Abstract
The objective of this study is to determine the incidence and degree of anterior clinoid process pneumatization, in addition highlighting to their clinical significance. Multidetector-row CT scans of the skull base were reviewed in 648 subjects between 2007 and 2008. The presence of pneumatized anterior clinoid process and its degree were studied and documented. These datas were istatistically analyzed. Pneumatization of the ACP was found in 62 of 648 patients (9.6%) including 32 (51.6%) men and 30 (48.4%) women. The age of these patients ranged from 21 to 82 years (mean, 41 ± 15.7 years). Pneumatization of the ACP occurred only on the left side in 14 cases (22.6%), only on the right side in 11 cases (17.7%), and bilaterally in 37 patients (59.7%). ACP pneumatization Type I, in which less than 50% of the ACP is pneumatized, was found in 47 of 124 sides (38%), Type II, in which more than 50% but not totally pneumatized ACP, was found in 28 of 124 sides (22.6%), and Type III, in which the ACP is totally pneumatized, was found in 22 of 124 sides (17.7%). The incidence of Type I in the general population was 6.6%, Type II was 3.5%, and Type III was 2.5%. Radiologically recognizing the degree of ACP pneumatization is important in decreasing the incidence of surgical complications during anterior clinoidectomy. Proper intraoperative management can be undertaken with special attention to the new classification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resection of the anterior clinoid process (ACP) is an essential step in the exposure of paraclinoid and some supraclinoid segments aneurysms of the internal carotid artery (ICA), in addition to some of the skull base tumors of the region. In these cases, ACP not only covers the clinoidal and supraclinoidal segments of ICA and ophthalmic artery, but also the proximal neck of the aneurysm. After removing this small bone, the distal dural ring can be dissected circumferentially, the optic strut can be drilled down further, and the ICA can be mobilized away from the optic nerve, thus, enabling the safe clipping of proximal ICA aneurysms. Complications after anterior clinoidectomy are due to the complex architecture of the skull base and its lesions. These include opening the paranasal sinuses and consequent rhinorrhea [26, 27, 35], visual disturbance, oculomotor paresis [12], injury to the optic nerve and/or internal carotid artery [11], injury of the ophthalmic artery [27], rupture of an aneurysm [21], and pneumocephalus. The potential risk of consequent rhinorrhea after an anterior clinoidectomy is due to communication between the air cells in pneumatized ACP with paranasal sinuses, which is repoted to be from 2.7 to 7% [14, 27, 32]. Because of this risk, pneumatization of ACP is considered an important issue in the surgery of the skull base generally, and anterior clinoidectomy especially. The incidence of pneumatization of the ACP has been reported as 4 to 29.3%, [3, 4, 6, 8, 13, 24, 30, 32], with higher rates of rhinorrhea after anterior clinoidectomy in these patients. Degrees of ACP pneuamtization is also important, however, less reported in the literature. To our best knowledge, this is the first report in the literature concerning the degrees of ACP pneumatization. In this article, the aim is to determine the incidence and the degrees of the ACP pneumatization, with highlighting their clinical significance.
Overview
The ACP is a bony projection on the medial part of the posterior border of the lesser sphenoid wing [28]. The term clinoid was derived from the ancient Greek word klineios, which means “resembling a bed post” [22]. ACP has a dense surface of cortical bone and a weak diploe of cancellous bone. However, if pneumatization of the sphenoid bone is widespread via the optic strut, it can form a hollow process with a thin bony wall. From a superior view, the ACP appears as a triangular mass with its base located in the medial part of the posterior border of the lesser sphenoid wing, and its [28]. tip projecting medioposteriorly. Anteriorly, the base of the ACP continues with the medial end of the sphenoid ridge. Medially, the base attaches to the body of the sphenoid bone by the posterior and anterior roots of the lesser sphenoid wing. The anterior root forms the roof of the OC. The posterior root (the optic strut), extends from the lower margin of the base of the ACP to the body of the sphenoid bone and is the main route for pneumatization of the ACP. The posterior root, the optic strut, forms the anterior part of the lateral and inferior walls of the OC and separates the optic canal from the superior orbital fissure [16] (Figs. 1 and 2).
