Abstract
Background
Post-traumatic hydrocephalus (PTH) is one of the primary complications during the course of traumatic brain injury (TBI). The aim of this study was to define factors associated with the development of PTH in patients who underwent unilateral decompressive craniectomy (DC) for TBI.
Methods
A total of 126 patients, who met the inclusion criteria of the study, were divided into two groups: patients with PTH (n = 25) and patients without PTH (n = 101). Their demographic, clinical, radiological, operative, and postoperative factors, which may be associated with the development of PTH, were compared.
Results
Multivariate logistic regression analysis revealed that cranioplasty performed later than 2 months following DC was significantly associated with the requirement for ventriculoperitoneal shunting due to PTH (p < 0.001). Also, a significant unfavorable outcome rate was observed in patients with PTH at 1-year follow-up according to the Glasgow Outcome Scale-Extended (p = 0.047).
Conclusions
Our results show that early cranioplasty within 2 months after DC was associated with a lower rate of PTH development after TBI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is an important public health issue because it is one of the most prevalent causes of death and disability in young adults [12, 13, 16]. Decompressive craniectomy (DC) serves as a life-saving procedure in cases of an uncontrollable increase in intracranial pressure and mass effect due to severe TBI [3, 14, 21, 30]. Although DC has gained popularity in the past few decades, the procedure is still associated with a marked complication rate [2, 17, 18, 33, 42].
Post-traumatic hydrocephalus (PTH) is one of the late complications of DC [2, 18, 33, 42]. It is associated with poor prognosis owing to the effects of the condition and complications of additional surgeries during the disease course [26, 27]. A comprehensive understanding of the pathophysiology and preventable causes of PTH after DC is mandatory to achieve optimal follow-up results. The present study aimed to retrospectively identify the factors associated with PTH requiring ventriculoperitoneal shunting (VPS) in patients who underwent unilateral DC for TBI.
Materials and methods
Patient population and study design
This study was reviewed and approved by the Erzincan Binali Yildirim University Clinical Research Ethics Committee (Number: 33216249-604.01.02-E.53219). Patients aged > 18 years who had undergone unilateral DC for TBI and who had survived 30 days after DC were included in the study. Exclusion criteria were as follows: the history of bilateral DC, the history of hydrocephalus before TBI, and bleeding diathesis. A total of 126 patients (84 men and 42 women) operated between January 2012 and January 2018 met the study criteria. Clinical, radiological, operative, and postoperative data of the patients were collected through clinical records and radiological examinations (Table 1). All patients were divided into two groups: patients with PTH (PTH group, n = 25) and patients without PTH (non-PTH group, n = 101). Patients who underwent VPS were included in the PTH group. Informed consent was obtained from all included participants.
Treatments were administered according to the guidelines recommended by the Brain Trauma Foundation [4, 7]. Specific radiological criteria were considered while making the DC decision. Patients with > 5-mm midline shift and compressed/absent cisterns on computed tomography (CT) were accepted as those requiring DC. Also, DC was performed in the presence of marked brain swelling and protrusion of cerebral tissues from the craniectomy area during the surgery. Subdural hematoma evacuation and DC were performed in the same session. A large fronto-temporo-parietal DC (larger than 12 × 15 cm in size) was performed with a medial border of at least 2–2.5 cm from the midline. A dural incision was performed in a stellate manner [15]. After subdural hematoma evacuation, duraplasty was performed with galea, the fascia lata, or an artificial graft. The cranial bone graft removed during DC was preserved inside the subcutaneous adipose tissue of the abdomen until cranioplasty was performed.
