Abstract
Vertebrobasilar artery dissecting aneurysms (VBDAs) are the most surgically challenging type of aneurysm. Cerebral revascularization is the ultimate treatment for complex VBDAs. We retrospectively analysed the characteristics, surgical outcomes and follow-up data of 21 patients who underwent cerebral revascularization to treat complex VBDAs from 2015 to 2022. According to the location of the aneurysm and the anatomic relationship between the VBDA and the PICA, VBDA patients were classified into four groups: aneurysms located at the VA with PICA involvement (10 patients), aneurysms located at the VA without PICA involvement (1 patient), aneurysms located at the basilar apex segment (1 patient) and aneurysms located at the basilar trunk segment (9 patients). A surgical algorithm for complex VBDAs was determined primarily by the location of the aneurysm, the status of the aneurysm and the ability of retrograde blood flow to reach the proximal vertebrobasilar artery. Surgical modalities for patients with aneurysms in the VA with PICA involvement included low-flow (OA-PICA) bypasses with aneurysm trapping, aneurysm excision or reconstructive clip in 8 patients and STA-PCA bypass combined with PICA preservation and aneurysm trapping in 2 patients. In patients with aneurysms in the VA without PICA involvement, aneurysm excision was performed without cerebral bypass. In patients with aneurysms in the basilar apex segment, high-flow bypass (ECA-RA-P2) with aneurysm trapping was performed. In patients with aneurysms in the basilar trunk segment, surgical modalities included high-flow bypasses (ECA-RA-P2 and LVA-RA-P2) with aneurysm trapping or proximal occlusion in 6 patients, ECA-RA-P2 bypass with partial proximal occlusion in 1 patient, ECA-RA-P2 bypass alone in 1 patient, and STA-PCA bypass with R-VA narrowing in 1 patient. Of the 21 patients, 20 experienced clinical improvement or no change, and 17 of 21 patients achieved favourable functional outcomes (mRS ≤ 2). However, one patient died of infarction and respiratory failure postoperatively. Aneurysms were completely obliterated in 13 patients, shrank in 5 patients and stabilized in 2 patients. The median follow-up period was 32.5 months. During the follow-up period, all bypasses were patent, and further clinical improvement was observed in 11 patients. Cerebral revascularization appears to be safe and effective for the treatment of complex VBDAs, and cerebral revascularization could act as a complementary treatment strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracranial dissecting aneurysms (IDAs) are not rare, especially in Eastern Asian populations, and constitute approximately 2–3% of all intracranial aneurysms [1, 2]. Vertebrobasilar artery dissecting aneurysms (VBDAs) constitute more than 60% of all intracranial dissecting aneurysms [3]. Symptomatic vertebrobasilar artery dissecting aneurysms, especially ruptured aneurysms, are serious life-threatening cerebrovascular diseases associated with a significantly poor prognosis [4]. Traditional therapies for symptomatic VBDA consist of microsurgical clipping, flow diversion, and endovascular parent artery occlusion [5]. Unfortunately, treating symptomatic complex vertebrobasilar artery dissecting aneurysms via simple microsurgical clipping or endovascular treatment is a significant challenge due to the broad, complex anatomy and potentially fatal complications of these aneurysms (e.g., infarction, haemorrhage, perforated artery occlusion and cranial nerve palsy) [6]. Cerebral revascularization has been increasingly used to treat complex aneurysms [7] and has shown encouraging results in the treatment of posterior circulation dissecting aneurysms [8, 9]. However, the safety and efficacy of cerebral revascularization for the treatment of vertebrobasilar artery dissecting aneurysms, especially basilar artery (BA) dissecting aneurysms and vertebral artery (VA) dissecting aneurysms with posterior inferior cerebellar artery (PICA) involvement, still need to be further evaluated. In this study, we report the results of cerebral revascularization for treatment of vertebrobasilar artery dissecting aneurysms and describe a feasible surgical algorithm for vertebrobasilar artery dissecting aneurysms.
Methods
Population and ethics
This retrospective review study was approved by the Institutional Ethics Committee of Tianjin Huan Hu Hospital. This study was a retrospective analysis of 21 patients with VBDA who were treated at Tianjin Huan Hu Hospital from 2015 to 2022. All cerebral revascularizations were performed by a senior neurosurgeon (XT). Patients were divided into four groups according to the location of the dissecting aneurysm and the anatomic relationship between the VBDA and PICA: VA with PICA involvement, VA without PICA involvement, basilar apex segment and basilar trunk segment.
Preoperative evaluation
Computed tomography angiography (CTA) or magnetic resonance angiography (MRA) with digital subtraction angiography (DSA) were performed to confirm the size, shape and parent vessel of the aneurysms. Patients managed with cerebral revascularization underwent computed tomography perfusion (CTP) or magnetic resonance perfusion (MRP) before surgery to unambiguously confirm blood flow reserve and assess tolerance to ischaemia in combination with preoperative symptoms. Radial artery grafts (RAGs) using an interposition graft were defined as high-flow bypasses, and other bypasses (superficial temporal artery (STA)-posterior cerebral artery (PCA) and occipital artery (OA)-posterior inferior cerebellar artery (PICA)) were defined as low-flow bypasses.