A cadaveric dissection of the skull base demonstrating the anterior clinoid process and its bony anatomic relations. The left anterior clinoid process is removed for further exposure of the related bony structures. ACP, anterior clinoid process; OC, optic canal; OS, optic strut; SF, sellar floor; SOF, superior orbital fissure; SWl, lesser sphenoid wing; TS, tuberculum sella
Materials and methods
Multidetector-row CT scans of the skull base were reviewed in 648 subjects between years 2007 and 2008. The CT scans included in this study were obtained patients admitted to our outpatient or emergency clinic with a suspicion of cranial pathologies. Images in axial plan obtained by using a Philips MX 8000 (Best, Netherland) scanner with 3-mm cuts were reviewed. Images were uploaded to Kodak Direct View Web Software version 5.2 © Eastman Kodak Company 2003, and image process and 3D constructions were done. The degree and the pattern of pneumatization of the anterior clinoid processes were studied and documented in both sides in 648 subjects (349 men, 299 women; age range, 18–92 years; mean age, 41 ± 19.3 years). Using the bone window, appropriate plans (axial, coronal, and sagittal) were selected for printing on a radiograph at 85% magnification. These images were studied and evaluated by two neurosurgeons (NT, OY) and a neuroradiologist (SA). All measurements were entered and analyzed in a Microsoft Excel spreadsheet (Redmond, WA, USA). All data were provided as the mean ± standard deviation (SD), and statistical analyses were performed using the paired two-tailed Student t test.
Results
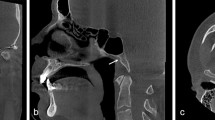
Pneumatization of the ACP was found in 62/648 patients (9.6%) including 32/62 (51.6%) men and 30/62 (48.4%) women. The age of these patients ranged from 21 to 82 years (mean, 41 ± 15.7 years). It occurred only on the left side in 14 cases (22.6%), only on the right side in 11 cases (17.7%), and bilaterally in 37 patients (59.7%). Pneumatization degrees were classified on the basis of the volume of pneumatization into four types: Type 0, non-pneumatized ACP (Fig. 3); Type I, less than 50% pneumatization of the ACP (Fig. 4); Type II, more than 50% but not totally pneumatized ACP (Fig. 5); and Type III, totally pneumatized ACP (Fig. 6). Type I occurred in 43/62 patients (69.3%), Type II in 23/62 patients (37%), and Type III in 16/62 patients (25.8%). When calculated on the basis of total 124 sides, pneumatization Type I occurred in 47/124 sides (38%), Type II in 28/124 sides (22.6%), and Type III in 22/124 sides (17.7%). The results of our study are summarized in Tables 1 and 2.
Discussion
Anatomic considerations
From the inferolateral aspect, the oculomotor nerve, the trochlear nerve, the three major branches of the ophthalmic nerve, and the abducens nerve form a neural bundle running under the lower margin of the ACP. The clinoidal segment of the ICA in between the proximal and distal dural rings is exposed after removal of the ACP. The deep (inner) dural layer separating the ACP from the roof of the cavernous sinus creates a clinoidal space (2–6 mm long) [28], which can be exposed following the removal of the process. The dura extending medially off the upper surface of the anterior clinoid forms the upper dural ring around ICA, and the dura lining the lower margin of the clinoid extends medially to form the lower dural ring, which extends toward the occulomotor nerve, and referred to carotico-occulomotor membrane. The clinoid segment of the carotid artery is enclosed with the dural sheath in between the two dural rings and referred as the carotid collar (Fig. 7).
A schematic drawing demonstrating the neurovascular relations of the right anterior clinoid process after its resection. a Lateral and b superior view. The dotted line indicates the location of the anterior clinoid process before resection. III: occulomotor nerve, IV: trochlear nerve, V1: ophthalmic nerve, V2: maxillary nerve, ICAc: clinoidal segment of the internal carotid artery, ICAh: horizontal segment of the internal carotid artery, ICAs: supraclinoid segment of the internal carotid artery, ON: optic nerve, LDR: lower dural ring, UDR: upper dural ring
The incidence of pneumatization of the ACP in our study is 9.6%, which was found similar to the results of previous studies (4 to 29.3%) [3, 4, 6, 8, 13, 24, 30, 32]. There was no significant difference in the side of the pneumatized ACP. Of these patients, 59.7% had bilateral pneumatized ACP. Moreover, there was no gender difference in the patients with pneumatized ACP (male to female ration 1:1). These results are found similar to those in previous reports [24]. Mikami et al. [24] had proposed a classification system for the pneumatization pattern of the ACP according to the route of pneumatization. According to Mikami et al. classification Type I; pneumatization via the optic strut (OS), Type II; via the anterior recess (AR), and Type III; via both the OS and AR (Fig. 5). Instead, we are proposing a new classification based on the degree of pneumatization of the ACP. In our study, the most common pneumatization pattern was Type I, which occurred in 47 sides (38%). On the other hand, Type II occurred in 28 sides (22.6%), and Type III in 22 sides (17.7%). Also, according to our results, the incidence of Type I in the general population is 6.6%, Type II is 3.5%, and Type III is 2.5%.