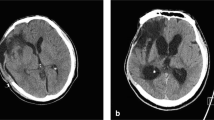
The diagnosis of PTH was based on the compliance of clinical symptoms with radiological images. Several clinical circumstances such as the decrease in the level of consciousness, persistent headache, nausea, vomiting, papilledema, focal neurologic deficits, and cognitive changes (decreased memory, decreased attention, and irritability) were considered to establish the diagnosis of PTH. While deciding if VPS should be performed, the definition of radiological PTH was established in reference to previous studies [9, 10]. The following were accepted as PTH criteria: radiologically determined lateral ventricular dilatation with an Evan’s Index of ≥ 30%, periventricular lucencies marked around frontal and occipital horns, decrease in intersulcal spaces at the convexity, and expansion of the third ventricle.
Cranioplasty timing was decided based on the choice of the surgeon and the patient’s recovery after initial TBI. All surgical interventions were performed under the supervision of two senior surgeons (AMM and AY). While the choice of one of the senior surgeons (AMM) for timing was early cranioplasty in patients with an appropriate clinical condition, that of the other senior surgeon (AY) was in favor of late cranioplasty.
Clinical data
Demographic data (sex and age), medical history, trauma mechanism, Glasgow Coma Scale (GCS) score, pupil reactions before DC, affected cerebral hemisphere side, the incidence of meningitis, and functional outcomes at the time of PTH diagnosis and at 12-month follow-up according to the Glasgow Outcome Scale-Extended (GOS-E) [40] were recorded and compared among all patients. The outcome was scored according to GOS-E scores: 1, death; 2, persistent vegetative state; 3, lower severe disability; 4, upper severe disability; 5, lower moderate disability; 6, upper moderate disability; 7, lower good recovery; and 8, upper good recovery [40]. Outcomes were classified as unfavorable (GOS-E score 1–4) or favorable (GOS-E score 5–8).
Radiological data
The maximum width of subdural hematoma, midline shift before DC, presence of contusion, status of basal cisterns, presence of subarachnoid hemorrhage (SAH), and presence of skull fractures were compared between the two groups. Midline shift was described as the length of the displacement of the septum pellucidum from the midline [25]. The basal cistern status was categorized as normal, compressed, or absent [37].
Operative and postoperative data
DC timing, duraplasty material, and cranioplasty timing and material were compared between the groups. DC timing was described as the time interval between the trauma and starting time of the operation for DC surgery plus 30 min, which presented the approximate time of decompression. Duraplasty material was classified into autologous (galea–pericranium and fascia lata) and artificial (bovine-derived pericardium membrane). Regarding cranioplasty timing, the timeframe was determined to distinguish early and late cranioplasty using the ROC curve and Youden’s index analyses. Cranioplasty material was classified into autologous (previously removed calvarial bone) and artificial (acrylic and titanium mesh).
Statistical analysis
Results are presented as numbers and percentages for categorical variables and as mean and standard deviation, median, or minimum–maximum for continuous variables. Chi-square or Fisher’s exact test was performed for between-group comparison of categorical variables. The normality of the distribution of continuous variables was confirmed using the Shapiro–Wilk test. Mann–Whitney U test was performed for the comparison of independent continuous variables. Multivariate logistic regression analysis was performed to determine the possible risk factors for PTH. Regarding multivariate logistic regression analysis, all variables significantly associated with PTH with a p value of < 0.25 in univariate analysis were selected. The statistical level of significance for all tests was set at 0.05. While the association between PTH development and cranioplasty timing was examined, the ROC curve was used and area under the curve (AUC) is presented. AUC values between 0.8 and 0.9 were accepted as excellent discrimination. Youden’s index was applied to specify the most appropriate threshold and optimal sensitivity and specificity. Statistical analyses were performed using IBM SPSS 19 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0; IBM Corp., Armonk, NY).
Results
PTH was observed in 25 (19.8%) of the 126 patients. The mean duration from DC to PTH diagnosis was 128 ± 42 (43–179) days. In 21 of these 25 patients, PTH developed after cranioplasty. In the remaining four patients, the decision of VPS timing was made according to the neurological status and brain bulging outside the craniectomy area. In two patients with marked bulging and neurological deterioration, VPS was performed a few days before cranioplasty. In the remaining two patients, cranial reconstruction and VPS were performed in same-session surgery.
