Abstract
An external ventricular drain (EVD) is used to facilitate cerebrospinal fluid (CSF) removal in medulloblastoma patients suffering from hydrocephalus. It is essential to recognize that EVD management plays a crucial role in influencing the incidence of drain-related complications. However, the ideal method for EVD management remains undetermined. Our research sought to examine the safety of EVD placement and the impact of EVD on the incidences of intracranial infections, postresection hydrocephalus, and posterior fossa syndrome (PFS). We conducted a single-center observational study involving a cohort of 120 pediatric medulloblastoma patients who were treated from 2017 to 2020. The rates of intracranial infection, postresection hydrocephalus, and PFS were 9.2%, 18.3%, and 16.7%, respectively. EVD did not influence the occurrence of intracranial infection (p = 0.466), postresection hydrocephalus (p = 0.298), or PFS (p = 0.212). A gradual EVD weaning protocol correlated with an elevated incidence of postresection hydrocephalus (p = 0.033), whereas a rapid weaning approach resulted in 4.09 ± 0.44 fewer drainage days (p < 0.001) than the gradual weaning strategy. EVD placement (p = 0.010) and intracranial infection (p = 0.002) were linked to delayed speech return, whereas a longer duration of drainage was conducive to the recovery of language function (p = 0.010). EVD insertion was not correlated with the incidence of intracranial infection, postoperative hydrocephalus, or PFS. The optimal EVD management method should encompass a rapid EVD weaning strategy, followed by prompt drain closure. We have presented additional evidence to improve the safety of EVD insertion and management in neurosurgical patients to ultimately facilitate the establishment of standardized institutional/national implementation and management protocols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is a principal clinical manifestation in pediatric patients with medulloblastoma. However, preresection techniques to divert cerebrospinal fluid (CSF), such as endoscopic third ventriculostomy (ETV), have been advocated by many pediatric neurosurgery centers [1,2,3]. Decreased ETV and ventriculoperitoneal shunt (VPS) placement are important goals, as these are neurosurgical procedures involving lifelong anatomical changes and implants, respectively. The implementation of interventions to circumvent the need for permanent diversion and to mitigate drain-related complications is not only beneficial for pediatric patients but also for health care systems. Notably, placement of an external ventricular drain (EVD) is a lifesaving procedure for acute hydrocephalus and intracranial hypertension in critical patients [4]. However, EVD monitoring increases the risk of complications in patients with posterior fossa tumors, and some analyses have indicated that patients with an EVD have a significantly higher risk of intracranial infection and postresection hydrocephalus [5, 6]. Compared with ETV and VPS, the safety and noninferiority of EVD application need to be elucidated in children with medulloblastomas. Moreover, there is still no consensus regarding the optimal strategy for managing an in-place EVD or for determining the most effective weaning method when no longer needed.
Posterior fossa syndrome (PFS) can develop after posterior fossa lesion removal, especially medulloblastoma [7]. It is still uncertain whether EVD is a risk factor for PFS. Previous studies have suggested that avoidance of EVD implantation does not reduce the risk of PFS [8]. Nevertheless, Khan et al. indicated that undergoing CSF diversion might increase the risk of neurologic injury [9]. A comprehensive understanding of EVD management in pediatric patients with medulloblastomas is lacking.
Herein, we present our retrospective analysis of a pediatric cohort examined at our institution to enhance our understanding of these subjects. The aim of this study was to clinically examine the association of EVD with intracranial infection, postresection hydrocephalus, and PFS. The secondary objective was to ascertain the impact of the EVD management strategy.
Materials and methods
Study cohort
Between January 1, 2017, and December 31, 2020, 166 patients who were younger than 18 years of age and with a newly confirmed diagnosis of medulloblastoma were seen at the Children's Hospital of Chongqing Medical University (CHCMU) and enrolled in the study. These children were assessed and treated in accordance with an institutional protocol that was based on the age and risk-stratified national consensus guideline (CCCG-MB-2017) for managing childhood medulloblastoma, which was conceived in 2016. This cohort study followed the STROBE guidelines [10]. An expert neurologist at our institution examined all the study participants after surgery, after undergoing craniospinal radiotherapy, and every 3–6 months thereafter until December 1, 2022. The CHCMU ethical review boards approved this retrospective examination of anonymized data, with the requirement for informed consent being waived (2,021,405).