Surgical algorithm for complex VBDA
The surgical algorithm for complex vertebrobasilar artery dissecting aneurysms was determined primarily by the location of the aneurysm, the status of the aneurysm and the ability of retrograde blood flow to reach the proximal vertebrobasilar artery. As previously reported [10,11,12], although low-flow bypass never permanently results in low flow, many surgeons prefer to use high-flow bypass to replace blood flow in the region of collateral circulation, conduct low-flow bypass for blood flow supplementation, and perform VA narrowing or partly proximal occlusion to reduce blood flow into the parent artery. To reduce the risk of cerebral ischaemia after surgery, we preferred to use high-flow bypass rather than low-flow bypass to replace blood flow in the region of collateral circulation after proximal occlusion in our centre because we found that low-flow bypass was not as reliable as high-flow bypass due to the higher incidence of postoperative ischaemia. As shown in Fig. 1, for aneurysms located in the BA or dominant VA, high-flow bypass was always performed to avoid ischaemic complications. For aneurysms located in the basilar apex segment, high-flow bypass combined with aneurysm trapping was performed if possible. If aneurysm trapping was impossible, high-flow bypass combined with proximal occlusion was performed. For aneurysms located in the basilar trunk segment, if intraoperative DSA revealed that the bypass graft could provide retrograde blood flow to the proximal vertebrobasilar artery, high-flow bypass combined with proximal occlusion was performed; otherwise, high-flow bypass alone or combined with partial proximal occlusion/VA narrowing was performed. DSA or CTA/MRA were performed 1 − 3 weeks after high-flow bypass alone and combined with partial proximal occlusion/VA to evaluate changes in the aneurysm. If the aneurysm disappeared or became smaller, the treatment was considered successful. Otherwise, parent artery occlusion was performed to reduce the blood flow provided by the parent arteries. Moreover, if patients suffered from ruptured aneurysms but aneurysm trapping was not possible, reconstructive clips were used to shrink the aneurysm or alter the haemodynamics of the ruptured aneurysm, as this approach could stabilize the ruptured aneurysm and reduce rebleeding. For aneurysms located in the VA with PICA involvement, low-flow bypass was performed to preserve the PICA, and aneurysms were trapped using interposition flow bypass (at the dominant VA) or trapped directly (at the nondominant VA). If aneurysm trapping was impossible, proximal occlusion was performed, and the specific location of the proximal occlusion was determined by the anatomic relationship between the VBDA and PICA. For aneurysms located in the VA without PICA involvement, if the dissecting aneurysm was located in the nondominant VA, aneurysm excision was performed without cerebral bypass. If the dissecting aneurysm was located in the dominant VA, interposition flow bypass or vascular reconstruction of the VA was performed. Additionally, for patients with a complex artery dissecting aneurysm located in the BA or dominant VA but were unable or unwilling to undergo high-flow bypass, if the CTP/MRP before surgery unambiguously confirmed that the blood flow reserve was good, a balloon occlusion test (BOT) was performed during DSA to confirm the presence of the anterior communicating artery (AcomA) and posterior communicating artery (PcomA). If the BOT did not induce any neurological symptoms and indicated that both the anterior communicating artery and posterior communicating artery were present and the compensatory capability of the anterior communicating artery (AcomA) and posterior communicating artery (PcomA) was good, low-flow bypass with proximal occlusion or partial proximal occlusion was performed; these patients were very likely to tolerate postoperative ischaemia because the blood flow reserve and compensatory capability of the collateral circulation were good. If the BOT was positive, high-flow bypass or vertebral artery constriction were performed. Notably, BOTs are not suitable for use in patients with ruptured aneurysms because they may increase the risk of rebleeding.
The algorithm of cerebral revascularization strategies for treating complex vertebrobasilar artery dissecting aneurysms. BA: basilar artery; VA: vertebral artery; PICA: posterior inferior cerebellar artery; OA: occipital artery. BA: basilar artery; STA: superficial temporal artery; DSA: digital subtracted angiography
Surgical procedure
For OA-PICA bypass, the diameter, flow velocity and tracing of the occipital artery were identified via ultrasound. An arc-shaped incision was made by a far-lateral approach up to the superior nuchal line and down to the C2 level. Under the microscope, the OA was separated carefully. To prevent cerebral vasospasm, the separated OA was immersed in papaverine heparin saline. After the occipital bone and arch of the posterior atlas were revealed, the suboccipital triangle was opened, and the arch of the posterior atlas on the operative side was removed to reveal the vertebral artery. Then, an OA-PICA end-to-side anastomosis was performed. For simple excision of a VA segment artery dissecting aneurysm, the vertebral artery was revealed without separating the OA, and the aneurysm was removed after the parent artery was clipped. For STA-PCA bypass, a standard frontotemporal craniotomy with temporal extension was used. The superficial temporal artery course was traced using a Doppler flow detector, and the superficial temporal artery was separated carefully under a microscope. After the craniotomy flap was created, the temporal bone was moved to the floor of the middle fossa. Then, the Sylvian fissure was split and opened, and cerebrospinal fluid was released. With minimal retraction of the temporal lobe, the PCA was revealed. Then, an STA-PCA end-to-side anastomosis was performed. For external carotid artery (ECA)-radial artery (RA)-P2 segment of the posterior cerebral artery (P2) high-flow bypass, the RA was extracted as a graft, and the P2 segment of the PCA without perforator arteries were chosen as the recipient vessel. The patency of the palmar arch was assessed by DSA and the Allen test preoperatively. With the help of a hand surgeon, a radial artery graft (RAG) with a length of 20 cm was performed during craniotomy. Moreover, the extended middle cranial fossa approach was used to expose the P2 segment of the PCA under general anaesthesia because the extended middle cranial fossa approach provided adequate anterior, frontal, temporal, and posterior surgical corridors. In detail, the patient was placed in the supine position, with the cheek bone at the highest point. First, the carotid sheath was opened in the neck, and the common carotid artery (CCA), internal carotid artery (ICA) and ECA were exposed. Then, a curved head incision starting approximately 1 cm anterior to the tragus and ending at the contralateral hairline was made. The zygomatic bone was transected, and the temporalis muscle was turned completely downwards. After a frontotemporal bone flap was made, the bones of the sphenoid ridge, anterior clinoid process and middle skull base were removed. If further exposure was necessary, grinding Glasscock’s triangle and Kawase’s triangle provided additional room for vascular anastomosis [13]. After that, the dura was opened carefully, and the P2 segment of the posterior cerebral artery was fully exposed after the temporal lobe was retracted. The graft was anastomosed to the ECA in an end-to-end fashion, and then the graft crossed the graft channel and was anastomosed to P2 in an end-to-side fashion. The sub-temporal approach is a classic surgical approach for basilar artery aneurysms, but we rarely use this approach for ECA-RA-P2 bypass because it may cause damage to the temporal lobe due to brain retraction [14].