Demographic, clinical, radiological, operative, and postoperative data of all patients are summarized in Table 1. There were no significant differences in age, sex, medical history (hypertension and diabetes mellitus), mechanism of trauma, GCS scores before DC, affected cerebral hemisphere side, pupil size, maximum width of subdural hematoma, midline shift, presence of contusion, presence of cranial fracture, the status of basal cisterns, presence of SAH, the time interval between the trauma and DC, duraplasty and cranioplasty material, and incidence of meningitis between the two groups (Tables 1, 2).
In both groups, the most common cause of trauma was traffic accidents (PTH group: 52%, non-PTH group: 48.5%), and the average GCS score at admission was < 8 (PTH group: 7.7, non-PTH group: 7.9). Among other clinical parameters, the rate of the dilated pupil was higher in the PTH group (40%) than in the non-PTH group (30.7%), but there was no significant difference (p = 0.373). The average subdural hematoma thickness was > 15 mm (PTH group: 16.8 mm, non-PTH group: 15.6 mm) and the average midline shift was > 10 mm (PTH group: 10.1 mm, non-PTH group: 10.6 mm) in both groups. The rate of traumatic SAK was higher in the PTH group (52%) than in the non-PTH group (38.6%), but there was no significant difference (p = 0.322). In addition, basal cisterns were affected (compressed or absent) in 96% of the patients in both groups. In the majority of patients in both groups, autologous duraplasty (PTH group: 96%, non-PTH group: 92.1%) and cranioplasty (PTH group: 96%, non-PTH group: 94.1%) materials were used. Further, the incidence of meningitis was higher in the PTH group than in the non-PTH group (8% vs. 3%), but the difference was not significant (p = 0.258).
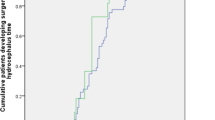
Regarding the relationship between cranioplasty timing and PTH development, ROC curve analysis revealed an AUC of 0.805 (95% CI 0.710–0.900; Table 3). According to this value, cranioplasty timing was considered excellent discrimination for PTH development. The optimal point according to Youden’s index was found to be 62 days. The possibility of PTH development was found to be higher in ≥ 62 days after DC (sensitivity: 0.800, specificity: 0.703; Table 3). According to these results, a timeframe of 2 months was determined to distinguish between early and late cranioplasty.
The mean cranioplasty timing was 101.9 ± 38.3 days in the PTH group and 68.1 ± 38.6 days in the non-PTH group. The time interval between DC and cranioplasty was significantly longer in the PTH group than in the non-PTH group (p < 0.001). In addition, 80% and 38.6% of the patients in the PTH and non-PTH groups, respectively, underwent cranioplasty later than 2 months. Early cranial reconstruction within 2 months after DC was associated with a lower rate of PTH development according to multivariate logistic regression analysis (p < 0.001; Table 2).
The unfavorable functional outcome rate according to GOS-E was 44% (11/25) in the PTH group at the time of diagnosis, and the median duration from DC to PTH diagnosis was 4.3 months. Therefore, the functional status of the non-PTH group within the fifth month (120–150 days) after DC was evaluated. The unfavorable functional outcome rate was 34.6% (35/101) in the non-PTH group, and no significant difference was observed between the two groups (p = 0.384). Finally, 56% of the PTH group and only 29.7% of the non-PTH group showed unfavorable functional outcomes at the 12-month follow-up. Multivariate logistic regression analysis revealed that PTH was associated with unfavorable functional outcomes according to GOS-E scores at the 12-month follow-up (p = 0.047; Table 2).
