Abstract
Vertebrobasilar (VB) intracranial dissecting aneurysms (IDAs) pose difficult therapeutic issues and are especially among the most difficult to manage surgically. There are, however, some cases where selective aneurysm obliteration by endovascular approach is impossible or is associated with an unacceptable risk of morbidity. This is particularly true when the aneurysm is dissecting, giant, or has a large neck. In such cases, surgical treatment may be the only alternative. Optimal management of these lesions is therefore challenging and treatment decisions have to be made on a case-by-case basis. Ideal treatment should be a complete surgical excision of the lesion; however, this procedure might only be possible after distal and proximal vessel wall occlusion which might not be tolerated by the patient depending on the location of the aneurysm. Therefore, formulation of recommendations concerning the surgical strategy remains still difficult due to inconsistency of surgical outcomes. The literature describing surgical strategy of VB IDAs is varying in quality and content, and many studies deal with only a few patients. In the presented review, the authors summarize the current knowledge on the incidence, pathogenesis, clinical presentation, and diagnostic procedures with special emphasis on surgical treatment of IDAs in posterior circulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Even still considered relatively rare, intracranial dissecting aneurysms (IDAs) are important causes of subarachnoid hemorrhage (SAH), stroke, or compression of intracranial structures [5, 139] affecting healthy young adults [46] with a high associated morbidity and mortality [67]. Nevertheless, their pathogenesis and management [76] remains unclear and debated, so far. Even though their conservative, endovascular, and surgical treatment have proved successful, with the availability of endovascular procedures, the method has become preferred in treating of IDAs in vertebrobasilar (VB) territory [141]. However, the purely endovascular treatment is unlikely to stop the disease process [156]. Ideal treatment should include a complete surgical excision of the lesion; however, this procedure might only be possible after a complete distal and proximal vessel occlusion which might not be tolerated by the patient [76]. Therefore, formulation of recommendations concerning the surgical strategy remains still difficult due to inconsistency of surgical outcomes [8, 25]. The aim of this paper was to conduct a systemic review of the literature and summarize the current knowledge on the incidence, pathogenesis, clinical presentation, diagnostic procedures, and surgical treatment of the IDAs in posterior circulation.
Search strategy
The strategy was designed by the authors to search data base PubMed for English- and Japanese-language articles using text words or terms including “intracranial,” “dissecting,” “dissection,” “aneurysms,” “posterior,” “circulation,” “vertebral,” “basilar,” “vertebrobasilar,” “superior cerebellar artery,” “anterior inferior cerebellar artery,” “posterior inferior cerebellar artery,” “posterior cerebral artery,” “surgical,” “treatment,” “therapy,” “methods,” “risk factors,” “pathophysiology,” “diagnosis,” and “prognosis.” The words/terms were used in both “AND” and “OR” combinations. The authors included studies with radiological, intraoperative, or pathological confirmation of dissection meanwhile studies that did not provide sufficient detail regarding diagnostic criteria for dissection were excluded. The search was not (a) designed to identify studies reporting on conservative or endovascular treatment and (b) restricted by date of publication, patient age, or number of subjects. The authors also performed a manual search of reference lists from eligible articles but did not seek to identify research abstracts from meeting proceedings or unpublished studies. The search was updated through November 2015.
Incidence
The IDAs, most frequently detected in Eastern Asian population [5], constitute 2–3 % of all cerebral aneurysms [96, 114]. The IDAs of VB territory occur at a rate of 1–1.5 per 100,000 individuals per year [136] and account for about 28 % of posterior circulation aneurysms [171]. The vertebral artery (VA) dissecting aneurysms often impinging the adjacent basilar artery (BA) [171], or posterior inferior cerebellar artery (PICA) [8, 25] represent the most frequent location of intracranial [8, 11], multiple [172], and posterior circulation dissections [46]. Bilateral VA dissecting aneurysms are reported to account for 7.4 to 16 % of all VA dissecting aneurysms [112, 171]. Whereas the intracranial VA is involved in 81.6 % of posterior circulation arterial dissections [46], the BA IDA represents a rarer entity [45] accounting for about 10.5 % of posterior circulation vessel dissections [122]. Meanwhile, the VA dissecting aneurysms occur in patients with mean age 49.7 ± 8.6 years [171] with a higher prevalence in males [68, 70, 90, 171], and the BA dissections tend to affect even younger people with mean age 31.7 years [90]. Since male predominance observed in children could not be explained by trauma, it suggests a sex influence on their pathogenesis [26]. Finally, whereas the incidence of SAH in VB IDAs ranges from 61 to 86 % [3, 58, 68, 70, 125, 171] and 24 % of them rebleed [171], ischemic events associated with the dissections were observed only in 26–62 % of patients [70, 150]. In addition, Ono et al. [111] reported that 18 % of initially unruptured IDAs showed clinical symptomatic recurrence and 90 % of them had major or minor strokes caused by occlusion of perforating arteries with a mean interval of 8.6 months. In children, the rate of recurrent ischemic events was observed in 15 % of cases of posterior circulation dissections with rare hemorrhagic complications [26].
Pathology and pathophysiology
A diversion of circulating blood into the weakened arterial wall may come from (a) a tear in the media due to rupture of the vasa vasorum [51] or new vessels formed in response to pathological process, such as atherosclerosis [124]; (b) the intimal surface causing mainly compression of the lumen by blood accumulation between the internal elastic lamina and media [12, 150]; or (c) the dissection plane lies between the media and the adventitia, causing outpouching of arterial wall [12]. Intracranial arteries are prone to subadventitial dissection [164] and subsequently to SAH [8] due to lack of an external elastic lamina and thin muscular/adventitial layer [164]. A recent observation that medial and subadventitial hemorrhage may also underlie ischemic symptoms indicates that some hemorrhagic transformations of unruptured IDAs may be caused by this mechanism [111]. Internal elastic lamina is the most important layer for determining the strength of the intracranial arteries; therefore, the vessel is more prone to damage if the elastic tissue is defective [28]. The intimal cushion formed at branch points in the cerebral circulation by splitting of the internal elastic lamina may serve as a starting point for dissection, especially if an underlying medial defect is present [39]. Dissection may lead to the formation of (a) intraluminal thrombus [12] with hypoperfusion and infarction [13] or (b) a false lumen that may be a source of emboluses [12, 76] or may occlude small perforators [76, 150]. Entry-exit type of dissections is less prone to rupture than entry-only type [151], since the former mentioned lesions contain a constant flow of blood through the pseudolumen and are clinically more stable [98].
Molecular and genetic factors
Congenital and acquired abnormalities of the arterial media and elastic tissue such as Ehlers-Danlos syndrome or fibromuscular dysplasia are seldom found in patients with dissecting aneurysms [138]. Occasionally, ultrastructural abnormalities resembling the aberrations found in Ehlers-Danlos syndrome type II or III have been found in nontraumatic cervical arterial dissections [10]. One study has also reported the association between alpha 1-antitrypsin deficiency and ruptured intracranial aneurysm or spontaneous dissection of the cervical internal carotid artery (ICA) [137]. Since a fewer than 8 % of patients have connective tissue disorders, it does emphasize the need to shift clinical focus away from seeking VA dissection only in patients with underlying conditions of this type [32].
Risk factors
Although in majority of cases the cause of IDA is unknown, several risk factors (e.g., hypertension, oral contraceptives, cigaret smoking, diabetes mellitus, head or neck trauma, syphilis, polycystic kidney disease, polyarteritis nodosa, systemic lupus erythematosus, or Moyamoya disease) are suspected to be associated with dissection [25, 52, 110, 114, 146, 150, 151, 166]. It has also been suggested that arterial wall edema during a migraine may contribute to the development of IAD as well [49].
Clinical presentation
Patients usually present with either SAH, signs coming from mass effect [12], or ischemic symptoms that have more benign course than those with SAH [37]. Fullerton et al. [26] reported on observation of a 100 % rate of cerebral ischemic presentation in children. A severe, recurrent, steady, or pulsating headache [37, 171] in the occiput with or without its irradiation to the ipsilateral neck preceding neurological disturbances by days to months [86] typically heralds dissections in posterior circulation. However, many patients do not reveal symptoms typically linked to VA IDAs [26] since a pooled prevalence of neck pain in VA dissections was only 46 % [32]. Minor neurological symptoms include diplopia, nystagmus, slight unilateral weakness, vertigo, nausea, vomiting, dysphagia, dysphonia, hemianopsia, dysarthria, Wallenberg’s or Terson’s syndrome, and Horner’s sign [12, 31, 91, 130, 135, 171]. Patients with BA dissection usually present with sudden coma [12] and tetraparesis and extensor plantar reflexes [11]. Cerebral peduncle in the midbrain or pontine tegmental area infarction occasionally causes hemiplegia or coma without pyramidal signs, respectively [11]. Fulminant extension of dissection in case of SAH is one of the important causes of sudden death of the patients [37].
Preoperative diagnosis
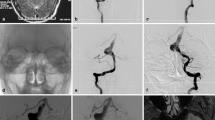
Dissections can be imaged either by digital subtraction angiography (DSA) or less invasive modalities, such as computed tomography (CT)/CT angiography (CTA) or magnetic resonance imaging (MRI)/MRI angiography (MRA). DSA is a gold standard for luminal imaging but, because of its invasive nature and advances in neuroimaging, it is mainly used if CTA or MRI is inconclusive, patients present with SAH, or if surgical or endovascular strategy is being considered [16].
Digital subtraction angiography
The common finding in subintimal dissections is a string sign characterized by a long, narrow column of contrast material; a flame-shaped tapering of the lumen; and occlusion of the artery. A subadventitial dissection is imaged as aneurysmal dilatation preceding or following a focal narrowing of the vessel lumen (“pearl and string sign”) [12]. Retention of contrast medium seen in the late angiographic phase due to its influx into the intramural lumina (intramural pooling sign) was defined as a diagnostic indication of arterial dissection [171] and double lumen sign was considered the most indicative of IAD and seems to be necessary for a decisive diagnosis [70, 135, 150, 171]. The string sign and the pearl and string sign show dynamic changes on follow-up angiography, while tapered occlusions or dissections evident as dilatation without luminal stenosis remain unchanged, irrespective of whether the saccular, fingerlike extensions parallel to the artery or fusiform luminal configuration are present [173].
