Abstract
The aim of this study was to analyze the long-term clinical and radiological outcomes of craniocervical decompression for patients affected by Chiari I-related syringomyelia. We performed a retrospective analysis of a group of patients affected by Chiari I-associated syringomyelia treated by craniocervical decompression (CCD). Surgical and technical aspects and preoperative factors predicting outcome were discussed. A total of 36 patients were reviewed. There were 17 men and 19 women (female/male ratio 1.11), and the mean age was 40.4 (range 18–68). The most important preoperative symptoms were related to myelopathy (pain, weakness, atrophy, spasticity, sensory loss, and dysesthesias). Most syrinxes were in the cervico-thoracic region (61.1%), and the majority of patients had tonsillar descent between the foramen magnum and C1. All patients underwent a craniectomy less than 3 cm in diameter followed by a duroplasty with dura substitute. No arachnoid manipulation was necessary. Three patients (8.1%) experienced cerebrospinal fluid leaks that resolved without complications. At a mean follow up of 40 months (range 16–72) 80.5% of patients exhibited improvement over their preoperative neurological examination while 11.1% stabilized. The syrinx shrank in 80.5% of patients. Chi-square test showed that preoperative syrinx extension and degree of tonsillar descent did not correlate with clinical and neuroradiological postoperative evolution. Treating syringomyelia associated in Chiari I malformation with CCD leads to a large percentage of patients with satisfying results and no irreversible complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Syringomyelia is defined as a cystic cavity within the spinal cord that slowly leads to chronic and sometimes irreversible myelopathy. This condition is very often associated to Chiari type I malformation (CMI), a pathologic caudal displacement of cerebellar tonsils through the foramen magnum and into the cervical canal. The mechanism of formation of the syrinx and the correlation with CMI has been poorly appreciated for a long time, and various treatments have been suggested accounting for nearly 15 different surgical approaches [1]. During the last decade, some authors elaborated theories [2–4, 6, 28–30, 32] centered on obstruction of cerebrospinal fluid (CSF) flow at the level of the foramen magnum. More recently, neuroradiological devices, such as cine-magnetic resonance imaging (MRI) for the study of CSF dynamics performed pre- and postoperatively, corroborated the surgical and pathological observations. Although no prospective randomized controlled study exists of the optimal surgical strategy for the treatment of syrinx and CMI, there is robust evidence from accumulated multicenter experience [7–11] that craniocervical decompression (CCD) represents a valid treatment. The goal of this approach is to enlarge the bony and dural posterior fossa and rebuild a new cisterna magna. While CCD has shown satisfactory results, there is still debate about the need for dura opening, the extent of the bony and dural decompression required, the need of arachnoid manipulation, and the method of dural closure. Unfortunately, many series in the literature compare patients with syringomyelia of different origin and analyze multiple surgical strategies together [12–14, 27].
Since 1996, we have operated on patients with CMI-associated syringomyelia using a standardized CCD and duroplasty with dura substitute. Here, we discuss our experience with our consecutive and uniformly treated series of patients with CMI associated syrinx, focusing on the surgical considerations and their clinical and radiological long-term results. We also performed a statistical analysis to investigate the role of preoperative predictors of outcome.
Patients and methods
We retrospectively reviewed our database concerning the period between 1996 and 2006. All of the patients with a diagnosis of syringomyelia and CMI were included. Because this analysis focused on adult patients, a lower age limit of 18 was set. Patients with associated hydrocephalus, brain tumors, history of severe head trauma, or previous cranial surgery, meningitis, and lumbo-peritoneal shunt were excluded. The data were collected by searching the hospital charts.