Follow-up evaluation
A CT scan was performed on the first postoperative day to check for intracranial haemorrhage or ischaemia, and CTA or DSA was performed within 1 week postoperatively to determine the patency of the graft and parent arteries and clarify the changes in the VBDA. The mRS score was used to evaluate functional outcome, and the mRS score was recorded on the day before the operation and 48 h after the operation. Outpatient follow-up angiography was conducted with DSA or CTA. During the follow-up period, mRS scores were collected to assess neurologic outcomes. The mRS scores collected at the latest follow-up were defined as the last mRS scores. A favourable functional outcome was defined as a last mRS score ≤ 2, and a poor functional outcome was defined as a last mRS score ≥ 3.
Results
Patient and aneurysm characteristics
The characteristics of the 21 patients are shown in Table 1. This study included 3 (14%) female and 18 (86%) male patients with a median age of 47 years (range, 14–68 years). In terms of the presentations of VBDA, 10 (47.6%) patients had cerebral ischaemic syndrome, 10 (47.6%) patients had brainstem/nerve compression, and 1 patient (4.8%) had a subarachnoid haemorrhage (SAH). The aneurysms were located at the VA with PICA involvement in 10 patients, at the VA without PICA involvement in 1 patient, at the basilar apex segment in 1 patient and at the basilar trunk segment in 9 patients. Dolichoectasia, segmental dilatation, fusiform dilatation, giant aneurysm, thrombosis and recurrent aneurysm were the common morphological characteristics. Of the entire cohort, 9 patients underwent high-flow bypass, 11 patients underwent low-flow bypass, and 1 patient underwent aneurysm excision without cerebral bypass.
Surgical characteristics of patients with complex vertebrobasilar artery dissecting aneurysms
The detailed surgical characteristics of patients with VBDA are shown in Tables 2 and 3. According to the location of the VBDA and the anatomic relationship between the VBDA and PICA, the patients were classified into the following groups: VA with PICA involvement, VA without PICA involvement, basilar apex segment or basilar trunk segment. Aneurysms were located at the VA with PICA involvement in 10 patients, the VA without PICA involvement in 1 patient, the basilar apex segment in 1 patient and the basilar trunk segment in 9 patients. The surgical algorithm for complex VBDA was determined primarily by the location of the aneurysm, the status of the aneurysm and the ability of retrograde blood flow to reach the proximal vertebrobasilar artery. The surgical modalities for patients with aneurysms in the VA with PICA involvement included low-flow (OA-PICA) bypasses with aneurysm trapping, excision or reconstructive clips in 8 patients and STA-PCA bypasses with PICA preservation and aneurysm trapping in 2 patients. In the patient with an aneurysm in the VA without PICA involvement, aneurysm excision was performed without cerebral bypass. In the patient with an aneurysm in the basilar apex segment, high-flow bypass (ECA-RA-P2) with aneurysm trapping was performed. In patients with aneurysms in the basilar trunk segment, surgical modalities included high-flow bypasses (ECA-RA-P2 and LVA-RA-P2) with aneurysm trapping or proximal occlusion in 6 patients, ECA-RA-P2 bypass with partial proximal occlusion in 1 patient, ECA-RA-P2 bypass alone in 1 patient, and STA-PCA bypass with R-VA narrowing in 1 patient.
Clinical outcomes and follow-up
As shown in Tables 2 and 3, mRS scores were collected on the day before the operation, 48 h after the operation and at the last follow-up. The median preoperative, postoperative and last mRS scores were 3 (range, 2–5), 2 (range, 1–6) and 2 (range, 0–6), respectively. Compared with the preoperative mRS scores, the last mRS scores decreased or remained unchanged in 20 of 21 patients but increased in 1 patient (Patient 16 in Table 3) due to postoperative complications. Of the 21 patients, 18 experienced postoperative clinical improvement or no change, but conditions worsened in 3 patients due to cerebral infarction or other complications. During the follow-up period, further clinical improvement was observed in most patients. Finally, at the last follow-up, 20 of the 21 patients had experienced clinical improvement or no change, and 17 of the 21 patients achieved favourable functional outcomes (mRS ≤ 2). Infarction, pneumonia, neuroparalysis, hydrocephalus and intracranial infection were the main postoperative complications. Specifically, two patients suffered from infarction (Patients 14 and 16 in Table 3), and one patient (Patient 16) died of infarction and respiratory failure. One patient suffered from CNIII paralysis (Patient 10 in Table 3) and one from hydrocephalus (Patient 12 in Table 3). Moreover, intracranial infection occurred in 2 patients (Patients 5 and 17 in Table 3), and pneumonia occurred in 11 patients. Postoperative DSA or CTA (performed within 1 week after all surgeries, such as bypass, endovascular proximal occlusion and VA narrowing) indicated that the aneurysms were completely obliterated in 13 patients, shrank in 5 patients and stabilized in 2 patients. The median follow-up period for the 20 remaining patients was 32.5 months (range, 12–75 months). All cerebral bypasses were patent, and no new deaths were reported during the follow-up period; further clinical improvement was observed in 11 patients.
In brief, high-flow bypasses with proximal occlusion in the basilar trunk segment were associated with more serious complications. For cerebral revascularization for complex VBDA, postoperative infarction could lead to death, and pneumonia was the most common complication. Furthermore, the prognosis of most patients improved with additional treatment during the follow-up period.
Representative cases
Patient 1
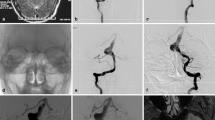
Therapeutic strategy for a dissecting aneurysm in the VA without PICA involvement: Aneurysm excision (Patient 1 in Table 3). A 14-year-old female presented with headache. Preoperative DSA and CTA revealed a giant dissecting aneurysm located at L-V4 without PICA involvement (Fig. 2A-D). An arc-shaped incision was made by a far-lateral approach, and the dissecting aneurysm was exposed and trapped by aneurysm clips (Fig. 2E-H). Then, the dissecting aneurysm was removed (Fig. 3I). DSA was performed immediately after surgery and showed that the aneurysm was obliterated, and the R-VA, BA and other important perforating branches were stable (Fig. 2J-L). No further neurological deficits were detected after the aneurysm was removed. Outpatient follow-up indicated that the patient achieved a favourable functional outcome (mRS score of 0).