The characteristics of the patients who underwent cranioplasty within 2 months after DC (early cranioplasty group) were comparable to those of the patients who underwent cranioplasty later than 2 months after DC (late cranioplasty group; Table 4). There was a significant difference between the two groups in terms of PTH development. Early cranioplasty was associated with a lower rate of post-traumatic hydrocephalus. The rate of PTH was 7.5% (5/67) in the early cranioplasty group and 33.9% (20/59) in the late cranioplasty group (p < 0.001). The between-group comparison was made in terms of functional status, including neurological deficits and symptoms. According to GOS-E, the rate of unfavorable functional outcome was 32.7% in the early cranioplasty group and 38.2% in the late cranioplasty group; however, no significant difference was observed between the two groups (p = 0.601). Moreover, no significant difference was observed in terms of other parameters between early and late cranioplasty groups.
Discussion
DC has gained popularity in neurosurgery practice in the past few decades. A prospective, randomized RESCUEicp trial showed that DC led to decreased mortality and increased vegetative state rates [22]. PTH is one of the major complications of DC after TBI. The development of PTH increases the unfavorable functional outcome rate during the follow-up [11, 31, 34]. Nasi et al. [31] reported an unfavorable functional outcome rate of 78%/48% in patients with PTH/without PTH; in another study by Su et al. [34], these rates were 60%/18%. In a study by Di et al. [11], the average GOS-E scores at 6 months after trauma were 3.3/6.8 in patients with/without PTH. In the present study, the unfavorable functional outcome rate at the 12-month follow-up was 56%/29.7% in patients with/without PTH.
Concerning the traditional perspective of cerebrospinal fluid (CSF) physiology, the majority of CSF is produced by ependymal cells and the choroid plexuses in the ventricles [28]. CSF circulates in ventricles, cisterns, and subarachnoid space and drains into the venous system with arachnoid granulations. According to novel insights, CSF circulation includes an additional pulsatile movement throughout the entire brain with fluid interchange among interstitial space, blood, and CSF. Aquaporins, astrocytes, and membrane transporters are suggested to be essential factors in brain CSF homeostasis [5, 6] CSF absorption by arachnoid granulations does not involve a directed flow, rather it requires pulsatile pressure because arachnoid granulations function as pressure-dependent unidirectional valves owing to their morphology [36, 39]. Moreover, the exchanges among vessels, interstitial space, and CSF throughout the brain are maintained under pulsatile pressure changes [6]. Large DC transforms the cranial vault from a closed system to an open box, resulting in the loss of pulsatile intracranial CSF dynamics. Flattening of the normal ICP waveform during intraparenchymal pressure monitoring after DC supports this condition in the clinical settings [39]. This may explain how early cranioplasty provides the balance of normal pressures and leads to the prevention of PTH development. Meanwhile, permanent dysfunction of arachnoid granulations occurs in CSF diversion cases, which provide long-term inhibition of pulsatile pressure [35]. Prolonged pressure disturbance in late cranioplasty may lead to permanent dysfunction of arachnoid granulations, resulting in PTH development even after cranioplasty. This notion is supported by the findings of the present study, wherein the rate of PTH development was lower in the early cranioplasty group than in the late cranioplasty group. Similar results have been demonstrated in previous studies. Waziri et al. assessed post-craniectomy hydrocephalus in decompression cases and reported that the average cranioplasty time was 75.3/22.3 days in patients with/without hydrocephalus [39]. Moreover, in their DC series, Nasi et al. reported that 92% of the patients who developed PTH underwent cranial vault restoration after 3 months [31]. In contrast, Honeybul et al. [19] concluded that cranioplasty time does not affect PTH development after DC. Indeed, the average cranioplasty time was approximately 3 months for both groups (PTH group: 86 days, non-PTH group: 96 days). Moreover, Honeybul et al. [19] suggested that the underlying mechanism of PTH is related to the severity of primary brain injury. Moreover, an observational study by Morton et al. [29] reported that cranial reconstruction performed later than 3 months was associated with a lower rate of hydrocephalus development. Although their study comprised a larger series, various indications for DC were included and no mechanism for a lower rate was proposed [29].