Computed tomography angiography
Thin-section multisection CTA better demonstrate extraluminal abnormalities [87] and frequently depict more findings indicative of dissection, such as more frequently found intimal flaps and pseudoaneurysms than MRI and MRA [158]. The lumen of VA dissection may be of normal caliber and the lesion is easily overlooked if only lumen-opacifying studies such as contrast MRA or DSA are performed and the only visible abnormality on CTA is a dorsal thickening of the arterial wall against the adjacent fat, the “suboccipital rind” sign [87]. The limitations of MRI/MRA, such as obscuration of mural hematoma by the hyperintense signal of thrombus within an occluded artery and lack of hyperintense signal within mural hematoma on T1WI in the first few days after onset of dissection may be overcome by CTA [20]. Chen et al. [50] found that CTA was especially sensitive and accurate for diagnosis of VA dissection with stenotic or expanded lumen when compared with conventional angiography. Comparing sensitivity and specificity, MR techniques and CTA for diagnosis of craniocervical arterial dissection are quite similar [117].
Magnetic resonance imaging/angiography
MRI of IDA include routine T1-weighted images (T1WI), T2-weighted images (T2WI) usually with spatial presaturation, either with or without fat suppression, and MRA with or without gadolinium enhancement [74, 81, 118, 121]. The intensity of an intramural hematoma on T1WI and proton images varies according to its age [74]. It appears isointense or slightly hyperintense during the first few days after onset, then becomes hyperintense in the subacute stage. The abnormal intensity of the hematoma resolves after several months [29]. Conventional MRI is able to detect the intramural hematomas in less than 35 % of VB IDAs because T1WI isointense hematoma in the acute stage is often obscured by surrounding tissue [71]. Contrary, Yoshimoto and Wakai [173] demonstrated that T1WI between 3 days and 2 months after the ischemic episodes shown intramural hematoma in 70 % of examinations. The T2WI seem to have less diagnostic value for imaging intramural hematoma in the posterior circulation because they are prone to image distortion due to hyperintense cerebrospinal fluid [74], bony structures, and air in mastoid bones [71]. An intimal flap is better visualized on MRI than angiography [56] and MRA seems to be useful in demonstrating pseudolumen, aneurysmal dilatation, and occlusion of the affected vessels [173]. Time-of-flight MRA can provide direct visualization of subacute intramural hematoma as a high-signal structure due to its short T1WI feature [74, 81, 117, 121]; however, differentiation between intraluminal flow, intramural hematoma, and surrounding tissue may be difficult due to (a) the intraluminal signal changes depending on flow velocity, presence of laminar flow and/or turbulence [20, 118] and (b) confusing high-signal intensity caused by the venous plexus and fat surrounding the VAs [74]. For the last decade, a variation of the black blood three-dimensional turbo spin-echo technique has been introduced with visualization grading scores of the intramural hematomas significantly higher than those of MRA [148]. Advanced high-resolution MRI provides detailed information of hidden structures such as intimal flap, inlet of the false lumen, size of the intramural hematoma, and ostium of small branching vessel [162]. It helps to differentiate between intramural hematoma and intraluminal thrombus [162]. Susceptibility-weighted technique provides images with higher resolution, flow compensation, less image distortion in the detection of micro bleeds and can differentiate hemorrhage from calcification [85].
Intraoperative diagnosis
The clinical diagnosis of VB IDAs is based on the characteristic angiographic features but is sometimes difficult to make with certainty [37]. Characteristic sign confirming preoperative diagnosis of dissection is a reddish discoloration of affected artery around aneurysmal dilation owning to hematoma in the vessel wall observed at surgery within acute stage [25, 37, 109, 135, 171]. Approximately 1 month after ictus, the aneurysm becomes whitish gray in color with shiny, smooth surface, firm, and not compressible due to organized intramural clot. Occasionally the vascularization of the outer wall is visible, too [171].
Differential diagnosis
Characteristic angiographic findings of aneurysmal dilatation were observed in 89 % of patients with SAH, but only in 14 % of cases with ischemic symptoms [37]. Limitation of CTA, MRA, and DSA is that they cannot differentiate between different vascular pathologies that exhibit similar luminal changes [87]. Occasionally, the lumen of affected artery appears normal in caliber on CTA, despite the vessel wall hematoma [87]. The string sign may be misdiagnosed as sign of spasm in patients with SAH [131]; however, the vasospasm would not be expected early (until fourth to tenth day) after bleeding and is often multifocal [79]. Moreover, the string sign may be distinguished from ordinary vasospasm by the presence of the mural irregularity [135]. Similarly, a localized abnormality in a single vessel would make an atherosclerotic lesion less likely. Vasculitis also tends to involve vessels in a diffuse manner and would be characterized by areas of arterial wall dilatation in addition to stenosis [79]. Since the term fusiform aneurysms may include both acute dissecting and chronic fusiform lesions [102] including giant so-called dolichoectatic aneurysms [19], a definitive diagnosis should be made on the basis of pathological dissection or angiographic and intraoperative findings. A certain subset of fusiform aneurysms may be caused by dissection of the aneurysmal wall [18, 100], but progressive dilatation of vessels due to defect of the internal elastic lamina may be an important factor in pathogenesis of these lesions as well [174]. Therefore, giant fusiform aneurysms should probably be regarded as distinct entities [30]. A fusiform or irregular aneurysmal dilation located at a non-branching site of an artery is very suggestive of IDAs if associated with a segmental stenosis [103]. However, additional radiological elements are needed to confirm the diagnosis of intracranial artery dissection, including rapid change in morphology [16]. Interestingly, Yamaura reported on that while 81 % VA dissecting aneurysms bleed and 24 % of these rebleed, no such feature was observed in atherosclerotic fusiform aneurysms [168]. Strictly, definite diagnosis of IDAs can be done only by pathological diagnosis [37].
Treatment strategy
As widely accepted guidelines are still missing, an interdisciplinary team of neurosurgeons and endovascular interventionalists should bear in mind patient’s medical and neurological condition, age, and personal preference, location and morphology of IDA, its relation to afferent and efferent vessels, critical structures, presence of neck calcification, dome-to-neck ratio [40], whether the PICA is reduplicated or its territory is supplied by an alternative vessel; whether the contralateral artery is present; whether there persists fetal posterior cerebral circulation [48]. In patients with ischemic symptoms, the treatment strategy should be focused on reduction of a risk of thromboembolic recurrent ischemic stroke by administration of anticoagulants or antiplatelet agents [46, 68] while stenting [12] and surgical treatment [37] is rarely indicated, because in patients without occlusions the arterial lumens usually open of their own accord over time, and anticoagulants are effective at preventing further thromboembolism. As ruptured IDAs are unstable and their tendency to rebleed is associated with high rate mortality, the endovascular approach is the first choice in their treatment [139]. However, a subset of IDAs not amenable to endovascular treatment still requires operative intervention [68]. Beside PICA-origin aneurysms, this subset includes giant, partially thrombosed, fusiform-like aneurysms, those with unfavorable neck-to-dome ratios, and those that have failed attempted endovascular treatment [175]. The purpose of surgery of IDAs is complete isolation of the dissection from the circulation in order to prevent rebleeding and extension of the dissection [37], avoid or reduce ischemic damage, and achieve neural decompression in case of giant aneurysm [97]. Management strategies that combine surgical and endovascular techniques in a complementary way can be designed accordingly [35]. Ventricular drainage can be a necessary emergent procedure, because many patients with rupture of VB IDAs have SAH complicated by hydrocephalus [37, 171].
VA dissecting aneurysm
Approximately 24 % of ruptured VA dissecting aneurysms may rebleed and 80 % of the patients die or become comatose, rebleeding should be prevented [168] and the lesion secured as soon as possible, since rebleeding occurs in early period [37, 155]. The factors that determine the appropriate treatment modalities for ruptured IDAs of the VA are guided by tolerance of the affected VA to a 20-min balloon test occlusion (BTO) and the involvement of the PICA [84].
PICA non-involvement and tolerance of BTO
Patients with IDA without PICA involvement who tolerate the BTO without compromising cerebral perfusion were treated using the Guglielmi Detachable Coils by a double-microcatheter technique with favorable outcomes [36, 40]. The occlusion of critical perforating vessels arising from the distal VA, especially the non-dominant VA, and from proximal and distal part of VB junction [88], may cause underlying treatment-related complications [36]. Affected VA can also be effectively proximally clipped [25, 135, 171], but only when a patient’s contralateral VA and posterior communicating arteries (PComA) are well developed and blood supply to the brain stem and cerebellum is sufficient [135, 161]. However, the blood flow from the contralateral VA may persist; this may retard thrombosis and organization of the dissection site, resulting in postoperative rebleeding [4, 25, 72]. Friedman and Drake [25] reported three VA occlusions by proximally placed clip to the PICA without deficit. The PICA filled retrogradely through (a) the partially thrombosed aneurysm, (b) its high origin just beyond the distal segment of the thrombosed aneurysm, or (c) a collateral vessel from the anterior inferior cerebellar artery (AICA). Interestingly, even trapping cannot prevent the postoperative rebleeding [55, 60]. Mechanism of development of such an aneurysms is not clearly understood, but three anticipated mechanisms have been mentioned: (a) a normal VA and BA developed a dissecting aneurysm due to hemodynamic stress after complete trapping; (b) a preexisting VA or BA dissecting aneurysms angiographically occult enlarged due to hemodynamic changes after trapping; and (c) incomplete trapping [60]. Finally, if the patient remains asymptomatic during the BTO but has evidence of cerebral perfusion compromise, a low- or high-flow bypass should precede parent vessel occlusion [40].