Clinical considerations
Carotid−ophthalmic and paraclinoid aneurysms and cavernous sinus tumors are still considered a technical challenge for safe exposure. Radical neck clipping of aneurysms and total removal of extensive tumors in the skull base, without retraction of the optic nerve and brain lobes, require removal of the anterior, middle, and posterior clinoid processes, optic strut, and/or petrous bone [18, 19, 35]. Anterior clinoidectomy is one of the most important steps in these procedures for the successful management of aneurysms or tumors. The removal of the ACP has been well described in literature [7, 11, 16, 34, 35]. Dolenc developed a combined extra- and intradural approach to remove the ACP and the roof of the optic canal in approaching the cavernous sinus, clinoidal space, and orbital apex [10, 11]. Takahashi et al. have also proposed an intradural en bloc removal of the ACP. This requires minimal drilling, thus, decreased risk of injury to the optic nerve and a shortened time for clinoidectomy [33]. During an anterior clinoidectomy, the ACP must be adequately removed for sufficient exposure of the related structures and pathologies, without injuring these normal structures.
Performing anterior clinoidectomy remains a challenging task with potential complications owing to its deep location between the ICA and CNs II and III. Injuries to CN II and III can be caused by unexpected motion of the drill or by heat, in the case of insufficient irrigation [20]. Even without intradural manipulation of CN III, Nutik [26] reported that occulomotor paresis and/or palsy occurred postoperatively in 3 of 30 patients. He also conveyed that the superior division of the CN III, which lies close to the ACP, was more likely to be injured. Moreover, decreased visual acuity and/or inferior visual field defects after anterior clinoidectomy is also reported [26].
A pneumatized optic strut and the degrees of the ACP pneumatization can be identified before surgery with computed tomographic images and, occasionally, with scout x-ray images from the angiogram. If not recognized before surgery, they can be identified during surgery by large air cells in this bone, by an internal layer of cortical bone within the optic strut, or by the mucosal lining of the sphenoid sinus [5]. These aerated channels within the ACP and optic strut often facilitate anterior clinoidectomy but indicate the need for repair later during the closure. The air cells in the pneumatized ACP can act as a safe limit for drilling, as entering these cells notifies the surgeon about the near location of the underlying neurovascular structures, thus more controlled drilling of the remaining thin internal layer of the cortical bone of the ACP is performed. The more the pneumatization degree of the ACP, the more wide safe limit, and more wide area for controlled drilling. Therefore, this may contribute in decreasing the risk of the underlying neurovascular structure injury. With preoperative evaluation of the degree of ACP pneumatization, the surgeon can estimate the extent of ACP drilling, thus, more controlled and safe removal of the ACP can be achieved.
One of the major complications of the removing of pneumatized ACP is the opening of the paranasal sinuses and the concomitant risk of rhinorrhea, with consequent high risk of sepsis [25]. This complication arises because the communication of the air cells in the pneumatized ACP with the paranasal sinuses. Although an extradural procedure reduces the risk of a CSF leak after the removal of a pneumatized ACP [26], this risk can be further decreased by preoperative radiologic evaluation of the pneumatization of the ACP, with a final attempt of reconstruction may be planned prior to surgery. Conservative management of CSF leaks in this area includes bed rest, serial lumbar punctures, and lumbar drainage. Surgical intervention is indicated if conservative management fails [1, 17, 30, 31]. Surgical technique for the treatment of CSF leaks after anterior clinoidectomy includes plugging the defect with bone wax, methylmethacrylate, polymer glue, fat, or tightly wedging a muscle plug into the optic strut (the ‘yo-yo’ technique) [5]. The direction of packing is also important; therefore, cranialization should be confirmed by fixing the muscle pieces where the mucous membrane protrudes [5, 9]. Moreover, with the development of endoscopic endonasal techniques in skull base surgery, endoscopic endonasal transsphenoidal repair of CSF leaks after the anterior clinoidectomy, can be performed easily [17]. After endoscopic endonasal anterior sphenoidectomy, the base of the ACP is represented as the bone depression in the sphenoid roof, located between the optic protuberance superiorly and the carotid protuberance inferiorly [2] (Fig. 8). The optico-carotid recess can then be reconstructed under direct endoscopic vision.