Preoperative and postoperative clinical evaluation
A senior neurosurgeon or neurologist performed the preoperative and postoperative neurological assessment. During the history gathering, attention was paid to the presence of other cases of syringomyelia and CMI among relatives. Because of the wide range of signs and symptoms, we preferred to summarize the clinical features using three classes: (1) posterior fossa overcrowding (PFO; headache, cervical pain, nystagmus, drop-attack, or syncope after effort); (2) long tract impairment (pain, weakness, atrophy, spasticity, sensory loss, and dysesthesias); (3) brainstem compression (BSC; cranial nerve impairment, hypoacusis, dysphagia, and sleep apnea). These symptoms were widely associated in many patients. The postoperative clinical evaluation was performed at 1, 3, and 6 months and yearly. Each patient’s clinical outcome was rated as “improved,” “unchanged”, or “worsened.” Anesthetic and early post-operative complications were recorded.
Neuroradiological preoperative and postoperative assessment
Preoperative neuroradiological assessment was achieved by 1.5 T or 1 T MRI of the entire axis. The measurement of the tonsillar herniation was accomplished using the standard basion-opisthion line as a reference for the foramen magnum plane. The degree of the tonsils’ downward displacement was classified as three types: (a) between foramen magnum and C1; (b) between C1 and C2; and (c) beyond C2. Other radiological features of CMI were also considered for diagnostic purpose: shape of tonsils (triangular or rounded); volume of the cisterna magna (reduced or absent); appearance of the perimedullary subarachnoid spaces. All of the possible associated malformations of the craniocervical region that could modify the surgical strategy were ruled out. The length of the syrinx was calculated on a T1 sagittal plane (TR 400–500 ms, TE 16–20 ms) to avoid considering edema or T2 hyperintensity related to compression of the cord as syrinx. The measurements of the syrinx were classified as cervical, cervico-thoracic, and holocord. The first postoperative MR was performed at 3 months, then at 6 months, and afterwards yearly. Syrinx modifications or absence of narrowing during follow-up were defined respectively as “collapsed” or “unchanged.” The time to narrowing of the syrinx following surgery was also recorded.
Surgical technique
The patient is placed in a prone position with the head in flexion. A midline incision from the inion to the arch of C2 is then performed. The dissection of the muscles is must be sub-periosteal, and the surgeon is careful not to cut the attachment on C2 to avoid weakening. A burr hole is placed at the level of the nuchal line on each side, and the craniectomy is then completed either by a high-speed drill or a rongeur. The craniotomy encompasses the foramen magnum and extends upward to the external occipital crest and inferior nucal line. The maximum diameter of the bone opening is 3 × 3 cm, but variations can exist according to the dimension of the posterior fossa and the degree of tonsillar descent. The posterior arch of C1 is eliminated in its most central part. Based on the position of the cerebellar tonsils, the posterior arch of C2 is sometimes also removed. The always-thickened craniocervical dural band is left in place and cut one layer during the opening of the dura to avoid accidental dural tearing and arachnoid damaging. Under the microscopic view, the dura is incised in a “Y” or “reversed L” shape (in case of asymmetrical tonsil’s descent) while trying not to cut the arachnoid. At this time, the collapsed cisterna magna is refilled by the CSF, and a normal pulsation of the tonsils ensues. Sometimes, we compressed the neck of the patient (as for a Queckenstedt test) to increase the intracranial pressure and favor the expansion of the subarachnoid space. We did not manipulate the subarachnoid space or the cerebellar structures. A patch of dura substitute is then tailored on the dimension of the dural opening and fixed with single stitches (3/0 silk or prolene).
Statistical analysis
We used the chi-square test to establish the presence of significant differences in the frequencies of preoperative tonsils’ descent and of the extension of syrinx (C, C-T, holocord) among patients grouped by clinical outcome (improved, unchanged, worsened) and radiological syrinx modification (collapsed, unchanged). Statistical significance was fixed at a p value of less than 0.05.