PICA involvement and tolerance of BTO
Even though some authors wrapped [171] or directly clipped neck of PICA-involved VA IDA with favorable outcome [37, 144], in patients with IDAs with PICA involvement who tolerate the BTO, aneurysm trapping and PICA transposition into the VA by interposing a superficial temporal artery (STA) [36] or radial artery (RA) graft [15] is performed either by end-to-end (STA) or end-to-side (STA or RA) anastomosis to PICA and end-to-side anastomosis (STA or RA) to VA facilitating anterograde flow to the PICA territory without requiring the extensive mobilization of brainstem perforating vessels and the proximal PICA [15, 36].
Intolerance of BTO
In the patient who are unable to tolerate the BTO, the trapping of the aneurysm via the posterior transpetrosal approach along with VA–posterior cerebral artery (PCA) bypass procedure may be performed by means of the superior petrosal sinus sacrifice, the tentorium cerebelli cutting and an end-to-side anastomoses between the P2 segment and the extradural VA using RA [36, 40] or vein graft [40]. The wider, proximal end of the RA should be attached to the VA and the smaller one, distal end to the PCA. Finally, trapping of the dissected portion of the VA is performed [36]. Provided that PICA is involved into dissection, trapping of the VA should be preceded by a VA–PCA anastomosis and revascularization of the PICA [36]. When clinically significant mass effect exists and the affected VA is dominant, revascularization along with decompression by aneurysmectomy or aneurysmotomy is demanded [7, 41, 43, 130, 161].
Neurophysiologic intraoperative monitoring
Since BTO cannot be applied to patients with severe SAH due to their deteriorated neurological condition [36], neurophysiologic intraoperative monitoring (NIOM) including electroencephalography (EEG) and evoked potentials are likely best used in combination and can be continuously monitored during the surgical procedure [84]. The final decision which type of NIOM will be used is determined by vascular territory at risk and the patient’s baseline neurologic status [84, 113]. Brainstem auditory evoked potential monitoring detects functional changes along the auditory brain stem pathway, however, ischemia in the cerebellum or PCA territories could be missed [84]. On the other hand, EEG provides a more global assessment of cerebral ischemia but cannot be used to monitor the posterior fossa region. It may be used to detect ischemia in the PCA territory, but its sensitivity is limited [84]. Alternative procedures to BTO include also the use of xenon CT [92] or single-photon emission computed tomography, but they are not very practical [134]. However, when supplemented with cerebral blood flow studies, modern series of carotid sacrifice after normal BTO show false-negative rates of up to 25 %. Moreover, in case of SAH, the cerebrovascular insufficiency may develop in consequence to vasospasm which could impair otherwise sufficient collateralization [113].
Surgical reconstruction of VA
Sano et al. [126] separated VA dissecting aneurysms into unilateral and circumferential group depending on whether the dissection involves only one side of the arterial wall or its entire circumference. The patients with unilateral dissecting aneurysms underwent surgical reconstruction of the VA, whereas patients with circumferential lesions were treated with either proximal clipping or trapping. Of the six patients in whom direct surgical clipping was performed, four had excellent outcomes, one fair outcome, and one died from cardiac failure 12 days after the surgery.
Unilateral/bilateral VA occlussion in bilateral dissecting aneurysms
Since an unilateral VA occlusion of bilateral VB dissections or bilateral VA dissecting aneurysms may be followed by development of stenotic or occlusive dissection or enlargement of a contralateral aneurysm occurs, follow-up angiography is necessary to assess the extent of aneurysm thrombosis, resolution of occlusive dissections, enlargement of contralateral dissecting aneurysms, and direction of flow in the BA. If recurrent or progressive aneurysm enlargement appears, prompt consideration of staged bilateral VA occlusion as definitive therapy is necessary [119, 172].
Wrapping
Even though results of wrapping techniques for saccular aneurysms are generally unsatisfactory [128, 142], Uhl et al. [155] reported on stabilization and elimination of further expansion and rebleeding of dissecting aneurysms following a wrapping of the entire lesion with a cuff resisting tensile stress during the 7 years follow-up period. The potential disadvantage of this method is that the clip might obliterate arterial branches [155] and damage the lower cranial nerves [73]. Well fitting cuffs formed from Dacron or PTFE (Gore-TexTM) strips are pulled through underneath the aneurysm. The ends are then approximated and secured with angled titanium aneurysm clips or malleable clips. Pulling the strip of material around the aneurysm is risky as it requires thorough dissection and removal of clot which may lead to rupture. To avoid this, a strip of PTFE may be first attached to one blade of a fenestrated angled aneurysm clip and then passed around the dissected artery [155]. Sliding a Sundt type encircling clip is certainly the wrapping method that imposes the smallest amount of mechanical stress onto the lower cranial nerves, but these clips are only useful if its size corresponds to the outer diameter of the dissected segment [155].
Surgical approaches
VA can be approached through suboccipital [135] or far lateral inferior suboccipital craniectomy [37] whereas the VB junction may be exposed using far lateral [38], transpetrosal [64], or combined far lateral-transpetrosal approach [38].
BA dissecting aneurysm
The BA IDAs pose difficult therapeutic problems and have even less favorable prognosis than IDAs arising from VA [24]. Furthermore, their rarity limits the acquisition of experience with management of these lesions. Surgical clipping of BA aneurysms has always been a complex procedure with high potential morbidity related to the deep location of the aneurysm and the proximity of critical perforators [65]. If an aneurysm poses a clippable neck, the most effective treatment is its direct obliteration [2, 130]. Unfortunately, in many cases, the aneurysm neck is extremely large and often filled with mural thrombus; therefore, an effective method of aneurysm exclusion from circulation is induction of its thrombosis [43]. Although the natural history of dissecting aneurysms is unique and differs from other vascular lesions, the ischemic effects of proximal ligation should be similar [2]. A standard treatment option for the unclippable giant VB aneurysms generally include endovascular [101] or surgical unilateral/bilateral VA occlusion either distally or proximally to the PICA, BA occlusion, or trapping [17, 38, 40, 43, 130, 161].
Trapping, wrapping, Hunterian ligating, and revascularization
Even though perforators arising from the wall of giant IDAs are thrombosed or nonfunctional [130] and of minimal importance [161], trapping should be avoided until the segment which should be isolated is very short and devoid of perforators [17]. Visualization of perforators behind the neck of a BA aneurysm is vital to safe clip application. However, often the size and projection of a BA aneurysm coupled with the operative approach through a deep surgical corridor preclude safe clipping [65]. While wrapping is associated with a persistent risk of aneurysmal rupture, proximal ligation/occlusion carries a risk of incomplete aneurysmal thrombosis and thromboembolic strokes [17, 18, 145]. Contrary to VA, in case of BA it may be dangerous, unless a sufficient collateral supply exist at least through 1 robust PComA [65].
Unilateral vs. bilateral VA occlussion
Unilateral ligation of a VA seems to be reasonable providing that the aneurysm fills principally or entirely through this VA and the other VA exists, unites with its fellow, and seems large enough to supply the BA. In case of VB aneurysms, a unilateral intracranial occlusion of a VA was performed in eight cases by Drake [17], with no morbidity and complete or nearly complete thrombosis in all but one aneurysm. Seven patients had excellent or fair results while one showed little recovery from an existing medullary syndrome. The best results were achieved when the artery was ligated intracranially just proximal to the aneurysm since an extracranial ligation of VA may be followed by development of an extensive collateral flow from deep cervical arteries with subsequent aneurysm enlargement [17]. If an aneurysm is filled from both VAs, their bilateral occlusion in the neck seems to be much less suitable with reported fair outcome only in about 30 % of patients [17]. Even bilateral PComA collateral flow from anterior to posterior circulation did not prevent development of an acute brain stem edema after bilateral VA occlusion [53].
BA occlusion
BA occlusion may be indicated as a fallback treatment if direct surgical or endovascular intervention is not considered safe due to a number of factors, including failure of previous treatments, large size of the aneurysm, posterior projection of the dome, neck-to-dome ratio > 1, incorporation of major vessels in the neck, and position of the thalamoperforators. Patients with recurrences after neck clipping or coil embolization are also potential candidates for this therapeutic option. Moreover, patient must either demonstrate spontaneous filling of at least one PCA via carotid arterial beds and the preoperative angiogram or trial BTO of the BA should document filling of the BA apex via at least one PComA. A surgical clip can be precisely placed at a single point on the BA between perforators under direct observation and the occlusion is thus stable and nonthrombogenic [65]. Since BA occlusion followed by positive outcome ranges from 40 to 80 % [17, 18, 65], one should keep in mind that the major risk from BA ligation is brain stem ischemia, which may cause disaster in approximately one third of patients [18, 43]. Steinberg et al. [143] reported that only 11 % of the patients treated with lower BA occlusion for predominantly giant intracranial aneurysms developed irreversible ischemic neurological deficits, whereas upper BA occlusions carried a 14 % risk of irreversible ischemic complications, with an additional 8 % of patients unable to tolerate complete BA occlusion but showing reversible ischemia with release of the tourniquet. The presence of only small PComAs correlated significantly with an increased risk of developing brain stem ischemia after upper (P 0.004) or lower (P 0.036) BA occlusion; two small PComA represented the highest risk group. Kellner et al. [65] reported that 80 % patients after proximal clip occlusion of the BA experienced no new postoperative neurological deficits whereas the rest made excellent recoveries at long-term follow-up. No patient experienced postoperative hemorrhage, rebleeding, or delayed neurological deterioration [65].
Revascularization
In circumstances where occlusion is not tolerated, extracranial-intracranial unilateral or bilateral anastomosis may provide the collateral flow that will allow safe occlusion of the BA [17]. High flow ICA to BA bypass with a saphenous vein may provide the collateral flow that will allow safe occlusion of the BA; occasionally complemented by aneurysmectomy [130]. However, a low flow bypass, such as STA anastomosed to either the SCA or the PCA may bear a risk of a high incidence of early graft occlusion and inadequate flow provided to support the upper basilar circulation [145].