Representative case with therapeutic strategy for a dissecting aneurysm in the VA without PICA involvement: aneurysm excision was performed without cerebral bypass. A-D DSA and CTA revealed a giant dissecting aneurysm located at L-V4 without PICA involvement. E An arc-shaped incision was made by a far-lateral approach. F Aneurysm was exposed. G-I Aneurysm was removed. J-L Postoperative DSA showed the aneurysm was obliterated and R-VA was stable
Representative case with therapeutic strategy for a dissecting aneurysm in the VA with PICA involvement: Low-flow bypass with aneurysm trapping. A-C Preoperative DSA and CTA revealed a dissecting aneurysm located in the R-VA with PICA involvement and distal end of R-VA was occluded. D An arc-shaped incision was made. E OA was separated. F PICA was exposed. G OA-PICA bypass anastomosis was performed. H Angiography with fluorescein sodium showed the bypass graft was patent. I Aneurysm was trapped. J-M Postoperative DSA and CTA showed the aneurysm was obliterated and bypass graft was patent
Patient 2
Therapeutic strategy for a dissecting aneurysm in the VA with PICA involvement: Low-flow bypass with aneurysm trapping (Patient 2 in Table 3). A 31-year-old man presented with hemianaesthesia. Preoperative DSA revealed a dissecting aneurysm located in the R-VA with involvement, and an obstruction was found at the distal end of the R-VA (Fig. 3A-C). An arc-shaped incision was made via a far-lateral approach (Fig. 3D). The OA was separated, the PICA was exposed (Fig. 3E-F), and OA-PICA bypass was performed (Fig. 3G-H). The aim of this direct anastomosis surgery was to preserve PICA flow. Then, the dissecting aneurysm was trapped by aneurysm clips (Fig. 3I). Postoperative DSA was performed immediately, and CTA was performed within 1 week after surgery. The results showed that the aneurysm was obliterated, and the bypass graft was patent (Fig. 3J-M). No further neurological deficits were detected after surgery. At discharge, the patient achieved clinical improvement.
Patient 3
Therapeutic strategy for a dissecting aneurysm in the basilar apex segment: High-flow bypass with aneurysm trapping (Patient 12 in Table 3). A critically ill female (58 years old, with a preoperative mRS score of 5) presented with sudden consciousness disturbance and was subsequently transferred to our hospital. CT showed a diffuse SAH, and preoperative DSA and CTA revealed a dissecting aneurysm located in the basilar apex segment (Fig. 4A-D). The radial artery (RA) was removed and used as an interposition graft (Fig. 4F). An extended middle cranial fossa approach was used, and ECA-RA-P2 bypass was performed to provide blood flow in the distal region. Then, the dissecting aneurysm was trapped by aneurysm clips. Postoperative DSA was performed immediately, and CTA was performed within 1 week after surgery. The results showed that the aneurysm was obliterated, the bypass graft was patent, and important perforating branches were sustained (Fig. 4G-K). Compared with the preoperative MRP (C), the postoperative MRP (L) indicated that cerebral ischaemia was not obviously aggravated after surgery. The patient suffered from hydrocephalus postoperatively, which was treated with cerebrospinal fluid diversion. The patient achieved clinical improvement, but she still suffered from a poor functional outcome (mRS = 4) at discharge. During follow-up rehabilitation, the patient achieved further clinical improvement, and the last mRS score was 3.
Representative case with therapeutic strategy for a dissecting aneurysm in the basilar apex segment: High-flow bypass with aneurysm trapping. A-D Pre-radiotherapy showed a dissecting aneurysm located in the basilar apex segment. E Preoperative MRP indicated that adequate perfusion could be found. F Radial artery was exposed and removed. G-K Postoperative DSA and CTA showed the aneurysm was obliterated and bypass graft was patent. L Postoperative MRP showed that adequate perfusion could be found
Patient 4
Therapeutic strategy for a dissecting aneurysm in the basilar trunk segment: High-flow bypass with proximal occlusion (Patient 18 in Table 3). A 47-year-old female presented with limb weakness. Preoperative DSA and CTA revealed a dolichoectasia dissecting aneurysm located in the basilar trunk segment (Fig. 5A-D). The extended middle cranial fossa approach was used, and ECA-RA-P2 bypass was performed during the first procedure (Fig. 5F-J). DSA was performed immediately after ECA-RA-P2 bypass, and CTA was performed within 1 week. DSA and CTA after cerebral bypass showed that the bypass graft was patent, and the VA, BA and other important perforating branches were stable (Fig. 5K‒M). Compared with the preoperative MRP, the MRP (N) after cerebral bypass indicated that cerebral ischaemia was alleviated after surgery. One week after the first operation (ECA-RA-P2 bypass), endovascular proximal occlusion of the bilateral vertebral arteries was performed during a second operation. MRP (R) after the second operation indicated that proximal occlusion of the bilateral vertebral arteries did not obviously aggravate cerebral ischaemia. DSA and CTA were performed after the second operation. The results showed that bilateral vertebral arteries were occluded, and the graft was patent (O-Q). No further neurological deficits were detected after surgery. At discharge, the patient’s mRS score was 3. Fortunately, the patient achieved a favourable functional outcome (mRS = 2) during follow-up care and treatment.
Representative case with Therapeutic strategy for a dissecting aneurysm in the basilar trunk segment: High-flow bypass with proximal occlusion. A-D Pre-radiotherapy revealed a dolichoectasia dissecting aneurysm located in the basilar trunk segment. E Preoperative MRP indicated that inadequate perfusion in the posterior circulation region. F The extended middle cranial fossa approach was used. G Aneurysm was exposed. H-I ECARA- P2 bypass was performed. J Angiography with fluorescein sodium showed the bypass graft was patent. K-M DSA and CTA after cerebral bypass showed that the bypass graft was patent. N MRP after cerebral bypass indicated that cerebral ischaemia was alleviated. O-P Bilateral vertebral arteries were occluded during a second operation. Q Postoperative DSA showed the graft was patent. R MRP after the second operation indicated not obviously aggravate cerebral ischaemia could be found
Patient 5
Therapeutic strategy for a dissecting aneurysm in the basilar trunk segment: High-flow bypass with partial proximal occlusion (Patient 19 in Table 3). A 63-year-old man suffered from a recurrent dissecting aneurysm after interventional embolization (Fig. 6A-B) and presented with limb weakness and intermittent respiratory dysfunction. An extended middle cranial fossa approach was used, and ECA-RA-P2 bypass was performed during the first procedure.