Results
Preoperative patients’ characteristics
During a period of 11 years (1996–2006), 39 patients were found to match the diagnosis of CMI associated syringomyelia. Two of these patients were not suitable for surgical treatment because of severe cardiovascular and liver dysfunction. One other patient refused the procedure. A total of 36 patients were treated by craniocervical osteo-dural decompression. There were 17 men and 19 women (female/male ratio 1.11) with a mean age of 40.4 (range 18–68). Long tract impairment was the most cited preoperative complaint (31 patients, 86.1%) followed by less frequent PFO (27.7%) and BSC (13.8%). Thirteen (36.1%) patients presented with combinations of symptoms. Table 1 summarizes the patients’ preoperative clinical and radiological characteristics as well as the intraoperative arachnoid tearing related complication rate. One patient presented a mild form of basilar invagination.
Surgical results
In all patients, an occipital craniectomy and C1 laminectomy was performed while paying maximal attention not to detach the muscles from C2 (Fig. 1). In two patients with a type C tonsillar position, a C2 laminectomy was also required. In some cases, a wide circular occipital sinus was encountered requiring a careful hemostasis either by cauterization or suture with 4/0 prolene. The exposure of the arachnoid plane demonstrated an opaque and thick arachnoid in some cases, but no attempt to dissect or open the arachnoid was made. The dura opening was covered with dura substitute in all cases. The mean time of the procedures was 2.3 h (range 1.5–4.4). There was no postoperative complication related to the surgical procedure (such as cerebellar contusion, hematoma of the surgical cavity, brainstem, or cranial nerves dysfunction). An accidental arachnoid tearing can occur during the opening of the dura mater. This event provokes an increase of the postoperative cervical and shoulder pain, prolonging the analgesic need and the hospital stay. Moreover, this is a predisposing factor to CSF leak that in our patients resolved with a lumbar external drainage in two cases and with surgical revision in one case. Their CSF samples did not show bacterial infections.
a Dissection of nuchal muscles is subperiosteal, and C2 attachments are left in place. Large arrow muscles attachment on C2; dashed arrow posterior arc of C1; square dashed arrow occipito-cervical band; thin arrow foramen magnum. b The arc of C1 is removed, and craniectomy is no larger than 3 cm in maximum diameters
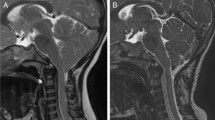
In two cases (5.5%), a re-operation was needed at 2 and 7 years. During the operation, both patients showed an unsatisfactory dura opening, hence a wider duroplasty was performed. Moreover, we documented that the dura manifested a very thick fibrosis around the insufficient dural openings (Fig. 2).
a Postoperative MR of a patient that experienced recrudescence of symptoms 2 years after first operation. Note the syringobulbia. b Intraoperative image. Once the previous dura patch was removed, a thick and stenosed dura was found. c Dura mater was removed, and a new and large patch was inserted. d The last MR shows rebuilding of a larger cisterna magna and the disappearance of syringobulbia
There was no mortality in the postoperative period; and in the early (24 h) postoperative course, no anesthesiology complication was recorded.
Clinical and neuroradiological long-term outcome
During a follow-up ranging from 8 to 68 months (mean 40 months), 29 patients (80.5%) improved their symptoms. As already reported in other series (26), headache is the first symptom to disappear, sometimes as soon as the discharge. On the other hand, advanced dysesthesic pain or arm or limb weakness has the worst prognosis, and normally only a partial recovery is observed. Four patients (11.1%) presented no modification of symptoms after decompression. The two patients with a C2 laminectomy did not show any persisting cervical pain, and a plain radiographic film with dynamic test did not demonstrate instability. As described above, two patients had a re-operation. They experienced a delayed relapse of preoperative symptoms, and MR showed persistent compression of the cerebellar tonsils (Fig. 2). Symptoms eventually subsided after the second operation. Concerning our three patients who had a worsened clinical outcome, we did not find peculiar elements that could explain such an unsatisfying outcome. In those patients, postoperative MR showed a correct rebuilding of the cisterna magna, and in two the syrinx collapsed.