BTO and BA aneurysm
If BA fills spontaneously and well from carotid injection then BA occlusion will be probably tolerated. However, even the existence of well-developed PComA either at angiography or operation does not ensure sufficient blood supply to brain stem after BA occlusion [17]. The existence of collateral circulation can be evaluated either by carotid artery compression called as Allcock’s maneuver [18], by BTO as described above for VA during angiography, or intraoperatively when the PComAs could be visualized [65]. While BTO predicts outcome following ICA occlusion with accuracy [157] and neurological deterioration during BTO of the ICA would usually contraindicate parent vessel occlusion, this test has less value in the BA due to the possible perforators obstruction by endovascular balloon. In these situations, patients have to be examined for visual problems indicating ischemia in the PCA distribution and the BTO failure. Symptoms consistent with brainstem ischemia (such as arm or leg weakness, cranial nerve lesions, or loss of consciousness) would not contraindicate surgical BA occlusion, as long as anatomical evidence of filling of the top of the BA via the carotid circulation is angiographically documented. In the case of complex BA tip aneurysms, therefore, the BTO retains its negative predictive value while the positive predictive value of the test is decreased [65]. The use of a temporary BTO with monitoring of auditory and somatosensory evoked potentials may be useful to identify appropriate candidates for this approach [2]. The monitoring of somatosensory and motor evoked potentials is important during the surgical BA occlusion [65].
Surgical approach
There are many surgical approaches that enable access to VB system with their inherited disadvantages. A suboccipital and subtemporal transtentorial approach need significant brain retraction [17] whereas a transoral, transmaxillary, and transclival exposure suited for some midbasilar aneurysms offer a narrow surgical field with a higher risk of cerebrospinal fluid leakage and infection [6, 44, 140]. A combined subtemporal-suboccipital route is suited for visualization of the rostral-to-caudal extent of the brain stem requires a transection of the tentorium and occasionally the transverse and sigmoid sinuses as well. Even though partial or total petrosectomy enables reconstruction of difficult giant VB and midbasilar aneurysms with no brain retraction, a permanent hearing loss and potential damage of facial nerve are its inherited disadvantages [14, 129, 130]. The facial nerve can be mobilized with recovery of normal or nearly normal function provided that at least two of its feeding arteries were preserved [130]. The orbitozygomatic approach extended by removal of the anterior and posterior clinoid process and dorsum sella, exposing the upper two fifths of the BA and far-lateral approach, exposing the lower two fifths of the BA [38, 78] can replace transpetrosal approaches [130]. They have lower morbidity rates than transpetrosal approaches [78]. If the exposure from these two approaches is insufficient because of the size or complexity of a particular aneurysm, then one of the transpetrosal approaches may be selected [78]. Either barbiturate-induced electroencephalic burst suppression and mild hypothermia [40] or deep hypothermic circulatory arrest [17, 78, 130] is critical to clipping large and giant BA aneurysms directly. If crucial dissection or debulking of thrombosed or atherosclerotic aneurysms is needed, adenosine (an endogenous purine nucleoside) may be administered intravenously with the aim to produce cardiac arrest for a few tens of seconds and subsequent profound hypotension for another tens of seconds. Administration of these therapeutic measures may be repeated if previous dose was without complication [33].
Peripheral cerebellar artery dissecting aneurysm
Isolated IDA confined to more peripheral cerebellar arteries have been observed only cursorily in the neurosurgical practice [31]. Majority of peripheral cerebellar artery dissecting aneurysms (PCADAs) arise from their proximal segment (88 %) and present with SAH (58 %) [31]. PICA is the most frequently affected vessel (65 %) followed by SCA (23 %) and AICA (12 %) [31]. Extremely quaint case in this region is represented by ruptured dissecting labyrinthine artery aneurysm successfully surgically treated by trapping without hearing loss or facial weakness [34].
Ischemic group
In patients with ischemic symptoms, some authors performed proximal occlusion of the parent artery [75, 95], trapping with bypass [99], resection and bypass [61], encasement [25], or circumferential wrapping [107] with good postoperative recovery. Other surgeons are inclined to treat the patients conservatively [159, 167].
Subarachnoid hemorrhage group
In patients with SAH, surgical occlusion of the parent artery by trapping [23, 120, 147] or entrapment [170] of the site of dissection, without [149, 163] or with [1, 77, 98, 107] bypass surgery, clipping [104, 163, 170], wrapping [57, 154], circumferential wrapping [107], or coil embolization [93, 123, 152] were applied with favorable results except in one patient who underwent coating of the dissected SCA [31, 93]. If circumferential wrapping with clipping reconstruction or trapping with distal revascularization are technically difficult, Nussbaum et al. [106] suggested a novel approach called remote distal outflow occlusion. The authors reported favorable outcomes and dramatic angiographic resolution of the aneurysmal dilation without ischemic injury or cerebellar edema following application of this method.
AICA
AICA dissecting aneurysms are divided into P group (pons) and C group (cerebellum). The pons group consists of pA (AICA anterior pontine segment), pL (lateral branch on the pons to the meatal loop), and pM (medial branch on the pons). Cerebellum group consists of m-loop (meatal loop), cL (lateral branch post meatal loop), and cM (medial branch on the cerebellum) [27]. Occlusion of the parent artery of AICA dissecting aneurysms pons group without revascularization may entail the risk of death. On the other hand, the parent artery of AICA IDAs cerebellum group may be occluded without severe consequences, but hearing loss and/or cerebellar infarction may be expected [27].
PICA
PICA occlusion distal to the telovelotonsillar segment generally does not result in brainstem injury and is well tolerated in majority of cases [1, 47, 59, 61, 82, 83, 106, 163]. Some patients have a very large PICA that also supplies the AICA territory [106]. When the perforators already are involved in the dissection, proximal occlusion will not cause brainstem infarction. However, if these perforators are still intact great care must be taken to preserve them during occlusion of the parent artery [31, 132].
SCA
The evaluation of cerebellar collateral blood flow is sometimes rather difficult. Using the BTO to assess occlusion of the small arteries of the posterior circulation has not been validated [77]. The more proximal the SCA segment, the more unpredictable the clinical repercussions can be after occlusion. More distal occlusions are more frequently associated with ischemic lesions, but this does not tend to cause clinical manifestations. Larger arteries with small adjacent ones, as well as the presence of a vasospasm, could indicate insufficient collateral blood circulation [77]. The safety of occlusion without revascularization in treatment of the SCA lesions remains unclear. Tohgi et al. [153] reported that after SCA infarcts, 61 % of cases resulted in independent outcomes, 26 %, in dependent outcomes, and 6 % of patients died. Consequences of ischemia in SCA territories were much less favorable than in PICA or AICA regions [153].
Surgical approach
PICA IDAs can be approached by a far-lateral suboccipital craniotomy with C1 laminectomy and partial condylar resection [107], AICA IDAs by lateral suboccipital retrosigmoid approach [27] or transpetrosal approach [64] and SCA IDAs by subtemporal [77] or combined subtemporal presigmoidal approach [105]. Of the revascularization methods, the PICA-PICA, PICA-occipital artery [27, 107] or STA-SCA bypasses [77] were applied.
PCA dissecting aneurysm
Isolated dissection of the PCA is rare [9, 26, 63, 79, 80, 89, 133]. They are predominant in females, with a female–male ratio of 3.1:1 [26, 63]. Isolated PCA dissections tend to present with ischemic symptoms and have a more benign clinical course and prognosis [26, 63, 80, 89, 108]. They are most commonly located near the P1–P2 junction, adjacent to the free border of the tentorium cerebelli [79] suggesting shearing injury of the artery. However, dissections of P3–P4 [54, 79] or P2 segment [79, 133] have been observed as well. Majority of cases are treated conservatively [54, 63, 89, 133]. Anticoagulation, specifically heparin [79, 133] or antiplatelet therapy [133] is recommend for patients with IDAs presenting with stable ischemic symptoms. Patients with ruptured IDAs, repeated intramural hemorrhage, enlargement of an associated aneurysm, or progressive neurologic deficits typically require treatment by surgical or endovascular means. Following placement of the Guglielmi detachable coils (GDCs) proximal to the aneurysm had favorable outcome 67 % of patients [79, 127]. In the surgical group, the P2–3 [8] and P3 [127] segments were involved. One patient underwent a left frontotemporal craniotomy and the abnormal segment was clipped on both sides and resected. In this patient, a hemiparesis developed postoperatively, which remained stable [8]. In the second case, a proximal ligation via temporal craniotomy was followed by thrombosis of the aneurysm without resulting in infarction in the region of the PCA. The occlusion of distal PCA where perforators branch off can be indicated with less risk of infarction especially in cases in which collateral supply of the distal posterior cerebral artery is rich [127]. Of the three patients with SAH managed conservatively, two remained asymptomatic at 6 and 12 months, respectively [116], while the third had rebleeding at 8 months [108]. This suggests that despite demonstration of angiographic improvement in short-term follow-up, the inherent vessel wall abnormality persists, predisposing to recurrent hemorrhage [108].
Complications and their avoidance
Since VB IDAs pose specific anatomical proximities which increase the chance of branching vessels injury, perforators or cranial nerves during surgical procedure and indocyanine green video-angiography visualization is limited to the vessels directly observed under operating microscope [165], the application of the endoscope in deep seated aneurysm may further increase the feasibility of the procedure to minimize the retraction of brainstem, vascular, and neural structures resulting in reduced morbidity [22, 115]. In patients with VA IDAs, the IX. and X. cranial nerves deficits were observed in 16 %, meanwhile XI., XII. cranial nerve paresis and sensory long tract lesions occurred in 5, 5, and 16 % of cases, respectively [171]. Yamaura et al. [169] found that such neurological deficits were commonly seen when the aneurysm was located within 10 mm of the midline or more than 13 mm posterior to the clivus. Thrombosis of the VA and its perforators to the medulla is known to play a more important role in the development of lateral medullary infarction than thrombosis of the PICA [21]. When the PICA is a major supplier of blood to the medulla, the PICA should remain patent proximal to the clip. When the VA is the main supplier to the lateral medullary region, clip occlusion should be performed proximal to the PICA origin so that the PICA will provide collateral perfusion to the distal segment of the VA. This procedure will prevent thrombosis of the distal VA and its perforators [168]. Therefore, the location at which a clip is applied should be carefully decided and its relation to the origin of PICA conditioning the blood supply to the medulla carefully considered.