PTH is one of the late complications of DC after TBI, and PTH usually develops after cranioplasty. In a series by Nasi et al. [31], VPS was performed after cranioplasty in 61% (23/34) of patients, whereas in the present series, it was performed in 84% (21/25) of patients. Both results support the idea that prolonged pressure disturbance causes irreversible dysfunction in arachnoid granulations [35], thereby maintaining the risk of PTH development even after cranioplasty. VPS and cranioplasty can be performed in same-session surgery. In the presence of severe PTH and obvious bulging of the brain parenchyma from the craniectomy area, cranioplasty should be performed a few days after the initial VPS. In the series by Nasi et al. [31], same-session surgery was performed in seven patients and VPS before cranioplasty was performed in four patients, whereas in the present series, same-session surgery and VPS before cranioplasty were performed in two patients each.
Several factors have been suggested to affect PTH development after DC. Both young [38] and old age [34] are reported to be associated with PTH development. Besides the presence of SAH [23], the presence of meningitis [20] and low GCS scores [11] have been suggested to affect PTH development. However, none of these factors was found to be associated with PTH development in our study.
Arachnoid adhesions formed after DC have been suggested to disrupt CSF distribution and cause PTH [8, 32]. Also, the probability of the adhesions was estimated to increase with potential foreign body reactions to artificial biomaterials used during duraplasty and cranioplasty [1]. These conditions raise the question of whether the use of artificial grafts increases the possibility of PTH development. To the best of our knowledge, the present study is the first to report the effects of cranioplasty and duraplasty materials on PTH development. Interestingly, we found no association between cranioplasty/duraplasty materials and PTH development.
Typically, the interval for early cranioplasty has been determined to be 3 months [19, 29]. However, some recent reports suggest that this limit should be modified to a shorter interval [24, 41]. In a study by Yang et al. [41], serial CT after TBI revealed that the radiologically earliest possible cranioplasty timing is within 2 months (34.6 ± 34.3 days) after DC. Kim et al. [24] investigated bone flap resorption and infection to determine the optimal cranioplasty timing and minimize complications using ROC curve analyses. They showed that early cranioplasty (< 45 days) was associated with a reduced complication rate [24]. In the present study, ROC curve analyses revealed that cranioplasty timing of ≤ 61 days after DC was associated with a lower rate of PTH development. Hence, the 2-month period was selected as the early cranioplasty limit.
This study has certain limitations. Several patients in the cohort were excluded because they did not comply with the study criteria; therefore, the exact rate of PTH development may be underrated. External validity (generalization) is problematic in the present study because despite the strong statistical result (p < 0.001), the sample size is not that large enough. Moreover, several factors that may affect PTH were possibly not included in the study owing to its retrospective nature. Therefore, prospective, multicenter studies may provide more precise conclusions.
Conclusions
According to the results of our study, early cranial reconstruction performed within 2 months after DC was associated with a lower rate of PTH. Besides, PTH was found to be associated with higher rates of unfavorable functional outcomes at 1-year follow-up. Early cranioplasty within 2 months may be recommended after DC to achieve favorable functional outcomes.
Abbreviations
- PTH:
-
Post-traumatic hydrocephalus
- TBI:
-
Traumatic brain injury
- DC:
-
Decompressive craniectomy
- VPS:
-
Ventriculoperitoneal shunting
- GCS:
-
Glasgow Coma Score
- GOS-E:
-
Glasgow Outcome Scale-Extended
- SAH:
-
Subarachnoid hemorrhage
- ROC:
-
Receiver-operating characteristic
- AUC:
-
Area under the curve
- CSF:
-
Cerebrospinal fluid
References
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100.
Ban SP, Son YJ, Yang HJ, Chung YS, Lee SH, Han DH. Analysis of complications following decompressive craniectomy for traumatic brain injury. J Korean Neurosurg Soc. 2010;48(3):244–50.