All of the patients performed at least three postoperative MR controls (three to six). The syringomyelia collapsed in 29 patients (80.5%; Fig. 3) and remained unchanged in seven (19.4%). We did not observe any widening of the syrinx. The mean time for the syrinx to shrink was 8 months (range 6–26 months). No patient experienced a cerebellar sagging. By using the chi-square test, we did not find any significant difference in the frequencies of preoperative syrinx extension among patients grouped by clinical outcome and syrinx postoperative modification (p = 0.551 and p = 0.574, respectively). Similarly, the preoperative degree of tonsillar descent did not show a correlation with clinical and neuroradiological postoperative evolution (p = 0.603 and p = 0.440, respectively).
Discussion
On average, recent surgical series demonstrate that more than two thirds of patients improve or stabilize their preoperative neurological status after CCD [7, 15–18]. Our experience is in agreement with this trend given that improved and stabilized patients accounted for 91.6%. Unlike other series [11–13, 19, 20], the present analysis derived from a consecutive and uniformly treated population in which the same surgical techniques treated only CMI related syringomyelia.
Some authors have continued to prefer the syringo-subarachnoidostomy as an adjunct to the CCD [14, 21, 31], but we think that a syringosubarachnoid shunt is disadvantageous. Spinal cord injury, accompanying myelotomy with insertion of the catheter, and shunt failure (up to 29% [14, 21, 22]) may occur, albeit not often. In addition, the syringosubarachnoid shunt cannot be indicated for patients in whom the Chiari I malformation is mainly responsible for clinical symptoms or in whom the cavity in the spinal cord is too small to insert a catheter.
Although the theoretical bases of the CCD are widely accepted (namely the re-building of a new cisterna magna), there is still debate about several surgical aspects. We advise performing a craniectomy that does not exceed 3 cm of maximum diameter so that cerebellar sagging is avoided. Some authors [7, 10, 11, 26], believing that the decompression of the PF is insufficient to restore a normal CSF flow, support the coartation of tonsils or dissection of the arachnoid. Even though their results in terms of neurological outcome are convincing, the extra-arachnoidal approach is safer. In series where decompression with intrarachnoidal manipulation was used, postoperative complications such as dysphagia (3.4%), hearing loss (5%), mesencephalic dysfunction (2.2%) have non-negligible rates [11, 16]. In our series, no patients had complications related to direct surgical damage.
Several observations dictate the choice to open and replace the dura. The first criterion is whether the thickened occipito-cervical dural band strongly contributes to the narrowing. Some authors [7, 12, 16, 23] reported an increased rate of syrinx resolution when suboccipital decompression is combined with duroplasty. The second criterion, as already reported [5], is whether this band shows an increase in collagen fibers, hyalinous nodules, and calcifications. In fact, in our two patients, that underwent a second operation, a thicker and more fibrous band was found. In his experience, Tubbs and collaborators [18] observed thickening and scarring of the occipito-cervical membrane in a patient re-operated on for the recurrence of symptoms.
Opening the dura somewhat enhances the risk of accidental arachnoid tearing. In three (8.3%) cases, we recorded CSF leaks that eventually resolved without more severe complications. This percentage is lower than those series reporting patients undergoing CCD with arachnoid manipulation that showed up to 16% of external CSF leaks [11, 16]. Hoffman and Souweidane had a 0% CSF leak rate [24] in a series of patients operated on by CCD and duroplasty. On the other hand, Perrini et al. [17], in describing a CCD with dura opening but without duroplasty, reported a 4% rate of CSF leaks with need of reoperation. They prefer not to perform duroplasty because they believe this maneuver involves a higher risk of arachnoid tearing. In our experience, accidental arachnoid opening mainly happens during the opening of the dura rather than during its closure, so duroplasty is useful to prevent CSF leaks.
