Abstract
Posttraumatic hydrocephalus (PTH) is common in patients undergoing decompressive craniectomy (DC) for traumatic brain injury (TBI), but the incidence, mechanisms, and risk factors have not been fully elucidated. This study aimed to determine the incidence of and the factors associated with PTH. We retrospectively reviewed patients who underwent DC for TBI at our institute between January 2014 and December 2018. We identified and compared the demographic, clinical, and radiological data, and 12-month functional outcome (as assessed by the Glasgow Outcome Scale [GOS]) between patients who developed PTH and those who did not. Logistic regression analyses were performed to identify risk factors for PTH. Additionally, the influence of PTH on unfavorable functional outcome was analyzed. PTH developed in 18 (18.95%) of the 95 patients who survived at 1 month after DC. A multivariate analysis indicated that postoperative intraventricular hemorrhage (odds ratio [OR] 4.493, P = 0.020), postoperative subdural hygroma (OR 4.074, P = 0.021), and postoperative hypothermia treatment (OR 9.705, P = 0.010) were significantly associated with PTH. The 12-month functional outcome significantly differed between the patients who developed PTH and those who did not (P = 0.049). Patients who developed PTH had significantly poorer 12-month functional outcomes than those who did not (P = 0.049). Another multivariate analysis indicated that subdural hemorrhage (OR 6.814, P = 0.031) and the presence of at least one dilated pupil before DC (OR 8.202, P = 0.000) were significantly associated with unfavorable functional outcomes (GOS grades 1–3). Although the influence of PTH (OR 5.122, P = 0.056) was not statistically significant in the multivariate analysis, it had a great impact on unfavorable functional outcomes. PTH considerably affects functional outcomes at 12 months after DC for TBI. Furthermore, postoperative imaging findings such as intraventricular hemorrhage and subdural hygroma can predict the development of PTH; therefore, careful observation is required during the follow-up period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is defined as an acute injury to the head caused by blunt or penetrating trauma or from acceleration/deceleration forces [1]. Although most TBIs are mild, severe TBI can cause death, severe disability, or a vegetative state in patients of all ages. TBI can be pathophysiologically classified into primary injury and secondary injury [2]. Primary injury is a direct injury caused by physical impact on the brain, which includes brain contusion, diffuse axonal injury, and vascular injury. Secondary injury, in contrast, is an indirect and constant injury caused by the primary injury, which results in intracranial hypertension and cerebral ischemia. Therefore, decompressive craniectomy (DC) can be performed to control medically intractable intracranial hypertension and prevent secondary cerebral ischemia in cases of severe TBI.
Although DC is a life-saving procedure that can effectively reduce the intracranial pressure (ICP), it could also cause various complications resulting in long-term neurological deterioration [3, 4]. In particular, posttraumatic hydrocephalus (PTH) is a nontrivial complication requiring additional surgical treatment, with an incidence of 2–46% depending on the diagnostic criteria [5,6,7,8,9,10]. Thus, it is crucial to determine the incidence of PTH after DC for TBI and to identify the factors affecting the development of PTH, which remain to be elucidated.
Herein, we conducted a retrospective study to examine the development of PTH after DC for TBI and analyzed the risk factors associated with PTH. Furthermore, we analyzed the factors predictive of unfavorable functional outcomes, including the development of PTH, in these patients.
Methods
This retrospective study included consecutive patients who underwent DC for TBI. This study was approved by the Institutional Review Board (IRB) of the local hospital (IRB No. 2019-05-032) and was carried out in accordance with the Declaration of Helsinki. The need to obtain informed consent was waived by the IRB as this was a recording-based study with no patient contact.