a Endoscopic view in a cadaveric specimen after anterior sphenoidectomy demonstrating the sellar floor and the base of the anterior clinoid process (optico-carotid recess). b Endoscopic view in an another cadaveric specimen after resecting the floor of the sellar fossa and the left cavernous sinus to demonstrate the endoscopic anatomy of the left anterior clinoid process and the related neurovascular structures. III: occulomotor nerve, VI: trochlear nerve, ACP: anterior clinoid process, C: clivus, CPc: paraclival portion of the carotid protuberance, CPs: parasellar portion of the carotid protuberance, ICA-A: anterior bend of internal carotid artery, ICA-P: posterior bend of internal carotid artery, OC: optic canal, OCR: optico-carotid recess, ON: optic nerve, OP: optic protuberance, Oph: ophthalmic artery, PG: pituitary gland, PS: planum sphenoidale, SF: sellar floor, TS: tuberculum sella
Mucoceles protruding from the paranasal sinus into the intracranial space through the anterior clinoidectomy bone defect, which result from chronic accumulation of mucous secretions and are lined by respiratory epithelium [23, 25], can compress adjacent structures and cause secondary defects in cranial nerve function. In the case of the optic nerve compression due to mucoceles, patients often present with ocular symptoms, including optic neuropathy, visual field defects, or blindness [9, 23, 29]. It is recommended to avoid tearing of the mucous membrane, check it in the pneumatized area, and push it to the paranasal sinuses. For this aim, Huynh-Le et al. [15] have proposed a method to avoid tearing the mucous membrane, by using a diamond drill in the anterior clinoidectomy.
Most, if not all, pneumatized ACPs communicate with the sphenoid sinus via the OS, as previously reported [24, 26]. This finding is similar to the results of our study. In contrast to the classification proposed by Mikami et al. [24], which is based on the root of the pneumatization of ACP and has influence on the surgical technique of reconstruction, our classification is based on the degree of pneumatization, which has influence on the preoperative planning for more controlled drilling of the ACP aiming to decrease the risk of injury of related neurovascular structures and evaluating the risk of the development of postoperative CSF leak, with a final attempt of reconstruction planned prior to surgery. Using multidector-row CT coronal reconstruction, types of ACP pneumatization are easily distinguished according to relationships between the optic canal and the pneumatization. Preoperative awareness of the pneumatization of the ACP and its types, and its relation to the paranasal sinuses is important for surgical planning in and around clinoidal space. A safe removal of ACP, without tearing the mucosa or injury of related neurovascular structures, and appropriate bony defect closure can be achieved if the surgeon recognizes the degree of ACP pneumatization prior to surgery. On the basis of this radiologic anatomic study, we believe that our proposed classification will have a great influence on the surgical application of anterior clinoidectomy.
Conclusion
Radiologically, recognizing the pneumatization of the ACP and OS is important in decreasing the incidence of surgical complications of an anterior clinoidectomy, and the proper intraoperative management to prevent these complications. Therefore, a safe removal of ACP, without tearing the mucosa or injury of related neurovascular structures, and appropriate bony defect closure can be achieved.