Our results demonstrate that preoperative extension of syrinx and the type of tonsillar descent does not correlate statistically to postoperative evolution of symptoms and MR modification after surgery. These results are in agreement with other investigators [4, 15], leading to our conclusion that surgeons cannot use these MR findings to predict outcome. Hence, as in other pathologies of the spinal cord, the first feature to consider when counseling a patient with CMI associated syringomyelia for surgery is the severity of the preoperative neurological status. Moreover, referring to the work of Attal et al. [15], the most important predictors of surgical outcome is the interval between onset of symptoms and CCD. An early surgical approach in symptomatic patients should be preferred. Nevertheless, most of the “improved” patients continue to present minor symptoms (such as dysesthesias or paresthesias) even when their syrinx shrinks. Indeed, the slow and progressive cavitation of the spinal cord produces a lesion that can be only partially reversed, even after a satisfying CCD. Recently, Wetjen et al. [25] published a prospective study that investigated the neuroradiological and clinical effect of CCD on syringomyelia during a long period of observation. They confirmed previous results that CCD progressively reduces syrinx volume and improves symptoms even in those cases where the narrowing is only partial. The persistence of symptoms does not mean a failure of the procedure but rather a confirmation of a chronic spinal damage. In our routine practice, if a patient presents persistence or worsening of symptoms after surgery, a MR of the posterior fossa is advised to primarily rule out an insufficient CCD. In the same way, if symptoms recur after surgery, a new MR is necessary to detect a re-stenosis (as described in our cases) probably due to insufficient duroplasty.
Conclusions
Treating syringomyelia associated with Chiari I malformation through craniocervical osteo-dural decompression with duroplasty results in a large percentage of patients with satisfying results and no irreversible complications. Although our experience is limited by a relatively small number of patients and the lack of prospective observation, it seems that our data agrees with larger studies. Particularly, the extension of the syrinx does not seem to predict clinical and MR results; hence, preoperative clinical conditions are the most reliable predictors of outcome. Consequently, a symptomatic patient must be identified early for this procedure. Further research is needed in order to better comprehend the pathophysiology of the syrinx in Chiari I malformation so that a larger percentage of patients can benefit from this procedure.
References
Aschoff A, St K (1993) 100 years syrinx surgery: a review. Acta neurochir (Wien) 123:157–159
Aboulker J (1979) Syringomyelia and intra-rachidian fluids. X. Rachidian fluid stasis (in French). Neurochirurgie 25(suppl 1):98–107
Gardner WJ (1977) Syringomyelia. Surg Neurol 7:370
Gardner WJ, McMurray FG (1976) “Non-communicating syringomyelia: a non-existent entity. Surg Neurol 6:251–256
Nakamura N, Iwasaki Y, Hida K, Abe H, Fujioka Y, Nagashima K (2000) Dural band pathology in syringomyelia with Chiari type I malformation. Neuropathology 20(1):38–43
Oldfield EH (2001) Syringomyelia. J Neurosurg 95(Suppl 1):153–155
Aghakhani N, Parker F, David P, Morar S, Lacroix C, Benoudiba F, Tadie M (2009) Long-term follow-up of Chiari-related syringomyelia in adults: analysis of 157 surgically treated cases. Neurosurgery 64(2):308–315
Badie B, Mendoza D, Batzdorf U (1995) Posterior fossa volume and response to suboccipital decompression in patients with Chiari I malformation. Neurosurgery 37:214–218
Feldstein NA, Choudhri TF (1999) Management of the Chiari I malformations with holocord syringohydromyelia. Pediatr Neurosurg 31:143–149
Fischer EG (1995) Posterior fossa decompression for Chiari I deformity, including resection of the cerebellar tonsils. Childs Nerv Syst 11:625–629
Klekamp J, Batzdorf U, Samii M, Bothe HW (1996) The surgical treatment of Chiari I malformation. Acta Neurochir 138:788–801