Patient population
The data of 154 consecutive patients who underwent DC for TBI at our institute between January 2014 and December 2018 were retrospectively reviewed. Patients with a history of hemorrhagic or ischemic stroke, meningitis, craniotomy or craniectomy, or cerebrospinal fluid (CSF) diversion such as ventriculoperitoneal (VP) shunt or endoscopic third ventriculostomy were excluded from this study. Surgery was performed in a standardized manner using a trauma flap by four neurosurgeons. The indications for DC were based on the Brain Trauma Foundation guidelines for the management of ICP following TBI, fourth edition [11]. Patients with intracranial lesions with a midline shift of > 10 mm; hematoma volume of > 30 or 10 cc in supratentorial or infratentorial lesions, respectively; or Glasgow Coma Scale (GCS) score of < 8 underwent surgery at admission. Patients with ICP that was consistently > 20 mmHg or progressive neurological deterioration underwent surgery. In most cases, DC with extensive dural expansion with allograft and hematoma evacuation, if it was surgically accessible, was performed. Postoperative hypothermia treatment was performed in patients with intractable brain swelling or extreme bulging of the brain tissue after DC on intraoperative findings.
Assessment of clinical variables
The baseline demographic characteristics examined in this study included age, sex, cause of trauma, and accompanying major extracranial injuries. We also reviewed the following preoperative computed tomography (CT) findings: the presence of subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), subdural hemorrhage (SDH), intracerebral hemorrhage (ICH), contusion, skull fracture, midline shift, cerebral hernia, effaced cistern ambient, hematoma expansion, contralateral hemorrhage, or skull fracture. The postoperative CT findings included the following: the distance between the midline and the bone flap, and the presence of postoperative IVH, postoperative infarction, or hygroma. The area of craniectomy (craniectomy area) was calculated from the skull X-ray taken postoperatively (largest transverse diameter × vertical diameter perpendicular to transverse diameter × π/4) [12]. In addition, various clinical factors were investigated as follows: Rotterdam score, GCS score at admission, initial platelet counts, international normalized ratio, activated partial thromboplastin time, pupil size and reactivity at admission, type of DC, CSF drain during craniectomy, postoperative hypothermia treatment, reoperation, and the timing of cranioplasty.
Outcome assessment
The patients were classified into two groups based on the development of PTH. The incidence of PTH and the factors associated with the development of PTH were the primary end-point of this analysis. The variables associated with the development of PTH were assessed including baseline demographic characteristics, radiological findings, and various clinical factors.
The definition of PTH was divided into two categories. One is defined as neuroimaging evidence of ventricle enlargement as assessed by the modified frontal horn index, with the largest width of the frontal horns divided by the bicortical distance in the same plane being ≥ 33% [13]. The other is defined as progressive ventricular dilatation, which was established using the criteria described by Gudeman et al. as follows: enlarged anterior horn of the lateral ventricles, enlarged temporal horns, enlarged third ventricle, normal or absent sulci, and periventricular lucencies on serial CT [14].
We also analyzed the clinical outcome such as the 12-month functional outcome as the secondary end-point. Functional outcome was measured using the Glasgow Outcome Scale (GOS). Under this rating system, a GOS score of 1 indicates death, 2 indicates a persistent vegetative state, 3 indicates severe disability (conscious but disabled), 4 indicates moderate disability (disabled but independent), and 5 indicates excellent recovery with return to baseline functional status. Additional analyses were performed to identify the factors that were predictive of the clinical outcome among the aforementioned variables including the development of PTH.
Statistical analysis
Baseline demographic characteristics, preoperative and postoperative CT findings, variable clinical factors, and clinical outcomes were compared between patients who developed PTH and those who did not using Student’s t test or the Mann-Whitney U test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables where appropriate. For discrete variables, the odds ratio (OR) and 95% confidence interval (CI) were calculated. The variables with a P value of < 0.25 on univariate analysis were included in the binary multivariate logistic regression analysis (forward conditional) to derive the potential factors independently associated with the development of PTH or unfavorable functional outcome.
All statistical analyses were performed using standard statistical processing software (SPSS, version 25.0; SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± standard deviation (SD). Differences with probability values of < 0.05 were considered statistically significant.