Representative case with therapeutic strategy for a dissecting aneurysm in the basilar trunk segment: High-flow bypass with partial proximal occlusion. A-B Pre-radiotherapy revealed a recurrent dissecting aneurysm located in the basilar trunk segment. C Preoperative MRP indicated that inadequate perfusion in the posterior circulation region. D-G DSA and CTA after cerebral bypass showed that the bypass graft was patent. H MRP after cerebral bypass indicated that cerebral ischaemia was not deteriorating. I-L L-VA was occluded, and the bypass graft was patent. M MRP after the second operation indicated not obviously aggravate cerebral ischaemia could be found
Because intraoperative DSA indicated that the bypass graft could not provide sufficient retrograde blood to the proximal BA/VA, proximal occlusion was not performed during the first procedure. DSA was performed immediately after the first procedure, and CTA was performed within 1 week. DSA and CTA after the first procedure showed that the bypass graft was patent, and the VA, BA and other important perforating branches were stable (Fig. 6D-G). Because the bypass graft could not provide sufficient retrograde blood to the proximal BA/VA, four weeks after the first operation (ECA-RA-P2 bypass), partial proximal occlusion (endovascular proximal occlusion of the L-VA) was performed during the second operation to minimize antegrade flow to the aneurysm. The reason that only partial proximal occlusion was performed during the second surgery was that we were worried about a fatal infarction after complete proximal occlusion. Fortunately, DSA and CTA after the second operation showed that the aneurysm stabilizing after the partial proximal occlusion, so further endovascular proximal occlusion of the R-V A was performed.
As shown in Fig. 6I-L, the L-VA was occluded, and the bypass graft was patent. Compared with the preoperative MRP (Fig. 6C), the postoperative MRP indicated that cerebral bypass (Fig. 6H) and proximal occlusion of the L-VA (Fig. 6M) did not obviously aggravate cerebral ischaemia. The patient achieved clinical improvement, but he still suffered from a poor functional outcome (mRS score of 3). Unfortunately, the patient did not experience further clinical improvement at the follow-up rehabilitation, and the last mRS score was 3.
Patient 6
Therapeutic strategy for a dissecting aneurysm in the basilar trunk segment: High-flow bypass only (Patient 20 in Table 3). A 44-year-old man presented with headache and vomiting. DSA and CTA revealed a dolichoectasia dissecting aneurysm located in the basilar trunk segment (Fig. 7A-C). Because intraoperative DSA indicated that a bypass graft could not provide retrograde blood to the proximal BA/VA, the patient underwent ECA-RA-P2 bypass alone via the extended middle cranial fossa approach (Fig. 7E-I). Postoperative CTA was performed on the 3rd day after surgery and showed that the bypass graft was patent (Fig. 7J). One month later, DSA was performed to confirm the changes in the VBDA. DSA revealed that the aneurysm was shrinking (Fig. 7M), which indicated successful treatment, and further partial or complete proximal occlusion was not necessary. If the aneurysm had increased in size, partial or complete proximal occlusion would be attempted. In clinical practice, we found that high-flow bypass alone could treat only some aneurysms successfully. As reported [15, 16], high-flow bypass could augment the reverse flow of the bypass graft with the antegrade flow of the vertebrobasilar artery system, altering intra-aneurysmal haemodynamics and promoting therapeutic intra-aneurysmal thrombosis. Compared with the preoperative MRP (D), the MRP (N) after bypass indicated that cerebral ischaemia was obviously aggravated after surgery. No further neurological deficits were detected after surgery. The patient achieved a favourable functional outcome (mRS score of 1).
Representative case with therapeutic strategy for a dissecting aneurysm in the basilar trunk segment: High-flow bypass only. A-C Pre-radiotherapy revealed a recurrent dissecting aneurysm located in the basilar trunk segment. D Preoperative MRP indicated that inadequate perfusion in the posterior circulation region. E-F ECA-RAG anastomosis was performed. G The conduit for RAG was made. H-I RAG-P2 anastomosis was performed. J-K Postoperative DSA and CTA showed that the bypass graft was patent. L-M Postoperative DSA showed that the aneurysm was shrinking. N Postoperative MRP indicated that cerebral ischaemia was alleviated
Patient 7
Therapeutic strategy for a dissecting aneurysm in the basilar trunk segment: Low-flow bypass with partial proximal occlusion (Patient 21 in Table 3). A 52-year-old man presented with dizziness. Preoperative DSA and CTA revealed a dolichoectasia dissecting aneurysm located in the basilar trunk segment (Fig. 8A-D). DSA indicated that the BOT was negative, both the AcomA and PcomA were present, and CTP/MRP before surgery unambiguously confirmed that the blood flow reserve was good. We have found that low-flow bypass is simpler and quicker than high-flow bypass is, and we wanted to try using low-flow bypass to treat complex postcirculation aneurysms, although low-flow bypass is believed to be less reliable than high-flow bypass at replacing blood flow in the region of the collateral circulation. In this patient, a combined surgical approach was used. Low-flow bypass (STA-PCA) was performed for blood flow supplementation, while partial proximal occlusion (R-VA narrowing) was conducted to reduce blood flow into the parent artery and avoid postoperative cerebral infarction. STA-PCA bypass was chosen for the following reasons: if low-flow bypass (STA-PCA) could not provide enough blood flow in the region of the collateral circulation, STA-PCA bypass could easily be changed to a high-flow bypass (RA-PCA). CTA was performed on the 2nd day after the first operation and showed that the bypass graft was patent and that the R-VA was narrowed (Fig. 8G-I). Four weeks later, DSA was performed to confirm the changes in the VBDA. DSA did not reveal that the aneurysm was shrinking (Fig. 8F); thus, further endovascular proximal occlusion of the R-VA was performed to reduce blood flow into the parent artery, and the L-VA was preserved to avoid postoperative infarction. DSA after the second surgery showed that the bypass graft was patent and that the aneurysm was shrinking (Fig. 8K-N). Compared with the preoperative MRP (E), the MRP after the first surgery (J) and second surgery (O) indicated that bypass and proximal occlusion of the R-VA did not obviously aggravate cerebral ischaemia. No further neurological deficits were detected after surgery. During follow-up care and treatment, the patient achieved a favourable functional outcome.