Prognosis
The most predictive factor for surgical outcome in patients with aneurysms of the VB system is the size of the aneurysm. Sixteen percent morbidity and mortality rates have been reported for small aneurysms (<12 mm), 25 % for large aneurysms (12 to 25 mm), and 50 % for giant aneurysms (>25 mm) [18, 42, 66, 94, 145]. Probability of rebleeding and morbidity/mortality rates of IDAs of the VB circulation were reported as high as 71 and 50 %, respectively [4, 17, 18, 73, 94, 97, 145, 160]. Kawaguchi and associates [62] reported in their series of VA dissecting aneurysms 21 % rebleeding rate during the first 72 h with 100 % mortality. Yamaura et al. [171] reported 24 % of rebleeding in time period from third to 17th postictus day with 80 % mortality rate. Aoki and Sakai [4] reported 30 % rebleeding with 80 % occurring in the first few hours and Mizutani et al. [97] reported 71 % rebleeding, with the mortality 46.7 %, compared to a mortality of 8.3 % of patients, who bled only one time. Acute and chronic dissecting aneurysms of the basilar trunk are known to have a higher mortality rate if aneurysm ruptured (30 vs. 0 %, respectively) [69]. Overall outcome in the patients with BA IDAs was poor, with a mortality rate of 53 % within days to months; an additional 12 % experiences moderate to severe disability, and only 26 % made a fair recovery [3]. Kim et al. [69] reported that 100 % of conservatively managed patients with acute BA IDAs experienced rebleeding with the mortality 67 %, while the rest 33 % were only moderately disabled. Interestingly, the chronic BA dissecting aneurysms, also known as chronic mural bleeding ectasias, are diagnosed incidentally in 64 %, as a cause of compressive syndrome or stroke in the remaining 36 % of cases [125]. Even though, these aneurysms have traditionally been considered to follow a benign course without clinical or imaging progression, they may increase in size over time, rupture, or cause a stroke in 25, 4, and 14 % of cases, respectively [125].
Conclusions
Surgical management of IDAs in posterior circulation is challenging because of difficult surgical access, frequent occurrence of a broad-neck, and the proximity of the perforating branches. Treatment decisions are often complicated by an unpredictable clinical course and controversies in treatment strategy. Nevertheless, the surgical treatment still plays a significant role in their treatment. A better understanding of their incidence, pathology, and natural history may help to improve their management strategy. Although in patients with ischemic symptoms, conservative therapy may be sufficient and effective, if recurrent or progressive ischemic neurological deficit or enlargement of the dissection occur despite appropriate medical therapy, the surgical treatment is justified. Since ruptured IDAs are unstable and their tendency to rebleed is associated with high mortality, the endovascular treatment is the first choice, however, a certain subset of IDAs are not amenable to endovascular approach and still require surgical strategy. The rebleeding occurs in an early period, particularly in the first 24 h, so the treatment should be performed as soon as possible. If the aneurysms pose a clippable neck, the most effective is direct clipping; in case it is unclippable, standard surgical treatment options include Hunterian ligation of VA or BA distally or proximally to the PICA, aneurysmal trapping or circumferential wrapping. Each procedure may be combined with revascularization. If circumferential wrapping with clipping reconstruction or trapping with distal revascularization is technically difficult and the neurosurgeon is confronted with peripheral PICA AIDAs, a remote distal outflow occlusion may be considered.
References
Ali MJ, Bendok BR, Tawk RG, Getch CC, Batjer HH (2002) Trapping and revascularization for a dissecting aneurysm of the proximal posteroinferior cerebellar artery: technical case report and review of the literature. Neurosurgery 51:258–263
Ali MJ, Bendok BR, Tella MN, Chandler JP, Getch CC, Batjer HH (2003) Arterial reconstruction by direct surgical clipping of a basilar artery dissecting aneurysm after failed vertebral artery occlusion: technical case report and literature review. Neurosurgery 52:1475–1481
Amin-Hanjani S, Ogilvy CS, Buonanno FS, Choi IS, Metz LN (1997) Treatment of dissecting basilar artery aneurysm by flow reversal. Acta Neurochir (Wien) 139:44–51
Aoki N, Sakai T (1990) Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke 21:1628–1631
Arauz A, Ruiz A, Pacheco G, Rojas P, Rodríguez-Armida M, Cantú C, Murillo-Bonilla L, Ruiz-Sandoval JL, Barinagarrementeria F (2013) Aspirin versus anticoagulation in intra- and extracranial vertebral artery dissection. Eur J Neurol 20:167–172
Archer DJ, Young S, Ottley D (1987) Basilar aneurysms: a new transclival approach via maxillotomy. J Neurosurg 67:54–58
Ausman JI, Lee MC, Chater N, Latchaw RE (1979) Superficial temporal to superior cerebellar artery anastomosis for distal basilar artery stenosis. Surg Neurol 12:277–283
Berger MS, Wilson CB (1984) Intracranial dissecting aneurysms of the posterior circulation. Report of six cases and review of the literature. J Neurosurg 61:882–894
Berthier E, Bourrat C (1993) Dissecting aneurysm of the posterior cerebral artery: case report and review of the literature. Cerebrovasc Dis 3:56–59
Brandt T, Hausser I, Orberk E, Grau A, Hartschuh W, Anton-Lamprecht I, Hacke W (1998) Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol 44:281–285
Caplan LR (1996) Posterior circulation disease: clinical findings, diagnosis, and management. Blackwell, Boston
Caplan LR (2008) Dissections of brain-supplying arteries. Nat Clin Pract Neurol 4:34–42
Caplan LR, Hennerici MG (1998) Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 55:1475–1482
Cass SP, Sekhar LN, Pomeranz S, Hirsch BE, Synderman CH (1994) Excision of petroclival tumors by a total petrosectomy approach. Am J Otol 15:474–484
Czabanka M, Ali M, Schmiedek P, Vajkoczy P, Lawton MT (2011) Vertebral artery-posterior inferior cerebellar artery bypass using a radial artery graft for hemorrhagic dissecting vertebral artery aneurysms: surgical technique and report of 2 cases. J Neurosurg 114:1074–1079
Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, Goeggel-Simonetti B, Engelter ST, Pezzini A, Bijlenga P, Southerland AM, Naggara O, Béjot Y, Cole JW, Ducros A, Giacalone G, Schilling S, Reiner P, Sarikaya H, Welleweerd JC, Kappelle LJ, de Borst GJ, Bonati LH, Jung S, Thijs V, Martin JJ, Brandt T, Grond-Ginsbach C, Kloss M, Mizutani T, Minematsu K, Meschia JF, Pereira VM, Bersano A, Touzé E, Lyrer PA, Leys D, Chabriat H, Markus HS, Worrall BB, Chabrier S, Baumgartner R, Stapf C, Tatlisumak T, Arnold M, Bousser MG (2015) Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 14:640–654
Drake CG (1975) Ligation of the vertebral (unilateral or bilateral) or basilar artery in the treatment of large intracranial aneurysms. J Neurosurg 43:255–274
Drake CG (1979) Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg 26:12–95
Drake CG, Peerless SJ (1997) Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg 87:141–162
Elijovich L, Kazmi K, Gauvrit JY, Law M (2006) The emerging role of multidetector row CT angiography in the diagnosis of cervical arterial dissection: preliminary study. Neuroradiology 48:606–612
Fisher CM, Ojemann RG, Roberson GH (1978) Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci 5:9–19
Fischer J, Mustafa H (1994) Endoscopic-guided clipping of cerebral aneurysms. Br J Neurosurg 8:559–565
Fransen P, de Tribolet N (1994) Dissecting aneurysm of the posterior inferior cerebellar artery. Br J Neurosurg 8:381–386
Friedman AH (1996) Arterial dissection. In: Wilkins RH, Rengachary SS (eds) Neurosurgery, 2nd edn. McGraw- Hill, New York, pp 2173–2176
Friedman AH, Drake CG (1984) Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg 60:325–334
Fullerton HJ, Johnston SC, Smith WS (2001) Arterial dissection and stroke in children. Neurology 57:1155–1160
Gi H, Inoha S, Uno J, Ikai Y, Koga H, Yamaguchi S, Nagaoka S (2007) Four cases of direct surgery for anterior inferior cerebellar artery aneurysms. No Shinkei Geka 35:571–578, Jpn
Glynn LE (1940) Medial defects in the circle of Willis and their relation to aneurysm formation. J Pathol Bacteriol 51:213–222
Gomori JM, Grossman RI, Goldberg HI, Zimmerman RA, Bialianuk LT (1985) Intracranial hematomas: imaging by high field MR. Radiology 157:87–93
Gonzalez AM, Narata AP, Yilmaz H, Bijlenga P, Radovanovic I, Schaller K, Lovblad KO, Pereira VM (2014) Blood blister-like aneurysms: single center experience and systematic literature review. Eur J Radiol 83:197–205