Results
A total of 151 patients who underwent DC for TBI met the inclusion criteria. Two patients who previously underwent DC and one that underwent craniotomy with tumor removal were excluded. Of the 95 patients who survived until 1 month after DC, 18 (18.95%) developed PTH. Ten PTH patients were diagnosed using the classical criteria by Gudeman and eight patients diagnosed using the other criteria. Thirteen patients (72.22%) were aged < 65 years and 15 (83.33%) were male in the PTH group. Further baseline demographics and clinical characteristics are detailed in Table 1. The prevalence of postoperative IVH, subdural hygroma, craniectomy area (cm3), and hypothermia treatment was significantly higher in the PTH group than in the no PTH group. The other variables were not significantly associated with the development of PTH. However, the presence of postoperative IVH (OR 5.677, P = 0.015), postoperative subdural hygroma (OR 4.133, P = 0.031), and postoperative hypothermia treatment (OR 18.180, P = 0.003) were significantly associated with the development of PTH in the multivariate analysis (Table 2).
The mean period from DC to the development of PTH was 7.42 ± 9.19 months with a range of 21 days to 31.5 months. In ten of the 18 patients with PTH, postoperative subdural hygroma preceded PTH, with a mean interval of 26 ± 25.3 days (range 3–83 days) from DC to the first CT image indicating subdural hygroma (Fig. 1). Fourteen patients developed PTH before cranioplasty, and four patients developed PTH after cranioplasty. The time intervals between cranioplasty and the development of PTH were 6.4, 13.6, 13.7, and 29.5 days, respectively. Among the 18 patients in the PTH group, ten underwent VP shunt placement. However, the remaining patients refused additional surgery such as VP shunt and underwent only conservative treatments.
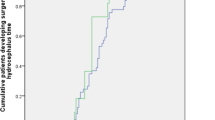
Of the 18 patients in the PTH group, two (11.11%) had favorable functional outcomes (GOS score of 4–5) at 12 months after DC, while 16 (88.89%) had unfavorable functional outcomes (GOS score of 1–3) (Fig. 2). Patients who developed PTH had significantly poorer 12-month functional outcomes than those who did not (P = 0.049) (Table 3). The multivariate analysis indicated that the patients with unfavorable functional outcome at 12 months after DC were more likely to have subdural hemorrhage (OR 6.814, P = 0.031) and at least one pupil dilated at admission (OR 8.202, P = 0.000) (Table 4).
Discussion
DC has been used to manage ICP since it was first described in 1901 by Kocher [15]. It is generally performed for malignant ischemic stroke, intracranial neoplasm, TBI, and spontaneous SAH or ICH. Although most studies have confirmed that DC effectively controlled the ICP and resulted in lower mortality in patients with TBI, it remains unknown whether DC can improve functional outcomes. In particular, various complications that occur after DC are also important factors that can worsen the patient’s long-term prognosis and further compromise quality of life. A recent systematic review of complications related to DC disclosed that the overall complication rate was 13.4% and the complications could be divided into three broad categories: hemorrhagic, infectious/inflammatory, and disturbances of CSF dynamics [3]. Hemorrhagic complications including new hematoma, remote hematoma, and hemorrhagic progression of contusion, and wound problems, such as abscesses or empyemas, and meningitis are included in the infectious/inflammatory category. Complications associated with abnormalities in CSF flow include hydrocephalus, subdural effusion, and paradoxical herniation.
PTH is a late-onset complication and is considered to be one of the major reasons for unexpected deterioration during postoperative rehabilitation. Therefore, it is vital to precisely determine the diagnostic criteria, incidence, and risk factors associated with the development of PTH for accurate diagnosis and subsequent intervention for PTH. However, according to one study, the incidence of PTH after DC varies widely from 2 to 46% according to the criteria used in each study [5,6,7,8,9,10]. Meanwhile, a recent meta-analysis evaluating 2402 patients undergoing DC for TBI concluded that the rate of hydrocephalus was 17.7% (13% in adults) [16]. In our series, the incidence of PTH in patients with TBI who underwent DC was 18.95%, which does not significantly deviate from the aforementioned incidence.