Representative case with therapeutic strategy for a dissecting aneurysm in the basilar trunk segment: Low-flow bypass with partial proximal occlusion. A-D Preoperative DSA and CTA revealed a dolichoectasia dissecting aneurysm located in the basilar trunk segment. E Preoperative MRP indicated that inadequate perfusion in the posterior circulation region. F-I STA-PCA bypass and R-VA narrowing were performed. J MRP after the first operation and showed that cerebral ischaemia was alleviated. K-M R-VA was occluded. N Postoperative DSA showed that the bypass graft was patent. O MRP after the second operation indicated not obviously aggravate cerebral ischaemia could be found
Discussion
There are currently no widely accepted guidelines or explicit medical evidence for decision-making in the treatment of complex vertebrobasilar artery dissecting aneurysms [17]. The location and morphology of dissecting aneurysms, surrounding structures, patient age, neurological condition, surgeon’s personal preference, etc., may impact therapeutic decisions [18, 19]. In theory, ideal therapeutic strategies for vertebrobasilar artery dissecting aneurysms should include complete occlusion of the aneurysm and maintenance of sufficient blood flow to the vertebrobasilar artery and important perforating branches; in particular, preservation of the PICA could be considered if the PICA is involved [20]. However, occlusion of the BA or dominant VA may lead to distal ischaemia [21], and revascularization of the brainstem makes these procedures further formidable challenges. Thus, an appropriate therapeutic strategy is key in treating VBDA. Anatomically, the vertebrobasilar artery can be subdivided into three arbitrary segments: the vertebral artery (VA), basilar trunk segment and basilar apex segment. The vertebral artery (VA) is adjacent to the PICA, the basilar trunk segment is adjacent to the anterior inferior cerebellar artery (AICA), and the basilar apex segment is adjacent to the superior cerebellar artery (SCA) and PCA [22, 23]. The perfusion of these important perforating branches should be maintained. Due to the diversity of anatomic and haemodynamic differences in the vertebrobasilar artery, a uniform treatment strategy cannot be applied for all dissecting aneurysms in the vertebrobasilar artery. Thus, we recommend multiple surgical strategies that depend on the location of the aneurysm: the VA with PICA involvement, the VA without PICA involvement, the basilar apex segment and the basilar trunk segment.
In the basilar apex segment, high-flow bypass with aneurysm trapping was performed in one patient. The dissecting aneurysm is isolated by trapping, and blood flow in the superior cerebellar artery (SCA) or posterior cerebral artery (PCA) is replaced by high-flow bypass grafts [24]. If the dissecting aneurysm cannot be trapped, proximal occlusion is performed. Proximal occlusion can reduce blood flow into the aneurysm, and elimination of the flow jet effect into the lumen of the aneurysm could facilitate complete thrombosis of the aneurysm or thrombosis with a small stable neck, which could obliterate or shrink the aneurysm [25, 26].
In the basilar trunk segment, high-flow bypass with aneurysm trapping/proximal occlusion, high-flow bypass with partial proximal occlusion, or high-flow bypass alone were conducted in our study. In theory, high-flow bypass grafts can provide retrograde blood flow to the proximal vertebrobasilar artery, which could prevent ischaemic complications caused by proximal vertebrobasilar occlusion [27, 28]. However, not all high-flow bypass grafts can provide retrograde blood flow to the proximal vertebrobasilar artery [29]. If a bypass graft alone can provide sufficient retrograde blood flow, high-flow bypass combined with aneurysm trapping or proximal occlusion is performed, and there are no concerns about postoperative cerebral ischaemia. Conversely, if the bypass graft cannot provide retrograde blood flow into the proximal vertebrobasilar artery, high-flow bypass alone or with partial proximal occlusion is performed to avoid the ischaemic complications caused by direct proximal occlusion. According to previous reports [16, 30], high-flow bypass can reduce antegrade blood flow to the aneurysm, which promotes thrombosis within the aneurysm and reduced pressure and wall shear stress in the aneurysm, and an approximately 40–70% reduction in pressure and wall shear stress occurs postoperatively. Furthermore, in addition to replacing blood flow in the region of the collateral circulation, high-flow bypass can provide retrograde blood flow to counteract the antegrade flow from the parent artery, which could also promote thrombosis within the aneurysm [31]. Additionally, low-flow bypass can be attempted in certain patients with good compensatory cerebral blood flow (BOT is negative). However, there is a concern that BOT may increase the risk of cerebral infarction and cause rebleeding in ruptured aneurysms. Furthermore, low-flow bypass may not provide sufficient supplemental blood flow, which may lead to fatal cerebral infarction.
In the VA segment, according to the anatomic relationship between the dissecting aneurysm and the PICA, aneurysms are divided into VA with PICA involvement and VA without PICA involvement. In addition to achieving complete occlusion of the aneurysm, preserving the PICA is also important. Occlusion of the PICA may cause ischaemia in the cerebellar and lateral parts of the medulla oblongata, causing Wallenberg syndrome [32]. The assessment of blood supply in the parent VA and contralateral VA is necessary. Theoretically, if dissecting aneurysms are located in the nondominant VA and the PICA is not involved, aneurysm excision can be performed without cerebral bypass because the flow in the contralateral VA is sufficient [33]. However, if dissecting aneurysms are located in the dominant VA, occlusion of the vertebral artery without interpositional flow bypass or vascular reconstruction of the VA will increase the risk of postoperative cerebral infarction since the flow through the contralateral VA is likely insufficient [34, 35]. Interposing a superficial temporal artery, occipital artery, saphenous vein graft or radial artery graft could be used for blood flow supplementation. For aneurysms with PICA involvement, we prefer OA-PICA bypass to preserve the PICA because this technique provides a wide surgical field and short surgical corridor, which increases the convenience and speed of the operation.
In brief, cerebral revascularization appears to be safe and effective for the treatment of complex vertebrobasilar artery dissecting aneurysms. However, cerebral revascularization is utilized mainly for complicated cases and often requires a high surgical skill level and more detailed evaluation before surgery; therefore, cerebral revascularization is not suitable for every patient. We believe that cerebral revascularization could complement treatment for vertebrobasilar artery dissecting aneurysms.