Management of EVD
The aim of preoperative EVD placement is to address hydrocephalus, alleviate intracranial hypertension, and avert injury due to acute intracranial pressure fluctuations post surgery. Placement of an EVD was decided based on the consulting neurosurgeon's clinical judgment, with respect to the patient's condition, time until tumor resection, and the anatomical feasibility of EVD insertion. The preoperative EVD placement criteria included severe clinical manifestations, such as frequent vomiting and reduced consciousness, upon admission that was attributable to hydrocephalus-induced intracranial hypertension. The radiographic hydrocephalus diagnosis was evaluated by an Evans index (the maximum frontal horn width divided by the maximal inner table width) exceeding 0.3 in magnetic resonance imaging (MRI) [11].
All EVDs were inserted in operation theatres under sterile conditions. Neuro-navigation was used in patients with small or distorted ventricles. None of the EVDs had an antibiotic-impregnated or silver-coated ventricular catheter, but all of them had an 18 mm diameter standard Ommaya reservoir (Medtronic, Minneapolis, Minnesota, USA), a butterfly scalp vein infusion set with a 20G noncoring needle and 20 cm of flexible tubing, a CSF reservoir bag with 1000 ml capacity, a 100 cm long connector, and a three-way connector. The ventricular catheter is connected with an 18 mm Ommaya reservoir chamber, and secured with a nonabsorbable suture. The Ommaya reservoir was placed subcutaneously near the hairline. The external parts consist of a scalp vein set and a CSF reservoir bag. The needle is inserted in a zig-zag fashion percutaneously to puncture the Ommaya reservoir for intermittent drainage. The flexible end of the scalp vein set is connected to the CSF reservoir bag with a 100 cm tube. The three-way connector is used to connect the scalp vein set with the tubing to facilitate repeated CSF samplings. The entire external assembly was changed aseptically and daily by the nursing team, including the needle for entry into the reservoir.
Intermittent (on-demand) drainage encompasses ensuring that the EVD remains closed, and only allowing the drain to be opened to facilitate CSF drainage when intracranial pressure (ICP) surpasses a predetermined threshold or the patient exhibits symptoms, thereby regulating the daily drainage volume between 50–200 ml. We measured ICP by the scalp vein set inserted in the Ommaya reservoir and connected to a pressure transducer (Transpac IV; ICU Medical, California, USA). The decision to wean was based solely on the managing physician's judgment and the patient's clinical condition. Patients underwent analysis within their designated rapid or gradual EVD weaning groups. Clinicians performed additional individualized secondary analyses predicated on the as-treated EVD weaning process. As-treated weaning strategies were established by utilizing data gleaned from the daily EVD collection instrument. For the as treated analysis, a rapid wean procedure included the following: keep the drain open at 5 cm H2O by default; attempt a rapid EVD wean trial when the ICP is consistently < 15 cm H2O for 24 h; and commence clamping within a 48-h window from the initiation of the weaning trial. The gradual EVD wean protocol was as follows: keep the EVD open at 0 cm above the tragus by default; open the EVD above the tragus when the ICP is < 15 cm H2O; raise the EVD by 5 cm per day until it is 15 cm above the tragus; determine weaning failure before raising further each day.
Intracranial infection complications
Intracranial infection definitions and classifications, ventriculitis and EVD catheter infection (ECI) were defined based on deep/organ space surgical site infection data from the Centers for Disease Control and Prevention [12]. An ECI diagnosis was based on a CSF biochemical profile indicating infection (progressive decline in CSF glucose, elevated protein levels, and increased pleocytosis), ≥ 1 positive CSF culture or Gram stain result, and no other symptoms except fever. The treating physician decided the need for antibiotic treatment. Patients with ventriculitis had all the ECI features, in addition to other clinical manifestations such as nuchal rigidity, photophobia, diminished mental status, seizures, or a moribund appearance.
Postresection hydrocephalus
The criterion for postresection hydrocephalus included failed EVD weaning due to elevated ICP, symptomatic deterioration (worsening mental status), radiographic failure (ventriculomegaly), or newly developed postoperative hydrocephalus necessitating VPS or ETV for permanent CSF diversion. The diagnosis of postresection hydrocephalus was based on the development of hydrocephalus between the first day following medulloblastoma resection and the first day of permanent CSF diversion.