Many studies have investigated the risk factors contributing to the development of PTH, and the results also varied among studies. A craniectomy < 25 mm from the midline; a large craniectomy; the presence, thickness, and distribution of SAH; subdural hygroma; the degree of hypoperfusion in the temporal lobe; repeated operations; and duration of coma were reported as risk factors [5, 6, 17,18,19,20,21,22,23]. Several studies also proposed that DC per se, extremely high ICP before DC, delayed cranioplasty, low initial GCS score, IVH, CSF infection, and old age were risk factors for PTH [6, 17,18,19, 21, 22, 24,25,26,27]. In contrast, some authors concluded that traumatic SAH and IVH were not associated with PTH, and a few studies suggested that younger patients were more likely to develop hydrocephalus requiring a VP shunt after DC [10, 28,29,30].
In the present study, we identified the risk factors associated with the development of PTH in a multivariate analysis using various suggested factors. Although preoperative IVH did not reach statistical significance, postoperative IVH was significantly associated with an increased risk of PTH. Postoperative IVH was identified in our study as a risk factor, which has never been reported before. The exact reason why IVH was not observed in the preoperative CT scan and occurred after surgery is not known; however, it is possible that bleeding could have occurred when the displaced brain parenchyma due to severe midline shift was repositioned by the surgical decompression. Additional studies are needed to explain the mechanism for this phenomenon.
With regard to postoperative subdural hygroma, a number of studies have already described the association with the development of PTH. In particular, Kaen et al. demonstrated that interhemispheric hygroma was a predictive radiological sign of hydrocephalus development within the initial 6 months after DC in patients with severe head injury with a sensitivity of 94% and a specificity of 96% [28]. Moreover, De Bonis reported that interhemispheric hygroma was present in 42% of patients with hydrocephalus, and temporally preceded the occurrence of ventricular enlargement [18]. Even in our study, > 50% (10/18) of the patients had subdural hygroma preceded by PTH. Many subdural hygromas resolve spontaneously; nevertheless, careful observation is required in patients presenting with subdural hygroma on serial CT scans because there is a speculative relationship with PTH.
One significant finding that differed from those of previous reports was the influence of postoperative hypothermia treatment. Hypothermia has been reported to be effective in reducing the ICP, but its role has not yet been established in the treatment of TBI. At our institute, there is no clear standard for conducting hypothermia treatment after DC. Indeed, the patients who underwent hypothermia treatment experienced severe brain swelling in the operating room or sustained persistent increased ICP after DC in the intensive care unit. No reports related to this association have been published previously, and the relationship between hypothermia and CSF flow dynamics is also not known. Regardless, Honeybul et al. found that the mechanism of PTH may be related to the severity of the primary brain injury [31]. In addition, Chen et al. reported that extra herniation after DC was independently associated with PTH [32]. Combining our findings with those of the aforementioned studies, we can indirectly infer that PTH occurs in patients with serious brain injury including extra herniation after DC or persistent increased ICP that are sufficiently severe to be considered for additional hypothermia treatment. This is because severe brain tissue damage is thought to cause more severe CSF circulation and absorption disturbances [32]. However, further research is needed to fully understand the mechanism regarding this.
In our study, an unfavorable functional outcome (GOS score of 1–3) was observed in 16 patients (88.89%) with PTH, which was significantly different from the rate of unfavorable outcome in the 49 patients (63.64%) without PTH. Regardless, only SDH and the presence of at least one dilated pupil predicted poorer functional outcome at 12 months postoperatively in the multivariate analysis. A large number of studies have investigated the incidence of and risk factors associated with PTH, but there is little information regarding its impact on functional outcomes. In one such aforementioned study, the authors demonstrated that hydrocephalus requiring a VP shunt was related to unfavorable functional outcome, which was observed in 86% of those with hydrocephalus but only 59% of those without hydrocephalus [31]. According to other recent studies, the occurrence of PTH was found to correlate significantly with unfavorable functional outcomes [33,34,35]. One study explained that this is because PTH directly impairs brain metabolism and function, and often leads to reduced clinical improvement and poorer functional outcome without timely detection and early management [32]. In addition to PTH, lower GCS scores on admission, high postoperative progressive hemorrhagic injury, bilateral craniectomy, older age, bilateral absence of pupil reactivity, reduced albumin, long duration of comatose state, and delayed cranioplasty were independent predictors that were correlated with unfavorable functional outcome [33, 35,36,37,38,39,40,41,42]. Although our results did not confirm that PTH is a significant independent risk factor that can predict unfavorable functional outcome in the multivariate analysis (P = 0.056), we identified several novel predictors, such as SDH and at least one dilated pupil, that were associated with unfavorable functional outcome. In addition, decrease of pupil reactivity and delayed cranioplasty were identified as predictive factors in the univariate analyses.