Blagodatsky MD, Larionov SN, Alexandrov YA, Velm AI (1999) Surgical treatment of Chiari I malformation with and without syringomyelia. Acta Neurochir 141:963–968
Guyotat J, Bret P, Jouanneau E, Ricci AC, Lapras C (1998) Syringomyelia associated with type I Chiari malformation. A 21-year retrospective study on 75 cases treated by foramen magnum decompression with special emphasis on the value of tonsil resection. Acta Neurochir 140:745–754
Iwasaki Y, Hida K, Koyanagi I et al (2000) Reevaluation of syringosubarachnoid shunt for syringomyelia with Chiari malformation. Neurosurgery 46(2):407–412
Attal N, Parker F, Tadié M, Aghakani N, Bouhassira D (2004) Effects of surgery on the sensory deficits of syringomyelia and predictors of outcome: a long-term prospective study. J Neurol Neurosurg Psychiatry 75:1025–1030
Munshi I, Frim D, Stine-Reyes R et al (2000) Effects of posterior fossa decompression with and without duraplasty on Chiari malformation-associated hydromyelia. Neurosurgery 46:1384–1390
Perrini P, Benedetto N, Tenenbaum R, Di Lorenzo R (2007) Extra-arachnoidal cranio-cervical decompression for syringomyelia associated with Chiari I malformation in adults: technique assessment. Acta Neurochir (Wien) 149:1015–1023
Tubbs RS, Wellons JC III, Oakes WJ et al (2003) Reformation of the posterior atlanto-occipital membrane following posterior fossa decompression with subsequent constriction at the craniocervical junction. Pediatr Neurosurg 38:219–221
Tognetti F, Calbucci F (1993) Syringomyelia: syringo-subarachnoid shunt versus posterior fossa decompression. Acta Neurochir (Wien) 123(3–4):196–197
Zhang ZQ, Chen YQ, Chen YA, Wu X, Wang YB, Li XG (2008) Chiari I malformation associated with syringomyelia: a retrospective study of 316 surgically treated patients. Spinal Cord 46:358–363
Hida K, Iwasaki Y, Koyanagi I, Sawamura Y, Abe H (2000) Surgical indication and results of foramen magnum decompression versus syringosubarachnoid shunting for syringomyelia associated with Chiari I malformation. Neurosurgery 46:407–413
Hida K, Iwasaki Y (2001) Syringosubarachnoid shunt for syringomyelia associated with Chiari I malformation. Neurosurg Focus 11 (1):Article 7
Versari PP, D'Aliberti G, Talamonti G, Collice M (1993) Foraminal syringomyelia: suggestion for a grading system. Acta Neurochir (Wien) 125(1–4):97–104
Hoffman CE, Souweidane MM (2008) Cerebrospinal fluid-related complications with autologous duraplasty and arachnoid sparing in type I Chiari malformation. Neurosurgery 62(3 Suppl 1):156–160
Wetjen NM, Heiss JD, Oldfield EH (2008) Time course of syringomyelia resolution following decompression of Chiari malformation Type I. J Neurosurg Pediatr 1(2):118–123
Alzate JC, Kothbauer KF, Jallo GI, Epstein FJ (2001) Treatment of Chiari I malformation in patients with and without syringomyelia: a consecutive series of 66 cases. Neurosurg Focus 11:1–9
Hayhurst C, Richards O, Zaki H, Findlay G, Pigott TJ (2008) Hindbrain decompression for Chiari-syringomyelia complex: an outcome analysis comparing surgical techniques. Br J Neurosurg 22(1):86–91
Milhorat TH, Johnson RW, Milhorat RH, Capocelli AL Jr, Pevsner PH (1995) Clinicopathological correlations in syringomyelia using axial magnetic resonance imaging. Neurosurgery 37:206–213
Milhorat TH, Kotzen RM, Anzil AP (1994) Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg 80:716–722
Oldfield EH, Muraszko K, Shawker TH, Patronas NJ (1994) Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg 80:3–15
Padovani R, Cavallo M, Gaist G (1989) Surgical treatment of syringomyelia: favorable results with syringosubarachnoid shunting. Surg Neurol 32:173–180
Sindou M, Chávez-Machuca J, Hashish H (2002) Cranio-cervical decompression for Chiari type I-malformation, adding extreme lateral foramen magnum opening and expansile duroplasty with arachnoid preservation. Technique and long-term functional results in 44 consecutive adult cases—comparison with literature data. Acta Neurochir (Wien) 144(10):1005–1019
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Ralf Alfons Kockro, Zurich, Switzerland