Neurologic evaluations and PFS definitions
Each child underwent a standardized test. A PFS diagnosis was predicated on post resection impaired verbalization, and mutism (lack of vocalization) and paucity of speech with incapacity to form three-word sentences were classified as PFS1 and PFS2, respectively. Children younger than 3 years of age were excluded from the PFS2 evaluations due to their limited linguistic prowess. These classifications (PFS1/PFS2) were endorsed in an international consensus statement, expert surveys and recent research [9, 13, 14].
Covariates
Chang et al. defined M0 as no evidence of metastatic disease and M + as indicative of metastatic disease [15]. Patients underwent next-generation sequencing molecular profiling of tumor tissue. Molecular subgroups were established through targeted mutational and chromosomal copy-number variant (CNV) analysis (Genetron Health, Beijing, China). Based on mutational/CNV-based assessments, the medulloblastomas were categorized into Wingless (WNT), Sonic Hedgehog (SHH), Group 3, and Group 4. A tumor's location was categorized as midline or lateral. The tumor volume was calculated on MRI using the ABC/2 formula in which parameter A represents the maximum diameter of the tumor measured on the axial image, parameter B represents the maximum diameter at 90° to A, and parameter C represents the number of slices of images multiplied by the slice thickness [16]. Approximately 72 h post resection, MRI was performed to ascertain the resection extent, focusing on the tumor's maximal cross-sectional area [17]. Surgeons and neuroradiologists defined gross total resection (GTR) as the absence of residual tumor tissue after resection. Near total resection (NTR) was characterized by no reactive enhancement or residual size ≤ 1.5 cm2, and subtotal resection (STR) was characterized by a residual size > 1.5 cm2.
Statistical methods
Categorical variables were denoted as numbers (percentages [%]) and assessed via the χ2 test or the Fisher exact test when the criteria for the χ2 test were unmet. Continuous variables were presented as the mean (standard deviation ± SD) or median (25th, 75th percentile) and compared between groups by utilizing Student's t test or equivalent nonparametric tests. The relationships between EVD and intracranial infection and the dichotomized PFS outcome (PFS1 + 2 vs. others) were assessed using binary logistic regression models. Cox proportional hazards models and cumulative incidence plots were used to determine whether EVD was a risk factor for post resection hydrocephalus. Given the predetermined patient cohort during the study period, adherence to the principle of approximately ten outcome events per variable in regression necessitates limiting the number of variables potentially linked to the outcome in subsequent analyses [18, 19]. Variables that reached the statistical significance threshold in the univariate models were included in the multivariate models.
The above analyses were performed using SPSS (version 25.0) and R 4.2.1. Each statistical test delineated herein was two-sided, with p values < 0.05 indicating statistically significant differences.
Results
General demographics
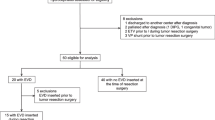
One hundred and twenty (72.3%) patients were centrally reviewed as medulloblastoma and with molecular subgroup prediction. An overview of the inclusion process and treatment methods for this study cohort is shown in Fig. 1. Patient demographical data are shown in Table 1. Compared with patients without an EVD for preoperative CSF diversion, patients with an EVD had more often a midline mass (66 of 75 [88.0%] vs 28 of 45 [62.2%]; p = 0.001), a larger tumor volume (mean ± SD, 40.6 ± 21.2 cm3 vs 29.3 ± 18.8 cm3; p = 0.015), and a radiographic hydrocephalus (50 of 75 [66.7%] vs 11 of 45 [24.4%]; p < 0.001). There was no difference in age, sex, disease stage, methylation subgroup or extent of tumor resection (p = 0.837, 0.208, 0.265, 0.071, and 0.686, respectively). Throughout the median 28.0-month follow-up period, nine patients were unaccounted for, resulting in a follow-up rate of 92.5%.
Features of intracranial infection
We identified 11 (9.2%) children with intracranial infections: ECI in eight (6.7%) and ventriculitis in three (2.5%). ECI and ventriculitis were substantiated at a mean ± standard deviation (SD) of 9.1 ± 4.4 days post-EVD implementation. EVD usage bore no correlation with infectious complications (p = 0.466, Fig. 2). The average catheter placement duration was longer in ECI/ventriculitis patients (6.4 ± 2.4 days) than in those without intracranial infections (4.7 ± 2.9 days); however, this disparity was not statistically significant (p = 0.071). We found no statistically significant differences in the incidence of ECI or ventriculitis in terms of sex, age, tumor location, tumor size, metastatic status, or extent of tumor resection, as depicted in Fig. 2. The univariate analysis showed that medulloblastoma molecular subgroups were not associated with a high risk of intracranial infection (Fig. 2).