Limitations
The study is a retrospective study and date is collected from a single-center. Moreover, twenty-one cases are too small a number to run meaningful statistical tests. Finally, microsurgical clipping, flow diverter, endovascular parent artery occlusion and other therapeutic methods do not show or discuss in detail in this article.
Conclusions
Cerebral revascularization appears to be safe and effective for the treatment of complex VBDA, and cerebral revascularization could act as a complementary treatment strategy for complex VBDAs.
Data availability
No datasets were generated or analysed during the current study.
References
Arauz A, Ruiz A, Pacheco G, Rojas P, Rodríguez-Armida M, Cantú C, Murillo-Bonilla L, Ruiz-Sandoval JL, Barinagarrementeria F (2013) Aspirin versus anticoagulation in intra and extracranial vertebral artery dissection. Eur J Neurol 20(1):167–172. https://doi.org/10.1111/j.1468-1331.2012.03825.x
Mizutani T (2011) Natural course of intracranial arterial dissections. J Neurosurg 114(4):1037–1044. https://doi.org/10.3171/2010.9.JNS10668
Takumi I, Mizunari T, Mishina M, Fukuchi T, Nomura R, Umeoka K, Kobayashi S, Teramoto A (2007) Dissecting posterior inferior cerebellar artery aneurysm presenting with subarachnoid hemorrhage right after anticoagulant and antiplatelet therapy against ischemic event. Surg Neurol 68(1):103–107. https://doi.org/10.1016/j.surneu.2006.08.063
Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, Goeggel-Simonetti B, Engelter ST, Pezzini A, Bijlenga P, Southerland AM, Naggara O, Béjot Y, Cole JW, Ducros A, Giacalone G, Schilling S, Reiner P, Sarikaya H, Welleweerd JC, Bousser MG (2015) Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 14(6):640–654. https://doi.org/10.1016/S1474-4422(15)00009-5
Liu P, Li Z, Hu L, Liu Y, Li P, Zhu W, Tian Y, Mao Y (2022) Clinical characteristics, endovascular choices, and surgical outcomes of intracranial vertebral artery dissecting aneurysms: a consecutive series of 196 patients. J Neurosurg 138(1):215-222 https://doi.org/10.3171/2022.4.JNS22609.
Fang YB, Lin A, Kostynskyy A, Agid R, Tymianski M, Radovanovic I, Krings T, Pereira VM (2018) Endovascular treatment of intracranial vertebrobasilar artery dissecting aneurysms: parent artery occlusion versus flow diverter. Eur J Radiol 99:68–75. https://doi.org/10.1016/j.ejrad.2017.12.009
Kikkawa Y, Kayahara T, Teranishi A, Shibata A, Suzuki K, Kamide T, Ikeda T, Kurita H (2019) Predictors of the resolution of cavernous sinus syndrome caused by Large/Giant cavernous carotid aneurysms after parent artery occlusion with High-Flow Bypass. World Neurosurg 132:e637–e644. https://doi.org/10.1016/j.wneu.2019.08.059
Kim YS, Kim TS, Yang IC, Joo SP (2019) Staged, combined management of ruptured vertebral artery dissecting aneurysms involving the posterior inferior cerebellar artery: report of 4 cases. 128:444–447. and Review of the Literature. World neurosurgeryhttps://doi.org/10.1016/j.wneu.2019.05.146
Yang Z, Song J, Li P, Zhu W (2021) How I do it? Posterior inferior cerebellar artery-intracranial vertebral artery reimplantation bypass and trapping of dissecting aneurysm involving the proximal posterior inferior cerebellar artery. Acta Neurochir 163(11):2973–2976. https://doi.org/10.1007/s00701-021-04918-9
Sia SF, Morgan MK (2013) High flow extracranial-to-intracranial brain bypass surgery. J Clin Neuroscience: Official J Neurosurgical Soc Australasia 20(1):1–5. https://doi.org/10.1016/j.jocn.2012.05.007
Ramanathan D, Starnes B, Hatsukami T, Kim LJ, Di Maio S, Sekhar L (2013) Tibial artery autografts: alternative conduits for high flow cerebral revascularizations. World Neurosurg 80(3–4):322–327. https://doi.org/10.1016/j.wneu.2012.01.035
Hendrikse J, van der Zwan A, Ramos LM, Tulleken CA, van der Grond J (2003) Hemodynamic compensation via an excimer laser-assisted, high-flow bypass before and after therapeutic occlusion of the internal carotid artery. Neurosurgery 53(4):858–865. https://doi.org/10.1227/01.neu.0000083552.45265.46
Aziz KM, van Loveren HR, Tew JM Jr, Chicoine MR (1999) The Kawase approach to retrosellar and upper clival basilar aneurysms. Neurosurgery 44(6):1225–1236
Goehre F, Kamiyama H, Noda K, Ota N, Tsuboi T, Miyata S, Matsumoto T, Yanagisawa T, Tokuda S, Tanikawa R (2016) Technical description of the medial and lateral anterior temporal Approach for the Treatment of Complex Proximal Posterior Cerebral Artery Aneurysms. World Neurosurg 86:490–496. https://doi.org/10.1016/j.wneu.2015.09.068
Haque R, Kellner C, Solomon RA (2009) Spontaneous thrombosis of a giant fusiform aneurysm following extracranial intracranial bypass surgery. J Neurosurg 110(3):469–474. https://doi.org/10.3171/2007.12.17653
Lee SH, Ahn JS, Kwun BD, Park W, Park JC, Roh SW (2015) Surgical Flow Alteration for the treatment of intracranial aneurysms that are unclippable, untrappable, and Uncoilable. J Korean Neurosurg Soc 58(6):518–527. https://doi.org/10.3340/jkns.2015.58.6.518
Balik V, Yamada Y, Talari S, Kei Y, Sano H, Suyama D, Kawase T, Takagi K, Takizawa K, Kato Y (2018) State-of-art in surgical treatment of dissecting posterior circulation intracranial aneurysms. Neurosurg Rev 41(1):31–45. https://doi.org/10.1007/s10143-016-0749-0
Frisoli FA, Srinivasan VM, Catapano JS, Rudy RF, Nguyen CL, Jonzzon S, Korson C, Karahalios K, Lawton MT (2021) Vertebrobasilar dissecting aneurysms: microsurgical management in 42 patients. J Neurosurg 1–9 Advance online publication. https://doi.org/10.3171/2021.9.JNS21397
Durongwatana N, Sriamornrattanakul K, Wongsuriyanan S, Akharathammachote N (2022) Microsurgical Treatment of vertebral artery dissection: Surgical Strategies and Treatment outcomes. World Neurosurg 159:e375–e388. https://doi.org/10.1016/j.wneu.2021.12.057
Cho WC, Lee HJ, Choi JH, Lee KS, Kim BS, Shin YS (2023) Clinical and radiological outcomes of vertebral artery dissecting aneurysms treated with endovascular treatments: a 12-year single-center experience. World Neurosurg 175:e904–e913. https://doi.org/10.1016/j.wneu.2023.04.040
Kalani MY, Spetzler RF (2016) Internal carotid artery-to-posterior cerebral artery bypass for revascularization of the brainstem. J Clin Neuroscience: Official J Neurosurgical Soc Australasia 24:151–154. https://doi.org/10.1016/j.jocn.2015.08.007
Ikram A, Zafar A (2023) Basilar artery infarct. StatPearls. StatPearls Publishing
Wang X, Tong X (2023) Vascular reconstruction related to the extracranial vertebral artery: the presentation of the concept and the basis for the establishment of the bypass system. Front Neurol, 14: 1202257 https://doi.org/10.3389/fneur.2023.1202257.