This is an interesting retrospective analysis of 36 consecutive cases summarizing the clinical and radiological outcome of decompression surgery for Chiari I associated syringomyelia. The results show first of all that straight forward posterior fossa decompression with duroplasty and without arachnoid opening results in a high percentage of clinical improvement and a low complication rate. Equally important, the radiological analysis shows that the pre-operative size of syrinx and position of tonsils do not correlate with the postoperative clinical or radiological course. In view of these results, I agree with the authors that early cranio-cervical decompression is the most appropriate first line surgical strategy. However, it would be interesting to further investigate the pre- and postoperative imaging features of those patients which did not benefit from decompression surgery in a larger series in order to define the cases in which extended surgical methods could be considered to further improve clinical outcome.
Jörg Klekamp, Quakenbrück, Germany
The authors present a series of 36 patients with Chiari I malformation and an average follow-up of 40 months. Surgical treatment involved a small decompression of the foramen magnum with a duraplasty but without arachnoid opening. The major argument for leaving the arachnoid or even the inner dura layer intact during this operation is avoidance of complications, i.e., cerebrospinal fluid (csf) fistulas in particular. In this paper, the authors claim no complications related to the surgical technique and yet they mention six accidental tears of the arachnoid (16.6%) and 3 csf fistulas (8.3%) that required either a lumbar drain or a surgical revision. In my understanding, these are complications related to the surgical technique. As with other similar papers on this subject, the technique of leaving the arachnoid intact does not automatically avoid csf fistulas. I always open the arachnoid during this operation. With appropriate closure of the wound, i.e., insertion of the duraplasty with running sutures and meticulous soft tissue closure, the rate of csf fistulas in my personal series of 194 patients is 3.1%.
The second argument is that clinical and radiological results are as good with this method as with potentially more dangerous techniques involving arachnoid dissection. This is probably a correct statement as far as short-term results are concerned. However, we have to remember, that we are dealing with a chronic disease. If we want to find out how to best treat our patients, we have to analyze long-term results applying survival statistics such as the Kaplan-Meier method and determine the rates of progression-free survival for each surgical technique. To my knowledge, none of the papers in the literature as well as this one provide such data. In this paper, the authors only mention two surgical revisions 2 and 7 years after the initial operation, i.e., a rate of 5.6%. Looking again at my series, four of 194 patients (2.1%) required surgical revisions 4–75 months after the initial operation. This low revision rate suggests that 97.9% were more or less satisfied. But that is not correct. With Kaplan-Meier statistics, the rate of patients free of progression was 82.9% for 15 years. As long as we do not have such data 5, 10, or 15 years after surgery for the different techniques advocated in the literature, this controversial discussion will continue.
Lotfi Hacein-Bey, Sacramento, USA
Although there is a wide consensus that symptomatic syringomyelia (with or without Chiari I malformation) should be generally treated with craniocervical decompression, there is some variety in the techniques advocated. The authors present a well-documented retrospective analysis of 36 patients with syringomyelia and Chiari I malformation treated during an 11-year period with a consistent surgical technique of duraplasty performed through a relatively small bone opening, and without arachnoid opening. Clinical presentation was posterior fossa overcrowding, long tract impairment or brainstem compression. Clinical and imaging follow-up are of reasonable duration and quality. Postoperative syrinx decrease rate was 80.5% and clinical improvement was observed in 92% of the patients in this series.