Risk factors for the development of intracranial infection. The results were based on single-variable logistic regression models. EVD: external ventricular drain; GTR: gross total resection; G3: Group 3; G4: Group 4; M0: no evidence of metastatic disease; M + : tumor was metastatic; NTR: near total resection; OR: odds ratio; SHH: Sonic Hedgehog; STR: subtotal resection; WNT: Wingless
Rate of postresection hydrocephalus
Every patient with an EVD underwent an attempted EVD wean protocol prior to conversion to VPS placement or ETV. Twenty-two patients required permanent CSF diversion, accounting for 18.3% of the study population—only 21.3% (16/75) developed postresection hydrocephalus. Six patients without preoperative EVD placement required permanent CSF diversion. In patients with postresection hydrocephalus, only 3/22 patients underwent ETV, and 19/22 underwent VPS. We observed no significant association of radiographic hydrocephalus with the requirement of permanent CSF diversion in the univariate regression (Fig. 3a).
Analysis of postresection hydrocephalus. a Risk factors for the development of postresection hydrocephalus. *: results were based on single-variable Cox models. ^: results were based on the final multivariable Cox model. b Distribution of the weaning strategy among patients with postresection hydrocephalus. c Distribution of drainage duration among different weaning strategies, and the difference between means. ECI: external ventricular drain catheter infection; EVD: external ventricular drain; GTR: gross total resection; G3: Group 3; G4: Group 4; HR: hazard ratio; M0: no evidence of metastatic disease; M + : the tumor was metastatic; NTR: near total resection; SHH: Sonic Hedgehog; STR: subtotal resection; WNT: Wingless
EVD application was not found to be associated with postresection hydrocephalus in the univariate Cox model (Fig. 3a). Our findings revealed that a gradual EVD weaning process correlated with an elevated hazard ratio (HR) concerning the occurrence of postresection hydrocephalus (HR: 2.488, 95% confidence interval [CI]: 1.078–5.743, p = 0.033, Fig. 3a and b), but there was no significant association between drainage duration or intracranial infection and postresection hydrocephalus (Fig. 3a). Patients with tumor metastasis had a higher risk of postresection hydrocephalus than those without metastasis (HR: 3.736, 95% CI: 1.618–8.628, p = 0.002, Fig. 3a). The multivariate analysis showed that a gradual weaning protocol (p = 0.010) and metastatic diseases (p = 0.001) increased the risk of postresection hydrocephalus (Fig. 3a). A notable impact of the weaning pattern on duration metrics was observed. Patients who were subjected to the gradual EVD weaning protocol (7.69 ± 2.58 days) had 4.09 ± 0.44 additional EVD days than those subjected to the rapid weaning protocol (3.61 ± 1.99 days, p < 0.001, Fig. 3c).
Risk factors for the development of PFS
PFS1 (complete mutism) was diagnosed in 14 children (11.7%), and PFS2 (partial mutism) was diagnosed in six children (5.0%). Univariate logistic regression analyses indicated that no variable predicted PFS development (Fig. 4a). Our data were then subjected to a sensitivity analysis after 18 participants younger than three years at the time of surgery were excluded. The relationship remained relatively unaltered (Fig. 4a). Moreover, there was no evidence that patients with an EVD (p = 0.127, Fig. 4b) had an increased risk of severe PFS. The severity of PFS was also not correlated with the duration of drainage (p = 0.793) or weaning strategy (p = 0.346).