Limitation of the study
We recognized some limitations in this study. First, the number of included patients was relatively small, which might have limited our ability to draw firm conclusions. Second, this study was retrospective in nature and utilized data only from hospital records, which could lead to bias regarding patient selection, data collection, and analysis. Finally, since the onset time of PTH varies among patients, evaluating the functional outcome at 12 months after DC may complicate the interpretation of the results. Therefore, further prospective and controlled studies with large populations are needed to obtain more reliable results regarding PTH and to determine the predictors associated with the development of PTH and functional outcome.
Conclusions
Our study revealed that postoperative IVH, subdural hygroma, and hypothermia treatment are risk factors associated with the development of PTH. Furthermore, SDH and at least one dilated pupil were predictive factors that were strongly associated with unfavorable functional outcome, and PTH was somewhat associated with unfavorable functional outcomes. Although these risk factors are not modifiable, it would be beneficial to conduct careful observation and initiate prompt management for patients with these risk factors in order to prevent unfavorable functional outcome caused by PTH.
References
Moon JW, Hyun DK (2017) Decompressive craniectomy in traumatic brain injury: a review article. Kor J Neurotrauma 13:1–8
Giammattei L, Messerer M, Cherian I, Starnoni D, Maduri R, Kasper EM, Daniel RT (2018) Current perspectives in the surgical treatment of severe traumatic brain injury. World Neurosurg 116:322–328
Brown D, Wijdicks E (2017) Decompressive craniectomy in acute brain injury. In: Handbook of clinical neurology. Elsevier, pp 299–318
Sasidharan GM, Shanbhag NC, Shukla DP, Konar SK, Bhat DI, Bhagavatula ID (2018) Complications of decompressive craniectomy. Front Neurol 9:977
Choi I, Park H-K, Chang J-C, Cho S-J, Choi S-K, Byun B-J (2008) Clinical factors for the development of posttraumatic hydrocephalus after decompressive craniectomy. J Kor Neurosurg Soc 43:227–231
De Bonis P, Sturiale CL, Anile C, Gaudino S, Mangiola A, Martucci M, Colosimo C, Rigante L, Pompucci A (2013) Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: a timeline of events? Clin Neurol Neurosurg 115:1308–1312
Fotakopoulos G, Tsianaka E, Siasios G, Vagkopoulos K, Fountas K (2016) Posttraumatic hydrocephalus after decompressive craniectomy in 126 patients with severe traumatic brain injury. Journal of Neurological Surgery Part A: Central European Neurosurgery 77:088–092
Honeybul S, Ho KM (2012) Incidence and risk factors for post-traumatic hydrocephalus following decompressive craniectomy for intractable intracranial hypertension and evacuation of mass lesions. J Neurotrauma 29:1872–1878
Ki HJ, Lee H-J, Lee H-J, Yi J-S, Yang J-H, Lee I-W (2015) The risk factors for hydrocephalus and subdural hygroma after decompressive craniectomy in head injured patients. J Kor Neurosurg Soc 58:254
Vedantam A, Yamal J-M, Hwang H, Robertson CS, Gopinath SP (2018) Factors associated with shunt-dependent hydrocephalus after decompressive craniectomy for traumatic brain injury. J Neurosurg 128:1547–1552
Carney N, Totten AM, O'reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N (2017) Guidelines for the management of severe traumatic brain injury. Neurosurgery 80:6–15