Gotoh H, Takahashi T, Shimizu H, Ezura M, Tominaga T (2004) Dissection of the superior cerebellar artery: a report of two cases and review of the literature. J Clin Neurosci 11:196–199
Gottesman RF, Sharma P, Robinson KA, Arnan M, Tsui M, Ladha K, Newman-Toker DE (2012) Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist 18:245–254
Groff MW, Adams DC, Kahn RA, Kumbar UM, Yang BY, Bederson JB (1999) Adenosine-induced transient asystole for management of a basilar artery aneurysm. Case report J Neurosurg 91:687–690
Gross BA, Frerichs KU, Du R (2013) Microsurgical treatment of a ruptured dissecting labyrinthine artery aneurysm. Clin Neurol Neurosurg 115:2277–2279
Hacein-Bey L, Connolly ES Jr, Mayer SA, Young WL, Pile-Spellman J, Solomon RA (1998) Complex intracranial aneurysms: combined operative and endovascular approaches. Neurosurgery 43:1304–1313
Hamada JI, Kai Y, Morioka M, Yano S, Todaka T, Ushio Y (2003) Multimodal treatment of ruptured dissecting aneurysms of the vertebral artery during the acute stage. J Neurosurg 99:960–966
Han DH, Kwon OK, Oh CW (1998) Clinical characteristics of vertebrobasilar artery dissection. Neurol Med Chir (Tokyo) 38 Suppl:107–113
Hanel RA, Spetzler RF (2008) Surgical treatment of complex intracranial aneurysms. Neurosurgery 62(Suppl 3):1289–1299
Hayman JA, Anderson RM (1966) Dissecting aneurysm of the basilar artery. Med J Aust 2:360–361
Hebb MO, Spetzler R (2009) Surgical management of giant intracranial aneurysms. In: Kalangu KKN (ed) Essential practice of neurosurgery, 2nd edn. Access Publishing Co., Ltd., Nagoya, pp 577–589
Heros R, Ameri AM (1984) Rupture of a giant basilar aneurysm after saphenous vein interposition graft to the posterior cerebral artery. J Neurosurg 61:387–390
Higashida RT, Hieshima GB, Halbach VV, Goto K, Dormandy B, Bell J, Cahan L, Bentson JR (1986) Intravascular detachable balloon embolization of intracranial aneurysms: indications and techniques. Acta Radiol 369:594–596
Hopkins LN, Budney JL, Castellani D (1983) Extracranial-intracranial arterial bypass and basilar artery ligation in the treatment of giant basilar artery aneurysms. Neurosurgery 13:189–194
Hosobuchi Y (1979) Direct surgical treatment of giant intracranial aneurysms. J Neurosurg 51:743–756
Hosoda K, Fujita S, Kawaguchi T, Shose Y, Yonezawa K, Shirakuni T, Hamasaki M (1991) Spontaneous dissecting aneurysms of the basilar artery presenting with a subarachnoid hemorrhage. J Neurosurg 75:628–633
Huang YC, Chen YF, Wang YH, Tu YK, Jeng JS, Liu HM (2009) Cervicocranial arterial dissection: experience of 73 patients in a single center. Surg Neurol 72(Suppl 2):S20–S27
Hudgins RJ, Day AL, Quisling RG, Rhoton AL Jr, Sypert GW, Garcia-Bengochea F (1983) Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg 58:381–387
Chang EC, Hoh BL, Ogilvy CS (2011) Microsurgery of vertebral artery, posterior cerebellar artery, and vertebrobasilar junction aneurysms. In: Winn RH (ed) Youmans neurological surgery, 6th edn. Elsevier, Philadelphia, pp 3871–3885
Chaves C, Estol C, Esnaola MM, Gorson K, O’Donoghue M, de Witt LD, Caplan LR (2002) Spontaneous intracranial internal carotid artery dissection. Report of 10 patients. Arch Neurol 59:977–981
Chen CJ, Tseng YC, Lee TH, Hsu HL, See LC (2004) Multisection CT angiography compared with catheter angiography in diagnosing vertebral artery dissection. AJNR Am J Neuroradiol 25:769–774
Chen L, Yau I, deVeber G, Dirks P, Armstrong D, Krings T (2015) Evolution of a chronic dissecting aneurysm on magnetic resonance imaging in a pediatric patient. J Neurosurg Pediatr 15:192–196
Chuang MJ, Lu CH, Cheng MH (2012) Management of middle cerebral artery dissecting aneurysm. Asian J Surg 35:42–48
Chung J, Park H, Lim YC, Hyun DK, Shin YS (2011) Endovascular treatment of basilar artery trunk aneurysms. Acta Neurochir 153:2137–2145
Inoue T, Nishimura S, Hayashi N, Numagami Y, Takazawa H, Nishijima M (2007) Postpartum dissecting aneurysm of the posterior cerebral artery. J Clin Neurosci 14:576–581
Inui Y, Oiwa Y, Terada T, Nakakita K, Kamei I, Hayashi S (2006) De novo vertebral artery dissecting aneurysm after contralateral vertebral artery occlusion—two case reports. Neurol Med Chir (Tokyo) 46:32–36
Iwama T, Andoh T, Sakai N, Iwata T, Hirata T, Yamada H (1990) Dissecting and fusiform aneurysms of vertebro-basilar system. Neuroradiology 32:272–279
Jafer J, Kamiryo T, Chiles BW, Nelson PK (1998) A dissecting aneurysm of the posteroinferior cerebellar artery: case report. Neurosurgery 43:353–356
Jin SC, Kwon DH, Choi CG, Ahn JS, Kwun BD (2009) Endovascular strategies for vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol 30:1518–1523
Kanou Y, Arita K, Kurisu K, Ikawa F, Eguchi K, Monden S, Watanabe K (2000) Dissecting aneurysm of the peripheral posterior inferior cerebellar artery. Acta Neurochir (Wien) 142:1151–1156
Katsuno M, Mizunari T, Kobayashi S, Takahashi H, Teramoto A (2009) Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery: case report. Neurol Med Chir (Tokyo) 49:468–470
Kawaguchi S, Sakaki T, Kamada K, Iwanaga H, Takehashi K, Tsujimoto M (1993) Dissecting aneurysm of the posterior inferior cerebellar artery: case report. Neurol Med Chir (Tokyo) 33:634–637
Kawaguchi S, Sakaki T, Tsunoda S, Morimoto T, Hoshida T, Kawai S, Iwanaga H, Nikaido Y (1994) Management of dissecting aneurysms of the posterior circulation. Acta Neurochir (Wien) 131:26–31
Kawahara I, Hiu T, Onizuka M, Toda K, Baba H, Yonekura M (2003) Isolated posterior cerebral artery dissection: case report and review of the literature. No Shinkei Geka 31:671–675, Jpn
Kawase T, Toya S, Shiobara R, Mine T (1985) Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg 63:857–861
Kellner CP, Haque RM, Meyers PM, Lavine SD, Connolly ES Jr, Solomon RA (2011) Complex basilar artery aneurysms treated using surgical basilar occlusion: a modern case series. Clinical article. J Neurosurg 115:319–327
Kempe LG (1979) Aneurysms of the vertebral artery. In: Pia HW, Langmaid C, Zierski J (eds) Cerebral aneurysms. Advances in diagnosis and therapy. Springer, Berlin, pp 119–120
Kim BC, Kwon OK, Oh CW, Bang JS, Hwang G, Jin SC, Park H (2014) Endovascular internal carotid artery trapping for ruptured blood blister-like aneurysms: long-term results from a single centre. Neuroradiology 56:211–217
Kim BM, Kim SH, Kim DI, Shin YS, Suh SH, Kim DJ, Park SI, Park KY, Ahn SS (2011) Outcomes and prognostic factors of intracranial unruptured vertebrobasilar artery dissection. Neurology 76:1735–1741
Kim BM, Suh SH, Park SI, Shin YS, Chung EC, Lee MH, Kim EJ, Koh JS, Kang HS, Roh HG, Won YS, Chung PW, Kim YB, Suh BC (2008) Management and clinical outcome of acute basilar artery dissection. AJNR Am J Neuroradiol 29:1937–4191
Kim CH, Son YJ, Paek SH, Han MH, Kim JE, Chung YS, Kwon BJ, Oh CW, Han DH (2006) Clinical analysis of vertebrobasilar dissection. Acta Neurochir (Wien) 148:395–404
Kim TW, Choi HS, Koo J, Jung SL, Ahn KJ, Kim BS, Shin YS, Lee KS (2013) Intramural hematoma detection by susceptibility-weighted imaging in intracranial vertebral artery dissection. Cerebrovasc Dis 36:292–298
Kitanaka C, Morimoto T, Sasaki T, Takakura K (1992) Rebleeding from vertebral artery dissection after proximal clipping. Case report. J Neurosurg 77:466–468
Kitanaka C, Sasaki T, Eguchi T, Teraoka A, Nakane M, Hoya K (1994) Intracranial vertebral artery dissections: clinical, radiological features, and surgical consideration. Neurosurgery 34:620–627
Kitanaka C, Tanaka J, Kuwahara M, Teraoka A (1994) Magnetic resonance imaging study of intracranial vertebrobasilar artery dissections. Stroke 25:571–575
Komiya H, Saeki N, Iwadate Y, Sunami K, Yamaura A (1988) Posterior inferior cerebellar artery dissecting aneurysm presenting with Wallenberg’s syndrome: case report. Neuro Med Chir (Tokyo) 28:404–408
Krings T, Choi IS (2010) The many faces of intracranial arterial dissections. Interv Neuroradiol 16:151–160
Lamis FC, De Paiva Neto MA, Cavalheiro S (2014) Fusiform superior cerebellar artery aneurysm treated with STA-SCA bypass and trapping. Surg Neurol Int 5(Suppl 4):S139–S142
Lawton MT, Daspit CP, Spetzler RF (1997) Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery 41:513–521
Lazinski D, Willinsky RA, TerBrugge K, Montanera W (2000) Dissecting aneurysms of the posterior cerebral artery: angioarchitecture and a review of the literature. Neuroradiology 42:128–133
Le Tu PT, Zuber M, Meder JF, Mas JL (1996) Dissection isolee de l’artere cerebrale posterieure. Rev Neurol (Paris) 152:542–547
Leclerc X, Lucas C, Godefroy O, Nicol L, Moretti A, Leys D, Pruvo JP (1999) Preliminary experience using contrast-enhanced MR angiography to assess vertebral artery structure for the follow-up of suspected dissection. AJNR Am J Neuroradiol 20:1482–1490