Analysis of posterior fossa syndrome. a Risk factors for the development of PFS. *: results were based on single-variable logistic models. #: results showed sensitivity analysis. b Distribution of PFS types 1 + 2 among use and nonuse EVD. c Kaplan‒Meier plots showing speech recovery in all 20 children with PFS and using EVD subgroups. ECI: external ventricular drain catheter infection; EVD: external ventricular drain; GTR: gross total resection; G3: Group 3; G4: Group 4; M0: no evidence of metastatic disease; M + : tumor was metastatic; NTR: near total resection; OR: odds ratio; PFS: posterior fossa syndrome; SHH: Sonic Hedgehog; STR: subtotal resection; WNT: Wingless
In the 14 PFS1 children, the median duration for speech recovery was 4.9 months (range 0.7–14.1), with three children exhibiting incomplete speech recovery 12 months post surgery. For the six PFS2 children, the median speech recovery time was 2.8 months (range 0.3–11.1). We employed multivariable-adjusted Cox regression analysis to investigate the risk factors that were linked to delayed speech recovery. For the association between EVD application and outcomes, drainage duration, weaning strategy, intracranial infection, postresection hydrocephalus, and PFS type were adjusted. EVD placement (p = 0.010, Fig. 4c) and ECI or ventriculitis (p = 0.002) were associated with delayed speech return. A longer length of drainage was more conducive to the recovery of language function (p = 0.010).
Discussion
This is the first systematic study of the safety and related complications of preoperative EVD application in pediatric patients with medulloblastomas and hydrocephalus. The rates of intracranial infection, postresection hydrocephalus, and PFS were 9.2%, 18.3%, and 16.7%, respectively. Unexpectedly, we found that the risk of each associated complication differed from that reported in the literature; thus, our results did not support that an EVD increased the risk of intracranial infection [20, 21] or postresection hydrocephalus [22]. We also found that the presence of EVD did not correlate with PFS or its severity [9]. Hence, the implementation of standard institutional/national approaches is essential to enhance the risk–benefit equilibrium.
Herein, we presented our algorithm to manage decompensated hydrocephalus in patients with medulloblastomas. If clinically indicated, we favor placing an EVD at presentation as a standardized neurosurgical procedure to allow safe delay of surgical intervention before attempting surgical excision of the medulloblastoma. Under certain conditions, CSF flow restoration is immediate; however, surgical intervention for medulloblastomas can be extensive or complex, so it is ideal to conduct such procedures during regular working hours with primary ancillary staff following thorough planning. An EVD allows emergency hydrocephalus therapy and venous sinus recoiling through the reopening of pericerebral subarachnoid spaces while ensuring brain relaxation and mitigating operative difficulties [6, 23]. Due to inadequate resources in neurosurgical units and tension between doctors and patients in China [24,25,26], an EVD is preferred among patients/parents and surgeons to mitigate the risk of complications during complex, emergency surgeries [27].
Additional research comparing ETV and VPS with EVD is warranted to validate our preference for EVD. Postresection hydrocephalus, often communicating due to protein precipitation and blood products in CSF [23], typically necessitates temporary rather than permanent diversion. As demonstrated in our results, only 21.3% of medulloblastoma patients who underwent preoperative EVD placement needed permanent shunting. Performing ETV on all patients with hydrocephalus could expose over 70% of them (based on our institutional subset) to unneeded permanent CSF diversion procedures. Preoperative VPS may also result in an unnecessary shunt removal operation in patients who did not need permanent shunting.
We also suggested that an EVD did not increase the risk of permanent CSF diversion following medulloblastoma surgery. We believe that data from this study and data from a prospective multicenter study can be used to support the strong recommendation for a rapid weaning protocol to decrease the need for permanent shunt placement and the duration of drainage [28]. The aim of our investigation was not to elucidate the physiological rationale behind the potential reduction in permanent shunt placement due to an EVD weaning protocol. A nonphysiological explanation might underlie the observed correlation between the weaning protocol and the postresection hydrocephalus rate. Weaning decisions relied solely on the managing physician's judgment and the patient’s clinical status. Practitioners conducting rapid weans could possibly tolerate asymptomatic, radiographic hydrocephalus to a certain extent, thereby decreasing the tendency for shunt placement. Patients tolerating rapid weans might not require permanent CSF diversion. Consequently, the relationship between the weaning protocol and postresection hydrocephalus may merely reflect the physician’s practices and the patients' conditions rather than the physiological factors. Intermittent drainage may have played an essential role in decreasing the risk of postresection hydrocephalus [29, 30]. However, the role of open drainage in postoperative draining of debris or blood and promoting the transparency of CSF cannot be ignored. Intermittent and open drainage methods were not compared because open drainage was not performed in this cohort. However, such comparisons can be made in preclinical and clinical investigations in the future.