Kim H, Lee HS, Ahn SY, Park SC, Huh W (2017) Factors associated postoperative hydrocephalus in patients with traumatic acute subdural hemorrhage. J Korean Neurosurg Soc 60:730–737
Woo J-Y, Lee S-B, Yoo D-S, Cho K-S, Huh P-W, Kang S-G, Kim D-S, Park C-K (2006) Differential diagnostic method between the external hydrocephalus and simple subdural hygroma. J Kor Neurotrauma Soc 2:31–36
Gudeman S, Kishore P, Becker DP, Lipper MH, Girevendulis A, Jeffries B, Butterworth J 4th (1981) Computed tomography in the evaluation of incidence and significance of post-traumatic hydrocephalus. Radiology 141:397–402
Kocher T (1901) Hirnerschütterung, hirndruck und chirurgische eingriffe bei hirnkrankheiten. A. Hölder
Fattahian R, Bagheri SR, Sadeghi M (2018) Development of posttraumatic hydrocephalus requiring ventriculoperitoneal shunt after decompressive craniectomy for traumatic brain injury: a systematic review and meta-analysis of retrospective studies. Med Arch 72:214–219
Cho B-R, Lee H-J, Lee H-J, Yi J-S, Yang J-H, Lee I-W (2012) Risk factors for the post-traumatic hydrocephalus following decompressive craniectomy in severe traumatic injury patients. Kor J Neurotrauma 8:110–114
De Bonis P, Pompucci A, Mangiola A, Rigante L, Anile C (2010) Post-traumatic hydrocephalus after decompressive craniectomy: an underestimated risk factor. J Neurotrauma 27:1965–1970
Honeybul S, Ho KM (2011) Long-term complications of decompressive craniectomy for head injury. J Neurotrauma 28:929–935
Jeon SW, Choi JH, Jang TW, Moon S-M, Hwang H-S, Jeong JH (2011) Risk factors associated with subdural hygroma after decompressive craniectomy in patients with traumatic brain injury: a comparative study. J Kor Neurosurg Soc 49:355–358
Jiao Q, Liu Z, Li S, Zhou L, Li S, Tian W, You C (2007) Influencing factors for posttraumatic hydrocephalus in patients suffering from severe traumatic brain injuries. Chin J Traumatol= Zhonghua chuang shang za zhi 10:159–162
Tian H-L, Xu T, Hu J, Y-h C, Chen H, Zhou L-F (2008) Risk factors related to hydrocephalus after traumatic subarachnoid hemorrhage. Surg Neurol 69:241–246
Yang X, Hong G, Su S, Yang S (2003) Complications induced by decompressive craniectomies after traumatic brain injury. Chinese Journal of Traumatology= Zhonghua chuang shang za zhi 6:99–103
Ding J, Guo Y, Tian H (2014) The influence of decompressive craniectomy on the development of hydrocephalus: a review. Arq Neuropsiquiatr 72:715–720
Iencean S, Ianovici N, Ciurea A (2009) Intracranial pressure monitoring study in severe traumatic brain injury and post-traumatic hydrocephalus. Romanian Neurosurgery 16:17–19
Mazzini L, Campini R, Angelino E, Rognone F, Pastore I, Oliveri G (2003) Posttraumatic hydrocephalus: a clinical, neuroradiologic, and neuropsychologic assessment of long-term outcome. Arch Phys Med Rehabil 84:1637–1641
Takeuchi S, Nagatani K, Wada K, Nawashiro H, Otani N, Osada H, Kobayashi H, Suzuki T, Shima K (2013) Is decompressive craniectomy a risk factor for ventriculomegaly? Brain Edema XV. Springer, In, pp 281–283
Kaen A, Jimenez-Roldan L, Alday R, Gomez PA, Lagares A, Alen JF, Lobato RD (2010) Interhemispheric hygroma after decompressive craniectomy: does it predict posttraumatic hydrocephalus? J Neurosurg 113:1287–1293
Low CY, Low YY, Lee KK, Chan SP, Ang BT (2013) Post-traumatic hydrocephalus after ventricular shunt placement in a Singaporean neurosurgical unit. J Clin Neurosci 20:867–872