References
Abuzayed B, Kafadar AM, Oğuzoğlu SA, Canbaz B, Kaynar MY (2009) Duraplasty using autologous fascia lata reenforced by on-site pedicled muscle flap: technical note. J Craniofac Surg 20(2):435–438
Abuzayed B, Tanriover N, Ozlen F, Gazioglu N, Ulu MO, Kafadar AM, Eraslan B, Akar Z (2009) Endoscopic endonasal transsphenoidal approach to the sellar region: results of endoscopic dissection on 30 cadavers. Turk Neurosurg 19(3):237–244
Arslan H, Aydinlioglu A, Bozkurt M, Egeli E (1999) Anatomic variations of the paranasal sinuses: CT examination for endoscopic sinus surgery. Auris Nasus Larynx 26:39–48
Bolger WE, Butzin CA, Parsons DS (1991) Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope 101:56–64
Chi JH, Sughrue M, Kunwar S, Lowton MT (2006) The “yo-yo” technique to prevent cerebrospinal fluid rhinorrhea after anterior clinoidectomy for proximal internal carotid artery aneurysms. Neurosurgery 59(Suppl 1):101–107
Citardi MJ, Gallivan RP, Batra PS, Maurer CR Jr, Rohlfing T, Roh HJ, Lanza DC (2004) Quantitative computer-aided computed tomography analysis of sphenoid sinus anatomical relationships. Am J Rhinol 18:173–178
Coscarella E, Baskaya MK, Morcos JJ (2003) An alternative extradural exposure to the anterior clinoid process: the superior orbital fissure as a surgical corridor. Neurosurgery 53:162–167
DeLano MC, Fun FY, Zinreich SJ (1996) Relationship of the optic nerve to the posterior paranasal sinuses: a CT anatomic study. AJNR Am J Neuroradiol 17:669–675
Deshmukh S, De Monte F (2007) Anterior clinoidal mucocele causing optic neuropathy: resolution with nonsurgical therapy. J Neurosurg 106:1091–1093
Dolenc V (1989) Anatomy and surgery of the cavernous sinus. Wien Springer, New York
Dolenc VV (1985) A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg 62:667–672
Dolenc VV (1999) A combined transorbital-transclinoid and transsylvian approach to carotid-ophthalmic aneurysms without retraction of the brain. Acta Neurochir Suppl 72:89–97
Gean AD, Pile-Spellman J, Heros RC (1989) A pneumatized anterior clinoid mimicking an aneurysm on MR imaging. Report of two cases. J Neurosurg 71:128–132
Hoh BL, Carter BS, Budzik RF, Putman CM, Ogilvy CS (2001) Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery 48:78–90
Huynh-Le P, Natori Y, Sasaki T (2004) Surgical anatomy of the anterior clinoid process. J Clin Neurosci 11:283–287
Inoue T, Rhoton AL, Theele D, Barry ME (1990) Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery 26:903–932
Kelley TF, Stankiewicz JA, Chow JM, Origitano TC, Shea J (1996) Endoscopic closure of postsurgical anterior cranial fossa cerebrospinal fluid leaks. Neurosurgery 39:743–746
Knosp E, Müller G, Perneczky A (1988) The paraclinoid carotid artery: anatomical aspects of a microneurosurgical approach. Neurosurgery 22:896–901
Kobayashi S, Kyoshima K (1989) Carotid cave aneurysms of the internal carotid artery. J Neurosurg 70:216–221
Kondo S, Okada Y, Iseki H, Hori T, Takakura K, Kobayashi A, Nagata H (2000) Thermological study of drilling bone tissue with a high-speed drill. Neurosurgery 46:1162–1168
Korosue K, Heros RC (1992) “Subclinoid” carotid aneurysm with erosion of the anterior clinoid process and fatal intraoperative rupture. Neurosurgery 31:356–360
Liddell HG, Scott R (1889) An ıntermediate Greek-English Lexicon, founded upon the seventh edition of Liddell and Scott’s Greek-English Lexicon. Harper & Brothers, New York
Lim CC, Dillon WP, McDermott MW (1999) Mucocele involving the anterior clinoid process: MR and CT findings. AJNR Am J Neuroradiol 20:287–290
Mikami T, Minamida Y, Koyanagi I, Baba T, Houkin K (2007) Anatomical variations in pneumatization of the anterior clinoid process. J Neurosurg 106:170–174
Nandapalan V, Watson ID, Swift AC (1996) Beta-2-transferrin and cerebrospinal fluid rhinorrhoea. Clin Otolaryngol Allied Sci 21:259–264
Noguchi A, Balasingam V, Shiokawa Y, McMenomey SO, Delashaw JB Jr (2005) Extradural anterior clinoidectomy. Technical note. J Neurosurg 102:945–950
Nutik SL (1988) Removal of the anterior clinoid process for exposure of the proximal intracranial carotid artery. J Neurosurg 69:529–534
Phuong Huynh-Le P, Natori Y, Sasaki T (2004) Surgical anatomy of the anterior clinoid process. J Clin Neurosci 11(3):283–287
Prepageran N, Subramaniam KN, Krishnan GG, Raman R (2004) Ocular presentation of sphenoid mucocele. Orbit 23:45–47