Moreover, children with large, midline tumors and a large Evans index value were more likely to undergo preoperative CSF diversion procedures. However, our patient population who underwent preoperative EVD were symptomatic and were diagnosed radiographically. Our criteria for preoperative EVD placement were strict, and EVD catheters were more frequently placed in our department (75/120, 62.5%) than in the department mentioned in Khan et al. (80/178, 44.9%). Nevertheless, their incidence of intracranial infection was consistent with our incidence of 9.2% [5]. Percutaneous access of a subcutaneous reservoir and frequent nonsterile manipulation might also contribute to a high intracranial infection rate [31, 32]. The Ommaya reservoir makes the EVD a closed system and avoids the need for additional ventricular entry, which could reduce the rate of retrograde infection. Moreover, shifting the puncture needle and drainage equipment daily under aseptic conditions could reduce the risk of an associated infection. In our department, EVDs were left for a maximum of 2 weeks [4]. Well-trained trainees and attending physicians could perform EVD procedures at the bedside. However, the operating room is the most sterile environment and should be used whenever feasible [5]; however, EVD procedures and reservoir puncture can be performed at the bedside if sterilization processes are improved. Despite inconsistencies in the literature [33,34,35], antibiotic-impregnated catheters to prevent infection should receive special consideration.
The risk of PFS1 + 2 was not higher in children with EVDs than in children without EVDs. EVD placement is a low-risk operation [36]. It is challenging to identify a correlation between an EVD and PFS. If there would have been a correlation, many patients with shunted hydrocephalus would have been diagnosed with PFS. Conversely, in our experience, EVDs improve surgical conditions and thereby avoid neurological damage. The current study found that EVD was associated with delayed speech recovery, and a longer drainage time promoted speech recovery. Our interpretation is that EVD insertion is related to clinically severe obstructive hydrocephalus and intracranial infection associated with brain injury. For those patients, a sufficient CSF shunt would have prevented neurologic injury and accelerated language function recovery.
There are significant limitations to our analysis. First, this single-center study involved a relatively small sample, limiting the power to test rare events, especially intracranial infection. Second, the retrospective and nonrandomized nature of the analysis introduces potential bias; we did not have a central blinded assessment of the intracranial infection and PFS. Third, as these results are limited to the experience of our team, they may not necessarily generalize broader patterns across all pediatric neurosurgery centers. For example, EVD may not be suitable for patients with medulloblastoma who require transfer to another healthcare facility due to the unavailability of local expertise or medical resources. However, the combination of EVD and Ommaya reservoir could safely address this issue, with intermittent drainage in the hospital transfer process. Last, the conclusion regarding the association between EVD weaning and permanent hydrocephalus treatment lacks sufficient support due to retrospective observational studies' limitations. Future research should include standardized protocols and controlled designs, possibly with randomization, to explore this relationship, which may affect efficacy and safety of the EVD weaning approach.
In our research, we contribute additional evidence to bolster the safety and precision of EVD management. The incidence of ECI/ventriculitis, postresection hydrocephalus, and PFS were 9.2%, 18.3%, and 16.7%, respectively, and remained uninfluenced by EVD utilization. We posit that EVD insertion prior to resection is the optimal approach to avert unnecessary procedures in cases of decompensated hydrocephalus, aligning with many surgeons’ practice patterns. Our study underscores the importance of optimizing sterilization processes during procedures and the sterility of drainage equipment systems to minimize infection risks. An optimal EVD management method might encompass an intermittent drainage protocol coupled with a rapid weaning strategy, followed by prompt drain closure. It is crucial to contemplate institutional/nationwide standard protocols that integrate these recommendations, refine the risk–benefit profile, and ensure consistent implementation.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Srinivasan HL, Foster MT, van Baarsen K, Hennigan D, Pettorini B, Mallucci C (2020) Does pre-resection endoscopic third ventriculostomy prevent the need for post-resection CSF diversion after pediatric posterior fossa tumor excision? A historical cohort study and review of the literature. J Neurosurg Pediatr 1–10. https://doi.org/10.3171/2019.12.Peds19539