Barthélemy EJ, Melis M, Gordon E, Ullman JS, Germano IM. Decompressive craniectomy for severe traumatic brain injury: a systematic review. World Neurosurg. 2016;88:411–20.
Brain Trauma Foundataion. American Association of Neurological Surgeons, Congress of Neurological Surgeons: guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24:1–106. https://doi.org/10.1089/neu.2007.9999.
Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10.
Bulat M, Klarica M. Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res Rev. 2011;65:99–112.
Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the management of severe traumatic brain injury Fourth Edition. Neurosurgery. 2017;80:6–15. https://doi.org/10.1227/NEU.0000000000001432.
Czosnyka M, Copeman J, Czosnyka Z, McConnell RS, Dickinson C, Pickard JD. Post-traumatic hydrocephalus: influence of craniectomy on the CSF circulation. J Neurol Neurosurg Psychiatry. 2000;68:246–8.
De Bonis P, Pompucci A, Mangiola A, Rigante L, Anile C. Post-traumatic hydrocephalus after decompressive craniectomy: an underestimated risk factor. J Neurotrauma. 2010;27(11):1965–70.
De Bonis P, Sturiale CL, Anile C, Gaudino S, Mangiola A, Martucci M, Colosimo C, Rigante L, Pompucci A. Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: a timeline of events? Clin Neurol Neurosurg. 2013;115(8):1308–12.
Di G, Hu Q, Liu D, Jiang X, Chen J, Liu H. Risk factors predicting posttraumatic hydrocephalus after decompressive craniectomy in traumatic brain injury. World Neurosurg. 2018;116:e406–e413413.
El-Menyar A, Mekkodathil A, Al-Thani H, Consunji R, Latifi R. Incidence, demographics, and outcome of traumatic brain injury in the middle east: a systematic review. World Neurosurg. 2017;107:6–21.
Ghajar J. Traumatic braın injury. Lancet. 2000;356:923–9.
Giammattei L, Messerer M, Cherian I, Starnoni D, Maduri R, Kasper EM, Daniel RT. Current perspectives in the surgical treatment of severe traumatic brain injury. World Neurosurg. 2018;116:322–8.
Güresir E, Vatter H, Schuss P, Oszvald Á, Raabe A, Seifert V, Beck J. Rapid closure technique in decompressive craniectomy. J Neurosurg. 2010;114(4):954–60.
Heegaard W, Biros M. Traumatic brain injury. Emerg Med Clin North Am. 2007;25(3):655–78.
Honeybul S. Complications of decompressive craniectomy for head injury. J Clin Neurosci. 2010;17(4):430–5.
Honeybul S, Ho KM. Long-term complications of decompressive craniectomy for head injury. J Neurotrauma. 2011;28(6):929–35.
Honeybul S, Ho KM. Incidence and risk factors for post-traumatic hydrocephalus following decompressive craniectomy for intractable intracranial hypertension and evacuation of mass lesions. J Neurotrauma. 2012;9(10):1872–8.
Hu Q, Di G, Shao X, Zhou W, Jiang X. Predictors associated with post-traumatic hydrocephalus in patients with head injury undergoing unilateral decompressive craniectomy. Front Neurol. 2018;14(9):337.
Hutchinson PJ, Corteen E, Czosnyka M, Mendelow AD, Menon DK, Mitchell P, Murray G, Pickard JD, Rickels E, Sahuquillo J, Servadei F, Teasdale GM, Timofeev I, Unterberg A, Kirkpatrick PJ. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study (www.RESCUEicp.com). Acta Neurochir Suppl. 2006;96:17–20.
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ. RESCUEicp Trial Collaborators: trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375(12):1119–30.
Jiao Q, Liu Z, Li S, Zhou L, Li S, Tian W, You C. Influencing factors for posttraumatic hydrocephalus in patients suffering from severe traumatic brain injuries. Chin J Traumatol. 2007;10(3):159–62.