Poca MA, Sahuquillo J, Mataro M, Benejam B, Arikan F, Baguena M (2005) Ventricular enlargement after moderate or severe head injury: a frequent and neglected problem. J Neurotrauma 22:1303–1310
Honeybul S, Ho KM (2014) Decompressive craniectomy for severe traumatic brain injury: the relationship between surgical complications and the prediction of an unfavourable outcome. Injury 45:1332–1339
Chen H, Yuan F, Chen SW, Guo Y, Wang G, Deng ZF, Tian HL (2017) Predicting posttraumatic hydrocephalus: derivation and validation of a risk scoring system based on clinical characteristics. Metab Brain Dis 32:1427–1435
Di G, Zhang Y, Liu H, Jiang X, Liu Y, Yang K, Chen J (2019) Postoperative complications influencing the long-term outcome of head-injured patients after decompressive craniectomy 9:e01179
Khalili H, Niakan A, Ghaffarpasand F, Kiani A, Behjat R (2017) Outcome determinants of decompressive craniectomy in patients with traumatic brain injury; a single center experience from southern Iran. Bull Emerg Trauma 5:190–196
Nasi D, Dobran M, Di Rienzo A, di Somma L, Gladi M, Moriconi E, Scerrati M, Iacoangeli M (2018) Decompressive craniectomy for traumatic brain injury: the role of cranioplasty and hydrocephalus on outcome. World Neurosurg 116:e543–e549
Chibbaro S, Di Rocco F, Mirone G, Fricia M, Makiese O, Di Emidio P, Romano A, Vicaut E, Menichelli A, Reiss A, Mateo J, Payen D, Guichard JP, George B, Bresson D (2011) Decompressive craniectomy and early cranioplasty for the management of severe head injury: a prospective multicenter study on 147 patients. World Neurosurg 75:558–562
Khan F, Valliani A, Rehman A, Bari ME (2018) Factors affecting functional outcome after decompressive craniectomy performed for traumatic brain injury: a retrospective, cross-sectional study. Asian J Neurosurg 13:730–736
Liang W, Xiaofeng Y, Weiguo L, Gang S, Xuesheng Z, Fei C, Gu L (2007) Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J Craniofac Surg 18:526–532
Nalbach SV, Ropper AE, Dunn IF, Gormley WB (2012) Craniectomy-associated progressive extra-axial collections with treated hydrocephalus (CAPECTH): redefining a common complication of decompressive craniectomy. J Clin Neurosci 19:1222–1227
Sun S, Zhou H, Ding ZZ, Shi H (2018) Risk factors associated with the outcome of post-traumatic hydrocephalus. Scand J Surg:1457496918812210
Yu P, Tian Q, Wen X, Zhang Z, Jiang R (2015) Analysis of long-term prognosis and prognostic predictors in severe brain injury patients undergoing decompressive craniectomy and standard care. J Craniofac Surg 26:e635–e641
Zhang K, Jiang W, Ma T, Wu H (2016) Comparison of early and late decompressive craniectomy on the long-term outcome in patients with moderate and severe traumatic brain injury: a meta-analysis. Br J Neurosurg 30:251–257
Acknowledgments
We would like to thank So Young Park, Byung Min Kim, Sang Woo Cho, Jae Min Lee, and Young Nam Kim for their assistance with this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board (IRB) of the local hospital (IRB No. 2019-05-032) and carried out in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was waived by the IRB as this was a recording-based study with no patient contact.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J.H., Ahn, J.H., Oh, J.K. et al. Factors associated with the development and outcome of hydrocephalus after decompressive craniectomy for traumatic brain injury. Neurosurg Rev 44, 471–478 (2021). https://doi.org/10.1007/s10143-019-01179-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01179-0