El-Ghandour NM (2011) Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in the treatment of obstructive hydrocephalus due to posterior fossa tumors in children. Child’s Nerv Syst 27:117–126. https://doi.org/10.1007/s00381-010-1263-2
Di Rocco F, Jucá CE, Zerah M, Sainte-Rose C (2013) Endoscopic third ventriculostomy and posterior fossa tumors. World Neurosurg 79:S18.e15-19. https://doi.org/10.1016/j.wneu.2012.02.018
Fried HI, Nathan BR, Rowe AS, Zabramski JM, Andaluz N, Bhimraj A et al (2016) The insertion and management of external ventricular drains: an evidence-based consensus statement : a statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care 24:61–81. https://doi.org/10.1007/s12028-015-0224-8
Dakson A, Kameda-Smith M, Staudt MD, Lavergne P, Makarenko S, Eagles ME et al (2021) A nationwide prospective multicenter study of external ventricular drainage: accuracy, safety, and related complications. J Neurosurg 1–9. https://doi.org/10.3171/2021.7.Jns21421
Saad H, Bray DP, McMahon JT, Philbrick BD, Dawoud RA, Douglas JM et al (2021) Permanent cerebrospinal fluid diversion in adults with posterior fossa tumors: incidence and predictors. Neurosurgery 89:987–996. https://doi.org/10.1093/neuros/nyab341
Ashida R, Nazar N, Edwards R, Teo M (2021) Cerebellar mutism syndrome: an overview of the pathophysiology in relation to the cerebrocerebellar anatomy, risk factors, potential treatments, and outcomes. World Neurosurg 153:63–74. https://doi.org/10.1016/j.wneu.2021.06.065
Renne B, Radic J, Agrawal D, Albrecht B, Bonfield CM, Cohrs G et al (2020) Cerebellar mutism after posterior fossa tumor resection in children: a multicenter international retrospective study to determine possible modifiable factors. Child’s Nerv Syst 36:1159–1169. https://doi.org/10.1007/s00381-019-04058-7
Khan RB, Patay Z, Klimo P, Huang J, Kumar R, Boop FA et al (2021) Clinical features, neurologic recovery, and risk factors of postoperative posterior fossa syndrome and delayed recovery: a prospective study. Neuro Oncol 23:1586–1596. https://doi.org/10.1093/neuonc/noab030
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 370:1453–1457. https://doi.org/10.1016/s0140-6736(07)61602-x
Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC (2016) Hydrocephalus in children. Lancet (London, England) 387:788–799. https://doi.org/10.1016/s0140-6736(15)60694-8
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. https://doi.org/10.1016/j.ajic.2008.03.002
Gudrunardottir T, Morgan AT, Lux AL, Walker DA, Walsh KS, Wells EM et al (2016) Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Child’s Nerv Syst 32:1195–1203. https://doi.org/10.1007/s00381-016-3093-3
Wickenhauser ME, Khan RB, Raches D, Ashford JM, Robinson GW, Russell KM, Conklin HM (2020) Characterizing posterior fossa syndrome: a survey of experts. Pediatr Neurol 104:19–22. https://doi.org/10.1016/j.pediatrneurol.2019.11.007
Chang CH, Housepian EM, Herbert C Jr (1969) An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology 93:1351–1359. https://doi.org/10.1148/93.6.1351
Yu YL, Lee MS, Juan CJ, Hueng DY (2013) Calculating the tumor volume of acoustic neuromas: comparison of ABC/2 formula with planimetry method. Clin Neurol Neurosurg 115:1371–1374. https://doi.org/10.1016/j.clineuro.2012.12.029
Lescher S, Schniewindt S, Jurcoane A, Senft C, Hattingen E (2014) Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurg Focus 37:E3. https://doi.org/10.3171/2014.9.Focus14479
Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48:1503–1510. https://doi.org/10.1016/0895-4356(95)00048-8
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49:1373–1379. https://doi.org/10.1016/s0895-4356(96)00236-3
Sáenz A, Badaloni E, Grijalba M, Villalonga JF, Argañaraz R, Mantese B (2021) Risk factors for surgical site infection in pediatric posterior fossa tumors. Child’s Nerv Syst 37:3049–3056. https://doi.org/10.1007/s00381-021-05256-y
Kourbeti IS, Vakis AF, Ziakas P, Karabetsos D, Potolidis E, Christou S, Samonis G (2015) Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg 122:1113–1119. https://doi.org/10.3171/2014.8.Jns132557