Lewis SB, Chang DJ, Peace DA, Lafrentz PJ, Day AL (2002) Distal posterior inferior cerebellar artery aneurysms: clinical features and management. J Neurosurg 97:756–766
Lister JR, Rhoton AL Jr, Matsushima T, Peace DA (1982) Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery 10:170–199
Liu AY, Lopez JR, Do HM, Steinberg GK, Cockroft K, Marks MP (2003) Neurophysiological monitoring in the endovascular therapy of aneurysms. AJNR Am J Neuroradiol 24:1520–1527
Lobel U, Sedlacik J, Sabin ND, Kocak M, Broniscer A, Hillenbrand CM, Patay Z (2010) Three-dimensional susceptibility-weighted imaging and two-dimensional T2*-weighted gradient-echo imaging of intratumoral hemorrhages in pediatric diffuse intrinsic pontine glioma. Neuroradiology 52:1167–1177
Lotze TE, Paolicchi J (2000) Vertebral artery dissection and migraine headaches in children. J Child Neurol 15:694–696
Lum C, Chakraborty S, Schlossmacher M, Santos M, Mohan R, Sinclair J, Sharma M (2009) Vertebral artery dissection with a normal-appearing lumen at multisection CT angiography: the importance of identifying wall hematoma. AJNR Am J Neuroradiol 30:787–792
Mahmood A, Dujovny M, Torche M, Dragovic L, Ausman JI (1991) Microvascular anatomy of foramen caecum medullae oblongatae. J Neurosurg 75:299–304
Maillo A, Diaz P, Morales F (1991) Dissecting aneurysm of the posterior cerebral artery: spontaneous resolution. Neurosurgery 29:291–294
Manz HJ, Luessenhop AJ (1983) Dissecting aneurysm of intra- cranial vertebral artery: case report and review of literature. J Neurol 230:25–35
Massimi M, Moret J, Tamburrini C, Di Rocco C (2003) Dissecting giant vertebro-basilar aneurysms. Childs Nerv Syst 19:204–210
Mathis JM, Barr JD, Jungreis CA, Yonas H, Sekhar LN, Vincent D, Pentheny SL, Horton JA (1995) Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol 16:749–754
Matsuyama T, Okuchi K, Norimoto K, Ueyama T (2002) Ruptured dissecting anterior inferior cerebellar artery aneurysm: case report. Neurol Med Chir (Tokyo) 42:214–216
McMurtry JG III, Housepian EM, Bowman FO Jr, Matteo RS (1974) Surgical treatment of basilar artery aneurysms: elective circulatory arrest with thoracotomy in 12 cases. J Neurosurg 40:486–494
Mizushima H, Sakaki K, Kunii N, Nishino T, Jinbo H, Abe T, Shimazu M, Matsumoto K (1994) Dissecting aneurysm in the proximal region of the posterior inferior cerebellar artery presenting as Wallenberg’s syndrome: case report. Neurol Med Chir (Tokyo) 34:307–310
Mizutani T (2011) Natural course of intracranial arterial dissections. J Neurosurg 114:1037–1044
Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T (1995) Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 36:905–913
Mizutani T, Kojima H, Asamoto S, Miki Y (2001) Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg 94:712–717
Nagahiro S, Goto S, Yoshioka S, Ushio Y (1993) Dissecting aneurysm of posterior inferior cerebellar artery: case report. Neurosurgery 33:739–742
Nagata I, Kikuchi H, Yamagata S, Miyamoto S, Kaneko T, Asato R (1990) Intraluminal thrombosis and growth-mechanism of giant intracranial aneurysms. No Shinkei Geka 18:1115–1120, Jpn
Nakahara T, Satoh H, Mizoue T, Kawamoto H, Kohmo Y, Kurisu K (1999) Dissecting aneurysm of basilar artery presenting with recurrent subarachnoid hemorrhage. Neurosurg Rev 22:155–158
Nakatomi H, Segawa H, Kurata A, Shiokawa Y, Nagata K, Kamiyama H, Ueki K, Kirino T (2000) Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms: insight on the mechanism of growth. Stroke 31:896–900
Narata AP, Yilmaz H, Schaller K, Lovblad KO, Pereira VM (2012) Flow-diverting stent for ruptured intracranial dissecting aneurysm of vertebral artery. Neurosurgery 70:982–988
Nishino A, Sakurai Y, Niizuma H, Satoh H, Kayama T (1991) Dissecting aneurysm of distal posterior inferior cerebellar artery: case report and review of the literature. No To Shinkei 43:381–386
Nussbaum ES, Defillo A, Zelensky A, Stoller R, Nussbaum L (2011) Dissecting peripheral superior cerebellar artery aneurysms: report of two cases and review of the literature. Surg Neurol Int 2:69
Nussbaum ES, Madison MT, Goddard JK, Lassig JP, Janjua TM, Nussbaum LA (2009) Remote distal outflow occlusion: a novel treatment option for complex dissecting aneurysms of the posterior inferior cerebellar artery. Report of 3 cases. J Neurosurg 111:78–83
Nussbaum ES, Madison MT, Myers ME, Goddard J, Janjua T (2008) Dissecting aneurysms of the posterior inferior cerebellar artery: retrospective evaluation of management and extended follow-up review in 6 patients. J Neurosurg 109:23–27
Ohki M, Nakajima M, Sato K, Kondo R, Saito S, Nakai O, Takeda K, Okumura H (1994) A case of dissecting aneurysm associated with mixed connective tissue disease. No To Shinkei 46:855–858, Jpn
Ohkuma H, Nakano T, Manabe H, Suzuki S (2002) Subarachnoid hemorrhage caused by a dissecting aneurysm of the internal carotid artery. J Neurosurg 97:576–583
Ohkuma H, Suzuki S, Kikkawa T, Shimamura N (2003) Neuroradiologic and clinical features of arterial dissection of the anterior cerebral artery. AJNR Am J Neuroradiol 24:691–699
Ono H, Nakatomi H, Tsutsumi K, Inoue T, Teraoka A, Yoshimoto Y, Ide T, Kitanaka C, Ueki K, Imai H, Saito N (2013) Symptomatic recurrence of intracranial arterial dissections follow-up study of 143 consecutive cases and pathological investigation. Stroke 44:126–131
Ono J, Yamaura A (1994) An analysis of intracranial dissecting aneurysms of the vertebrobasilar system. No Shinkei Geka Journal 3:128–134, Jpn
Pancucci G, Potts MB, Rodríguez-Hernández A, Andrade H, Guo L, Lawton MT (2015) Rescue bypass for revascularization after ischemic complications in the treatment of giant or complex intracranial aneurysms. World Neurosurg 83:912–920
Peluso JP, van Rooij WJ, Sluzewski M, Beute GN, Majoie CB (2008) Endovascular treatment of symptomatic intradural vertebral dissecting aneurysms. AJNR Am J Neuroradiol 29:102–106
Perneczky A, Boecher-Schwarz HG (1998) Endoscope-assisted microsurgery for cerebral aneurysms. Neurol Med Chir Suppl (Tokyo) 38 Suppl:33–34
Pozzati E, Padovani R, Fabrizi A, Sabattini L, Gaist G (1991) Benign arterial dissections of the posterior circulation. J Neurosurg 75:69–72
Provenzale JM, Sarikaya B (2009) Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. Am J Roentgenol 193:1167–1174
Provenzale JM, Sarikaya B, Hacein-Bey L, Wintermark M (2011) Causes of misinterpretation of cross-sectional imaging studies for dissection of the craniocervical arteries. Am J Roentgenol 196:45–52
Redekop G, TerBrugge K, Willinsky R (1999) Subarachnoid hemorrhage from vertebrobasilar dissecting aneurysm treated with staged bilateral vertebral artery occlusion: the importance of early follow-up angiography: technical case report. Neurosurgery 45:1258–1263
Rivas JJ, Domínguez J, Bravo P, Pérez J, Avila AP (2007) Dissecting aneurysm of the posterior cerebellar artery. Neurocirugia (Astur) 18:232–237
Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M (2008) Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics 28:1711–1728
Ruecker M, Furtner M, Knoflach M, Werner P, Gotwald T, Chemelli A, Zangerle A, Prantl B, Matosevic B, Schmidauer C, Schmutzhard E, Willeit J, Kiechl S (2010) Basilar artery dissection: series of 12 consecutive cases and review of the literature. Cerebrovasc Dis 30:267–276
Saito M, Ezura M, Takahashi A, Yoshimoto T (2000) An arterial dissection of the distal anterior inferior cerebellar artery treated by endovascular therapy. No Shinkei Geka 28:269–274, Jpn
Sakata N, Takebayashi S, Kojima M, Masawa N, Suzuki K, Takatama M, Kusumi Y, Mitsumata M (2001) Different roles of arteriosclerosis in the rupture of intracranial dissecting aneurysms. Histopathology 38:325–337
Saliou G, Sacho RH, Power S, Kostynskyy A, Willinsky RA, Tymianski M, terBrugge KG, Rawal S, Krings T (2015) Natural history and management of basilar trunk artery aneurysms. Stroke 46:948–953
Sano H, Kato Y, Okuma I, Yamaguchi S, Ninomiya T, Arunkumar R, Kanno T (1997) Classification and treatment of vertebral dissecting aneurysm. Surg Neurol 48:598–605
Sasaki O, Koizumi T, Ito Y, Sorimachi T, Koike T, Tanaka R (1992) Dissecting aneurysm of the posterior cerebral artery treated with proximal ligation. Surg Neurol 37:394–401
Seiler RW, Reulen HJ, Huber P, Grolimund P, Ebeling U, Steiger HJ (1988) Outcome of aneurysmal subarachnoid hemorrhage in a hospital population: a prospective study including early operation, intravenous nimodipine and transcranial Doppler ultrasound. Neurosurgery 23:598–604
Sekhar LN, Estonillo R (1986) Transtemporal approach to the skull base: an anatomical study. Neurosurgery 19:799–808