Liu Y, Shi X, Kc KIS, Sun Y, Liu F, Qian H, Zhang J (2018) Microsurgical Treatment for Complex Basilar Artery Aneurysms with Long-Term Follow-Up in a series of 35 cases. World Neurosurg 111:e710–e721. https://doi.org/10.1016/j.wneu.2017.12.158
Mai JC, Tariq F, Kim LJ, Sekhar LN (2013) Flow diversion radial artery bypass graft coupled with terminal basilar artery occlusion for the treatment of complex basilar apex aneurysms: operative nuances. Neurosurgery 72(2 Suppl Operative):ons116–ons126. https://doi.org/10.1227/NEU.0b013e31827bf2d8
Ravina K, Strickland BA, Buchanan IA, Rennert RC, Kim PE, Fredrickson VL, Russin JJ (2019) Postoperative antiplatelet therapy in the treatment of Complex Basilar Apex aneurysms Implementing Hunterian Ligation and Extracranial-to-intracranial bypass: review of the literature with an illustrative case report. World Neurosurg 123:113–122. https://doi.org/10.1016/j.wneu.2018.11.237
Zhang J, Feng Y, Zhao W, Liu K, Chen J (2021) Safety and effectiveness of high flow extracranial to intracranial saphenous vein bypass grafting in the treatment of complex intracranial aneurysms: a single-centre long-term retrospective study. BMC Neurol 21(1):307. https://doi.org/10.1186/s12883-021-02339-w
Wang X, Tong X, Liu J, Shi M, Shang Y, Wang H (2023) Petrous Carotid to Upper posterior circulation bypass for the Treatment of Basilar Trunk Aneurysm: a Novel High-Flow Intracranial-Intracranial Skull Base bypass for posterior circulation. 24(3):301–309 Operative neurosurgery (Hagerstown, Md.). https://doi.org/10.1227/ons.0000000000000510
Orita E, Murai Y, Sekine T, Takagi R, Amano Y, Ando T, Iwata K, Obara M, Kumita S (2019) Four-dimensional Flow MRI Analysis of Cerebral Blood Flow before and after High-Flow Extracranial-Intracranial bypass surgery with Internal Carotid Artery Ligation. Neurosurgery 85(1):58–64. https://doi.org/10.1093/neuros/nyy192
Lu X, Huang Y, Zhou P, Zhu W, Wang Z, Chen G (2021) Cerebral revascularization for the management of complex middle cerebral artery aneurysm: a case series. Experimental Therapeutic Med 22(2):883. https://doi.org/10.3892/etm.2021.10315
Kurşun B, Uğur L, Keskin G (2018) Hemodynamic effect of bypass geometry on intracranial aneurysm: a numerical investigation. Computer methods and programs in biomedicine. 158:31–40. https://doi.org/10.1016/j.cmpb.2018.02.008
van den Berg R, Doorschodt TC, Sprengers ME, Vandertop WP (2015) Treatment of dissecting aneurysms of the PICA: anatomical considerations and clinical outcome. J Neuroradiol = J de Neuroradiologie 42(5):291–297. https://doi.org/10.1016/j.neurad.2014.10.001
Shi L, Xu K, Sun X, Yu J (2016) Therapeutic progress in treating vertebral dissecting aneurysms involving the posterior inferior cerebellar artery. Int J Med Sci 13(7):540 https://doi.org/10.7150/ijms.15233.
Inoue T, Tamura A, Saito I (2015) Trapping and V3-radial artery graft-V4 bypass for ruptured dissecting aneurysm of the vertebral artery. NeuroSurg Focus 38(VideoSuppl1). https://doi.org/10.3171/2015.V1.FOCUS14465. Video1
Wongsuriyanan S, Sriamornrattanakul K (2020) Blind-Alley Formation and Occipital Artery-Posterior Inferior Cerebellar Artery Bypass for the Treatment of Unclippable Vertebral Artery Aneurysms with Posterior Inferior Cerebellar Artery Involvement. World neurosurgery, 138, e539–e550 https://doi.org/10.1016/j.wneu.2020.02.174.
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
Li-tian Huang: write manuscript and make drafting the figures; Xiaoguang Tong: conception and design of the study; Meng Zhang: material preparation and data collection.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This is a retrospective review study. Collection of retrospective data was approved by Institutional Ethics Committee of Tianjin Huanhu Hospital. For this type of study, no ethical approval is required
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Lt., Zhang, M. & Tong, X. Cerebral revascularization for complex vertebrobasilar artery dissecting aneurysms. Neurosurg Rev 47, 138 (2024). https://doi.org/10.1007/s10143-024-02365-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02365-5