Hosainey SAM, Lykkedrang BL, Meling TR (2022) Long-term risk of shunt failure after brain tumor surgery. Neurosurg Rev 45:1589–1600. https://doi.org/10.1007/s10143-021-01648-5
Won SY, Dubinski D, Behmanesh B, Bernstock JD, Seifert V, Konczalla J et al (2020) Management of hydrocephalus after resection of posterior fossa lesions in pediatric and adult patients-predictors for development of hydrocephalus. Neurosurg Rev 43:1143–1150. https://doi.org/10.1007/s10143-019-01139-8
Sun T, Gao L, Li F, Shi Y, Xie F, Wang J et al (2017) Workplace violence, psychological stress, sleep quality and subjective health in Chinese doctors: a large cross-sectional study. BMJ Open 7:e017182. https://doi.org/10.1136/bmjopen-2017-017182
Huang M, Wang J, Ni X, Chen G, Kong L (2016) Neurocritical care in china: past, present, and future. World Neurosurg 95:502–506. https://doi.org/10.1016/j.wneu.2016.06.102
[Anonymous] (2010) Chinese doctors are under threat. Lancet (London, England) 376: 657. https://doi.org/10.1016/s0140-6736(10)61315-3
Braddock CH 3rd (2010) The emerging importance and relevance of shared decision making to clinical practice. Med Decis Mak 30:5s–7s. https://doi.org/10.1177/0272989x10381344
Chung DY, Thompson BB, Kumar MA, Mahta A, Rao SS, Lai JH et al (2022) Association of external ventricular drain wean strategy with shunt placement and length of stay in subarachnoid hemorrhage: a prospective multicenter study. Neurocrit Care 36:536–545. https://doi.org/10.1007/s12028-021-01343-9
Olson DM, Zomorodi M, Britz GW, Zomorodi AR, Amato A, Graffagnino C (2013) Continuous cerebral spinal fluid drainage associated with complications in patients admitted with subarachnoid hemorrhage. J Neurosurg 119:974–980. https://doi.org/10.3171/2013.6.Jns122403
Rao SS, Chung DY, Wolcott Z, Sheriff F, Khawaja AM, Lee H et al (2019) Intermittent CSF drainage and rapid EVD weaning approach after subarachnoid hemorrhage: association with fewer VP shunts and shorter length of stay. J Neurosurg 132:1583–1588. https://doi.org/10.3171/2019.1.Jns182702
Mead PA, Safdieh JE, Nizza P, Tuma S, Sepkowitz KA (2014) Ommaya reservoir infections: a 16-year retrospective analysis. J Infect 68:225–230. https://doi.org/10.1016/j.jinf.2013.11.014
Szvalb AD, Raad II, Weinberg JS, Suki D, Mayer R, Viola GM (2014) Ommaya reservoir-related infections: clinical manifestations and treatment outcomes. J Infect 68:216–224. https://doi.org/10.1016/j.jinf.2013.12.002
Lang SS, Zhang B, Yver H, Palma J, Kirschen MP, Topjian AA et al (2019) Reduction of ventriculostomy-associated CSF infection with antibiotic-impregnated catheters in pediatric patients: a single-institution study. Neurosurg Focus 47:E4. https://doi.org/10.3171/2019.5.Focus19279
Pople I, Poon W, Assaker R, Mathieu D, Iantosca M, Wang E et al (2012) Comparison of infection rate with the use of antibiotic-impregnated vs standard extraventricular drainage devices: a prospective, randomized controlled trial. Neurosurgery 71:6–13. https://doi.org/10.1227/NEU.0b013e3182544e31
Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C, Hamilton AJ (2003) Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg 98:725–730. https://doi.org/10.3171/jns.2003.98.4.0725
Ortolano F, Carbonara M, Stanco A, Civelli V, Carrabba G, Zoerle T, Stocchetti N (2017) External ventricular drain causes brain tissue damage: an imaging study. Acta Neurochir 159:1981–1989. https://doi.org/10.1007/s00701-017-3291-0
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Zaiyu Zhang, Yuxin Wu, and Xueling Zhao. The first draft of the manuscript was written by Zaiyu Zhang. Yuxin Wu, Xueling Zhao, Jianju Zhou, Xuan Zhai, Lusheng Li, and Ping Liang revised it critically for important intellectual content. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Children's Hospital of Chongqing Medical University (No. 2021405).
Consent to participate
Written informed consent was obtained from the patients or their parents.
Consent to publish
This manuscript did not contain any individual person's data in any form (including any individual details, images, or videos).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Wu, Y., Zhao, X. et al. The insertion and management of an external ventricular drain in pediatric patients with hydrocephalus associated with medulloblastoma. Neurosurg Rev 46, 170 (2023). https://doi.org/10.1007/s10143-023-02080-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02080-7