Kim JH, Hwang SY, Kwon TH, Chong K, Yoon WK, Kim JH. Defining “early” cranioplasty to achieve lower complication rates of bone flap failure: resorption and infection. Acta Neurochir (Wien). 2019;161(1):25–31.
Liao CC, Chen YF, Xiao F. Brain midline shift measurement and its automation: a review of techniques and algorithms. Int J Biomed Imaging. 2018;2018:4303161.
Marmarou A, Foda Ma, Bandoh K, Yoshihara M, Yamamoto T, Tsuji O, Zasler N, Ward JD, Young HF. Posttraumatic ventriculomegaly: hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J Neurosurg. 1996;85(6):1026–35.
Mazzini L, Campini R, Angelino E, Rognone F, Pastore I, Oliveri G. Posttraumatic Hydrocephalus: a clinical, neuroradiologic, and neuropsychologic assessment of long-term outcome. Arch Phys Med Rehabil. 2003;84(11):1637–41.
McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg. 2009;59(3):369–83.
Morton RP, Abecassis IJ, Hanson JF, Barber JK, Chen M, Kelly CM, Nerva JD, Emerson SN, Ene CI, Levitt MR, Chowdhary MM. Timing of cranioplasty: a 10.75-year single-center analysis of 754 patients. J Neurosurg. 2017;128(6):1648–52.
Münch E, Horn P, Schürer L, Piepgras A, Paul T, Schmiedek P. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47(2):315–23.
Nasi D, Gladi M, Di RA, Somma L, Moriconi E, Iacoangeli M. Risk factors for post-traumatic hydrocephalus following decompressive craniectomy. Acta Neurochir (Wien). 2018;160(9):1691–8.
Shi SS, Zhang GL, Zeng T, Lin YF. Posttraumatic hydrocephalus associated with decompressive cranial defect in severe brain-injured patients. Chin J Traumatol. 2011;14(6):343–7.
Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26(6):E7.
Su TM, Lan CM, Lee TH, Hsu SW, Tsai NW, Lu CH. Risk factors for the development of posttraumatic hydrocephalus after unilateral decompressive craniectomy in patients with traumatic brain injury. J Clin Neurosci. 2019;63:62–7.
Takahashi Y. Withdrawal of shunt systems—clinical use of the programmable shunt system and its effect on hydrocephalus in children. Child’s Nerv Syst. 2001;17(8):472–7.
Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg. 2009;63(6):867–75.
Valadka AB, Bullock MR, Marion DW, Huang JH, Ranalli N, Zager EL, Wilberger JE. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–82.
Vedantam A, Yamal J-M, Hwang H, Robertson CS, Gopinath SP. Factors associated with shunt-dependent hydrocephalus after decompressive craniectomy for traumatic brain injury. J Neurosurg. 2018;128(5):1547–52.
Waziri A, Fusco D, Mayer SA, McKhann GM, Connolly ES. Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery. 2007;61(3):489–93.
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85.
Yang NR, Song J, Yoon KW, Seo EK. How early can we perform cranioplasty for traumatic brain injury after decompressive craniectomy? A retrospective multicenter study. World Neurosurg. 2018;110:e160–e167167.
Yang XF, Wen L, Shen F, Li G, Lou R, Liu WG, Zhan RY. Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir (Wien). 2008;150(12):1241–7.
Acknowledgements
The language editing of this article is partly supported by the Turkish Neurosurgical Society.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, and beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
This study was reviewed and approved by the Erzincan Binali Yildirim University Clinical Research Ethics Committee (Number: 33216249-604.01.02-E.53219). All procedures were performed in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Ozoner, B., Kilic, M., Aydin, L. et al. Early cranioplasty associated with a lower rate of post-traumatic hydrocephalus after decompressive craniectomy for traumatic brain injury. Eur J Trauma Emerg Surg 46, 919–926 (2020). https://doi.org/10.1007/s00068-020-01409-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-020-01409-x