Although better outcomes have been reported in the literature, the main merit of this article is to show that reasonable outcomes may be obtained in patients with syringomyelia who are treated with the consistent use of a relatively minimal surgical technique.
As our understanding of the physiology of syringomyelia increases, and as newer surgical techniques become available, continued improvements in clinical outcomes can be expected.
Richard G. Fessler, Chicago, USA
Dr. Spena et al., report the retrospective results of their decompression/dura-plasty technique for the treatment of Chiari I malformation in a group of 36 patients. They state that 80.5% of their patients experienced improvement of their neurologic symptoms over their preoperative neurological examination with a mean follow-up of 40 months (range 16–72). Their conclusion, based on these results, is that treating Syringomyelia associated in Chiari I malformation leads to a large percentage of patients with satisfying results with no irreversible complications. These results are not inconsistent with previous short-term results reported for a variety of techniques of treating Chiari malformation. Important observations noted by the authors include their observation that there appeared to be no correlation between the presence of syringomyelia and clinical outcome, and that the amount of pre-operative cerebellar descent and symptoms did not correlate with post-operative clinical result.
However, several technical details do make validity of these conclusions suspect. First, of course, the data was collected retrospectively. Second, the data was collected by the operating surgeons, thus introducing potential bias. Third, it is not discernable from the text, exactly what “improved, unchanged, or worsened” actually means, given the multiple symptoms followed by the authors. Finally, although the authors report that their follow-up averages 40 months, the “patients and methods” section reports that clinical evaluation was done at “one, three, and six months and at one year.” It is difficult to know how to reconcile this difference.
It is well-known that certain aspects of Chiari syndrome, such as muscle weakness and atrophy, ataxia, dorsal column dysfunction, hypesthetic pain, and cranial neuropathies are notoriously difficult to treat. Thus, having more detail on exactly what got better, what remained unchanged, what worsened, and how these impacted the patient’s quality of life are critical to interpreting outcome. Moreover, late failures and progression of symptoms are also common following treatment of Chiari malformation. Thus, assuming that the author’s data do reflect a true, unbiased improvement in their patient’s symptoms, a more accurate conclusion could only claim short term benefit. Long-term success rate needs to be determined after at least several more years of clinical follow-up.
Ricardo Botelho, São Paulo, Brazil
Magnetic resonance imaging brought clarity to the diagnosis of Chiari malformation and has revolutionized the treatment evaluation of this disease. The adequacy of neural decompression and return of cerebrospinal fluid flow at craniocervical junction may be, now, verified. The new factor recently suggested to evaluate the adequacy of surgery is the maintenance and expansion of the cisterna magna. The presence of this cisterna has been considered important in the prevention of post-operative cerebellar ptosis, functioning as a cushion buffer for structures of cranial posterior fossa. The arachnoid preservation technique in surgery for Chiari malformation has the potential to avoid adhesions between the dura and the neural structures that would occur with arachnoid opening and neural dissection techniques, recreating the Cisterna magna.
Because of the relative rarity of Chiari malformation, several samples of patients in different series have been necessary to demonstrate efficacy of the described technique. The work of Spena and Cols. provides additional evidence of such intervention in Chiari malformation. The authors have obtained more than 80% reduction in syringomyelia cavities, high clinical improvement and low complication rates. Technique of arachnoid preservation must be considered an important option in surgery for Chiari malformation, as demonstrated by these authors, among others.
Rights and permissions
About this article
Cite this article
Spena, G., Bernucci, C., Garbossa, D. et al. Clinical and radiological outcome of craniocervical osteo-dural decompression for Chiari I-associated syringomyelia. Neurosurg Rev 33, 297–304 (2010). https://doi.org/10.1007/s10143-010-0260-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-010-0260-y