Sapci T, Derin E, Almac S, Cumali R, Saydam B, Karavus M (2004) The relationship between the sphenoid and the posterior ethmoid sinuses and the optic nerves in Turkish patients. Rhinology 42:30–34
Senyuva C, Yucel A, Okur I, Cansiz H, Sanus Z (1996) Free rectus abdominis muscle flap for the treatment of complications after neurosurgical procedures. J Craniofac Surg 7:317–321
Sirikci A, Bayazit YA, Bayram M, Mumbuc S, Gungor K, Kanlikama M (2000) Variations of sphenoid and related structures. Eur Radiol 10:844–848
Takahashi JA, Kawarazaki A, Hashimoto N (2004) Intradural en-bloc removal of the anterior clinoid process. Acta Neurochir (Wien) 146:505–509
Yasargil MG, Gasser JC, Hodosh RM, Rankin TV (1977) Carotid–ophthalmic aneurysms: direct microsurgical approach. Surg Neurol 8:155–165
Yonekawa Y, Ogata N, Imhof HG, Olivecrona M, Strommer K, Kwak TE, Roth P, Groscurth P (1997) Selective extradural anterior clinoidectomy for supra- and parasellar processes. Technical note. J Neurosurg 87:636–642
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Tiit Mathiesen, Stockholm, Sweden
Drs Bashar et al. have provided a careful review of extradural anterior clinoidectomy with an analysis of the incidence of pneumatization. Extradural anterior clinoidectomy is an essential procedure for enhanced radicality and exposure in pterional and anterolateral approaches. Awareness of the pneumatization of the clinoid is greatly necessary as it influences the drilling, mucosal handling, and need for sealing to avoid CSF leakage into the sphenoid sinus. This article discusses these issues well. There are, however, two major weaknesses.
1. Drs Bashar et al. propose a new classification of the pneumatization. Their classification is based on the extent of pneumatization. Unfortunately they provide no thoughts or observations of how the surgical maneuvers would (or should) differ between classes I, II, and III; the new classification does not seem to make any practical sense. Whenever a classification is suggested, an investigation of its practical applicability and applications are warranted! I also miss a comparison between the new classification and the classification proposed by Mikami et al., which instead deals with the anatomical connections between the areated clinoid process and the sphenoid sinus. Surgically, the Mikami classification makes more sense, since it imposes an awareness of which potential fistulous connections need to be sealed. The present article does not make a case for incorporation of the Bashar classification of pneumatization into the neurosurgical curriculum.
2. Drs Bashar et al. conclude that recognizing pneumatization of the anterior clinoid is important for several reasons. The article, however, only shows that their method identified pneumatization of the anterior clinoid process in 9.6% of their population. The authors should instead have discussed whether the data are applicable for the general population and whether radiological findings agreed with clinical findings.
Although I agree with the conclusion, it was postulated already in the introduction and does not follow from the analyses that were undertaken by the authors. Still, the review serves as an excellent reminder of the benefits ant pitfalls in anterior clinoid removal.
Kazuhiko Nozaki, Shiga, Japan
The authors analyzed the incidence and degree of anterior clinoid process pneumatization in 648 cases and classified the pneumatization of anterior clinoid process (ACP) into four types. The incidence and degree of ACP pneumatization are important in terms of the risk of CSF leak after drilling of ACP. The presence of pneumatization on the side of optic strut or anterior root seems to make drilling easier but the risk of postoperative CSF leakage increases, and intraoperative adequate repair is mandatory. Extended drilling of pneumatized anterior clinoid process should be avoided if the procedure cannot produce necessary operative space. The degree and extension of pneumatization in the skull base should be carefully checked in each patient using high resolution CT in order to determine the adequate extent of drilling.
Rights and permissions
About this article
Cite this article
Abuzayed, B., Tanriover, N., Biceroglu, H. et al. Pneumatization degree of the anterior clinoid process: a new classification. Neurosurg Rev 33, 367–374 (2010). https://doi.org/10.1007/s10143-010-0255-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-010-0255-8