Sekhar LN, Chandler JP, Alyono D (1998) Saphenous vein graft reconstruction of an unclippable giant basilar artery aneurysm performed with the patient under deep hypothermic circulatory arrest: technical case report. Neurosurgery 42:667–673
Senter HJ, Sarwar M (1982) Nontraumatic dissecting aneurysm of the vertebral artery. Case report. J Neurosurg 56:128–130
Seyama H, Nishida T, Yamamoto M, Mori H, Satow T, Yamada J, Nakajima N, Takahashi JC, Iihara K, Murao K, Miyamoto S (2006) Therapeutic strategy for isolated dissecting aneurysms of the posterior inferior cerebellar artery: report of three cases and review of literature. No Shinkei Geka 34:1001–1006, Jpn
Sherman P, Oka M, Aldrich E, Jordan L, Gailloud P (2006) Isolated posterior cerebral artery dissection: report of three cases. AJNR Am J Neuroradiol 27:648–652
Shimizu H, Matsumoto Y, Tominaga T (2010) Parent artery occlusion with bypass surgery for the treatment of internal carotid artery aneurysms: clinical and hemodynamic results. Clin Neurol Neurosurg 112:32–39
Shimoji T, Bando K, Nakajima K, Ito K (1984) Dissecting aneurysm of the vertebral artery. Report of seven cases and angiographic findings. J Neurosurg 61:1038–46
Schievink WI (2001) Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 344:898–906
Schievink WI, Prakash UB, Piepgras DG, Mokri B (1994) Alpha 1-antitrypsin deficiency in intracranial aneurysms and cervical artery dissections. Lancet 343:452–453
Schievink WI, Wijdicks EF, Michels VV, Vockley J, Godfrey M (1988) Heritable connective tissue disorders in cervical artery dissections: a prospective study. Neurology 50:1166–1169
Sikkema T, Uyttenboogaart M, Eshghi O, De Keyser J, Brouns R, van Dijk JMC, Luijckx GJ (2014) Intracranial artery dissection. Eur J Neurol 21:820–826
Solomon RA, Stein BM (1988) Surgical approaches to aneurysms of the vertebral and basilar arteries. Neurosurgery 23:203–208
Sonmez O, Brinjikji W, Murad MH, Lanzino G (2015) Deconstructive and reconstructive techniques in treatment of vertebrobasilar dissecting aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol 36:1293–1298
Steiger HJ, Medele R, Bruckmann H, Schroth G, Reulen HJ (1999) Interdisciplinary management results in 100 patients with ruptured and unruptured posterior circulation aneurysms. Acta Neurochir (Wien) 359–367
Steinberg GK, Drake CG, Peerless SJ (1993) Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms: immediate results and long-term outcome in 201 patients. J Neurosurg 79:161–173
Sugita K, Kobayashi S, Inoue T, Banno T (1981) New angled fenestrated clips for fusiform vertebral artery aneurysms. J Neurosurg 54:346–350
Sundt TM Jr, Piepgras DG, Houser OW, Campbell JK (1982) Interposition saphenous vein grafts for advanced occlusive disease and large aneurysms on the posterior circulation. J Neurosurg 56:205–215
Suzuki I, Nishino A, Nishimura S, Numagami Y, Suzuki H, Utsunomiya A, Suzuki S, Uenohara H, Sakurai Y (2005) Nontraumatic arterial dissection of the anterior cerebral artery: six cases report. No To Shinkei 57:509–515, Jpn
Takahashi I, Takamura H, Gotoh S, Sasaki H, Makino K, Suzuki N, Nishihara I, Ishikawa T, Andoh M (1992) Dissecting aneurysm of posterior inferior cerebellar artery: a case report. No Shinkei Geka 20:277–281, Jpn
Takano K, Yamashita S, Takemoto K, Inoue T, Kuwabara Y, Yoshimitsu K (2013) MRI of intracranial vertebral artery dissection: evaluation of intramural haematoma using a black blood, variable-flip-angle 3D turbo spin-echo sequence. Neuroradiology 55:845–851
Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Otani N, Yoshino Y, Yatsushige H (2009) Trapping of ruptured dissecting aneurysm of distal anterior inferior cerebellar artery-case report. Brain Nerve 61:203–207, Jpn
Tawk RG, Bendok BR, Qureshi AI, Getch CC, Srinivasan J, Alberts M, Russell EJ, Batjer HH (2003) Isolated dissections and dissecting aneurysms of the posterior inferior cerebellar artery: topic and literature review. Neurosurg Rev 26:180–187
Thines L, Zairi F, Taschner C, Leclerc X, Lucas C, Bourgeois P, Lejeune JP (2006) Subarachnoid hemorrhage from spontaneous dissection of the anterior cerebral artery. Cerebrovasc Dis 22:452–456
Tikkakoski T, Leinonen S, Siniluoto T, Koivukangas J (1997) Isolated dissecting aneurysm of the left posterior inferior cerebellar artery: endovascular treatment with a Guglielmi detachable coil. AJNR Am J Neuroradiol 18:936–938
Tohgi H, Takahashi S, Chiba K, Hirata Y (1993) The Tohoku Cerebellar Infarction Study Group. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. Stroke 24:1697–1701
Ueki K, Teraoka A, Yoshida S, Hori T (1987) Dissecting aneurysm of the posterior inferior cerebellar artery: a case report. No Shinkei Geka 15:1215–1219, Jpn
Uhl E, Schmid-Elsaesser R, Steiger HJ (2003) Ruptured intracranial dissecting aneurysms: management considerations with a focus on surgical and endovascular techniques to preserve arterial continuity. Acta Neurochir (Wien) 145:1073–1084
van Rooij WJ, Sluzewski M (2009) Endovascular treatment of large and giant aneurysms. AJNR Am J Neuroradiol 30:12–18
van Rooij WJ, Sluzewski M, Metz NH, Nijssen PC, Wijnalda D, Rinkel GJ, Tulleken CA (2000) Carotid balloon occlusion for large and giant aneurysms: evaluation of a new test occlusion protocol. Neurosurgery 47:116–122
Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G (2008) Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol 29:1753–1760
Wakamoto H, Orii M, Miyazaki H, Ishikawa N (2002) A dissecting aneurysm of the posterior inferior cerebellar artery was reduced spontaneously during conservative therapy. No Shinkei Geka 30:425–429, Jpn
Wakhloo AK, Lanzino G, Lieber BB, Hopkins LN (1998) Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery 43:377–379
Wakui K, Kobayashi S, Takemae T, Kamijoh Y, Nagashima H, Muraoka S (1992) Giant thrombosed vertebral artery aneurysm managed with extracranial-intracranial bypass surgery and aneurysmectomy: case report. J Neurosurg 77:624–627
Wang Y, Lou X, Li Y, Sui B, Sun S, Li C, Jiang P, Siddiqui A, Yang X (2014) Imaging investigation of intracranial arterial dissecting aneurysms by using 3 T high-resolution MRI and DSA: from the interventional neuroradiologists’ view. Acta Neurochir (Wien) 156:515–525
Wetjen NM, Link MJ, Reimer R, Nichols DA, Giannini C (2005) Clinical presentation and surgical management of dissecting posterior inferior cerebellar artery aneurysms: 2 case reports. Surg Neurol 64:462–467
Wong GK, Tang HB, Poon WS, Yu SC (2010) Treatment of ruptured intracranial dissecting aneurysms in Hong Kong. Surg Neurol Int 1:84
Yamada Y, Kato Y, Ishihara K, Ito K, Kaito T, Nouri M, Oheda M, Inamasu J, Hirose Y (2015) Role of endoscopy in multi-modality monitoring during aneurysm surgery: a single center experience with 175 consecutive unruptured aneurysms. Asian J Neurosurg. doi:10.4103/1793-5482.151518
Yamashita M, Tanaka K, Matsuo T, Yokoyama K, Fujii T, Sakamoto H (1983) Cerebral dissecting aneurysms in patients with Moyamoya disease. J Neurosurg 58:120–125
Yamashita Y, Hayashi S, Saitou H, Teramoto A (2001) Dissecting aneurysm of posterior inferior cerebellar artery-studied by serial angiography. No Shinkei Geka 29:1057–1062, Jpn
Yamaura A (1988) Diagnosis and treatment of vertebral aneurysms. Neurosurg 69:345–349
Yamaura A, Ise H, Makino H (1981) Radiometric study on posterior inferior cerebellar aneurysms with special reference to accessibility by the lateral suboccipital approach. Neurol Med Chir 21:721–733
Yamaura A, Isobe K, Karasudani H, Tanaka M, Komiya H (1991) Dissecting aneurysms of the posterior inferior cerebellar artery. Neurosurgery 28:894–898
Yamaura A, Watanabe Y, Saeki N (1990) Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 72:183–188
Yasui T, Sakamoto H, Kishi H, Komiyama M, Iwai Y, Yamanaka K, Nishikawa M (1998) Bilateral dissecting aneurysms of the vertebral arteries resulting in subarachnoid hemorrhage: case report. Neurosurgery 42:162–165
Yoshimoto Y, Wakai S (1997) Unruptured intracranial vertebral artery dissection clinical course and serial radiographic imagings. Stroke 28:370–374
Yu YL, Moseley IF, Pullicino P, McDonald WI (1982) The clinical picture of ectasia of the intracerebral arteries. J Neurol Neurosurg Psychiatry 45:29–36
Zada G, Giannotta S (2011) Basilar trunk aneurysms. In: Winn RH (ed) Youmans neurological surgery, 6th edn. Elsevier, Philadelphia, pp 3886–3893
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balik, V., Yamada, Y., Talari, S. et al. State-of-art in surgical treatment of dissecting posterior circulation intracranial aneurysms. Neurosurg Rev 41, 31–45 (2018). https://doi.org/10.1007/s10143-016-0749-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-016-0749-0