diamond AW (2006) Variation in the diet of introduced Norway rats (Rattus norvegicus) inferred using stable isotope analysis. J Zool 271:463–468
Médail F (1998) Flore et végétation des îles satellites (Bagaud, Gabinière, Rascas) du Parc National de Port-Cros (Var, S.E. France). Scientific Reports of the Port-Cros National Park, France 17:55–80 (in French with English abstract)
Moller H, Craig JL (1987) The population ecology of Rattus exulans on Tiritiri Matangi Island, and a model of comparative population dynamics in New Zealand. NZ J Zool 14:305–328
Moreno R, Jover L, Munilla I, Velando A, Sanpera C (2010) A three-isotope approach to disentangling the diet of a generalist consumer: the yellow-legged gull in northwest Spain. Mar Biol 157:545–553
Orgeas J, Vidal E, Ponel P (2003) Colonial seabirds change beetle assemblages on a Mediterranean island. Ecoscience 10:38–44
Palmer M, Pons GX (1996) Diversity in western Mediterranean islets: effects of rat presence on a beetle guild. Acta Oecol 17:297–305
Parnell A, Inger R, Bearhop S, Jackson AL (2008) SIAR: stable isotope analysis in R. http://cran.r-project.org/web/packages/siar/index.html
Parnell AC, Ringer R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS One 5:e9672
Phillips DL, Eldridge PM (2006) Estimating the timing of diet shifts using stable isotopes. Oecologia 147:195–203
Polis GA, Anderson WB, Holt RT (1997a) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316
Polis GA, Hurd SD, Jackson CT, Sanchez-Piñero F (1997b) El Niño effects on the dynamics and control of an island ecosystem in the Gulf of California. Ecology 78:1884–1897
Popa-Lisseanu AG, Delgado-Huertas A, Forero MG, Rodríguez A, Arlettaz R, Ibáñez C (2007) Bats’ conquest of a formidable foraging niche: the myriads of nocturnally migrating songbirds. PLoS ONE 2:e205
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154
Quevedo M, Svanbäck R, Eklo P (2009) Intrapopulation niche partitioning in a generalist predator limits food web connectivity. Ecology 90:2263–2274
Rufaut CG, Gibbs GW (2003) Response of a tree weta population (Hemideina crassidens) after eradication of the polynesian rat from a New Zealand Island. Restor Ecol 11:13–19
Ruffino L (2010) Ecologie, dynamique de population, comportement et impact d’un rongeur introduit Rattus rattus sur les îles de Méditerranée. PhD dissertation, Université Paul Cézanne, Aix-en-Provence (in French with English abstract)
Ruffino L, Bourgeois K, Vidal E, Duhem C, Paracuellos M, Escribano F, Sposimo P, Baccetti N, Pascal M, Oro D (2009) Invasive rats and seabirds: a review after 2,000 years of an unwanted coexistence on Mediterranean islands. Biol Invasions 11:1631–1651
Schofield MR, Barker RJ, MacKenzie DI (2009) Flexible hierarchical mark-recapture modeling for open populations using WinBUGS. Environ Ecol Stat 16:369–387
Sol D, Lefebvre L (2000) Behavioural flexibility predicts invasion success in birds introduced to New Zealand. Oikos 90:599–605
Sol D, Timmermans S, Lefebvre L (2002) Behavioural flexibility and invasion success in birds. Anim Behav 63:495–502
Spencer SR, Cameron GR (1983) Behavioural dominance and its relationship to habitat patch utilization by the hispid cotton rat (Sigmodon hispidus). Behav Ecol Sociobiol 13:27–36
Stapp P (2002) Stable isotopes reveal evidence of predation by ship rats on seabirds on the Shiant Islands, Scotland. J Appl Ecol 39:831–840
Stapp P, Polis GA (2003) Marine resources subsidize insular rodent populations in the Gulf of California, Mexico. Oecologia 134:496–504
Tobin ME, Sugihara RT, Koehler AE, Ueunten GR (1996) Seasonal activity and movements of Rattus rattus (Rodentia, Muridae) in a Hawaiian macadamia orchard. Mammalia 60:3–13
Towns DR, Parrish GR, Westbrooke I (2003) Inferring vulnerability to introduced predators without experimental demonstration: case study of Suter’s skink in New Zealand. Conserv Biol 17:1361–1371
Towns D, Atkinson IAE, Daugherty CH (2006) Have the harmful effects of rats been exaggerated? Biol Invasions 4:863–891
Towns D, Parrish G, Tyrrell CL, Ussher GT, Cree A, Newman DG, Whittaker TH, Westbrooke I (2007) Responses of tuatara (Sphenodon punctatus) to removal of introduced pacific rats from islands conservation biology. Conserv Biol 21:1021–1031
Towns DR, Wardle DA, Mulder CPH, Yeates GW, Fitzgerald BM, Parrish GR, Bellingham PJ, Bonner KI (2009) Predation of seabirds by invasive rats: multiple indirect consequences for invertebrate communities. Oikos 118:420–430
Urton EJM, Hobson KA (2005) Intrapopulation variation in gray wolf isotope (δ15N and δ13C) profiles: implications for the ecology of individuals. Oecologia 145:317–326
Van Aarde RJ, Jackson TP (2007) Food, reproduction and survival in mice on sub-Antarctic Marion Island. Polar Biol 30:503–511
Vasquez DP (2005) Exploring the relationship between invasion success and niche breadth. In: Cadotte MW, McMahon SM, Fukami T (eds) Conceptual ecology and invasions biology. Springer, New York, pp 317–332
Vidal V, Médail F, Roche P, Tatoni T, Vidal P (1998) Impact of gull colonies on the flora of the Riou archipelago (Mediterranean islands of the south-east France). Biol Conserv 84:235–243
Vilà M, D’Antonio CM (1998) Fruit choice and seed dispersal of invasive vs noninvasive Carpobrotus (Aizoaceae) in coastal California. Ecology 79:1053–1060
Williamson M (1996) Biological invasions. Chapman & Hall, London
Acknowledgments
We are very grateful to all the people who helped in the field and for processing laboratory samples. We specially thank J.L. Chapuis for training us in microhistological analyses of diet, M. Pascal and O. Lorvelec for helping in the field, and J.F. Giroux and two anonymous referees for comments and advice on earlier drafts of the manuscript. We thank all the PCNP staff who enabled this long-term study on the natural reserve of Bagaud Island. Research was funded by the Agence Nationale pour la Recherche (ANR) with program ALIENS. Funds were provided by a PhD fellowship granted by Ecole Doctorale des Sciences de l’Environement to LR, a postdoctoral fellowship granted by the New Zealand Foundation for Research, Science and Technology to JCR, and Consejo Superior de Investigaciones Científicas (CSIC) to SC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruffino, L., Russell, J.C., Pisanu, B. et al. Low individual-level dietary plasticity in an island-invasive generalist forager. Popul Ecol 53, 535–548 (2011). https://doi.org/10.1007/s10144-011-0265-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-011-0265-6