Abstract
Purpose
We hypothesize that delayed phase imaging does not provide additional diagnostic information in patients who undergo multi-phasic CTA for suspected active bleeding.
Methods
Data on patients who underwent multiphasic CTA (pre-contrast, arterial, porto-venous, and delayed phases) for suspected acute bleed were retrospectively collected between January 2019 and November 2021. CTA images were reviewed by a general radiologist, an interventional radiologist, and a body imaging radiologist independently. Each reader evaluated if delayed phase images provided additional information that would change the final impression of the CTA report. Additional information regarding bleeding location, time needed for delayed image acquisition, and radiation exposure were also obtained.
Results
A total of 104 patients with CTAs were analyzed with an average age of 58 years ± 22. Studies rated with absent additional findings on delayed images were 102 (98.1%) by the interventional radiologist, 101 (97.1%) by the body imaging radiologist, and 100 (96.1%) by the general radiologist with percent agreement of 96.15% (kappa 0.54, p < 0.001). All the findings were characterized as unlikely to be clinically significant. Mean time added to complete a delayed phase images was 3.61 ± 3.4 min. The average CT dose length product (DLP) for the total exam was 3621.78 ± 2129.57 mGy.cm with delayed acquisition adding a mean DLP of 847.75 ± 508.8 mGy.cm.
Conclusion
Delayed phase imaging does not provide significant additional diagnostic information in evaluating patients with suspected active bleeding but is associated with increased examination time and radiation exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CT has become an integral part of the evaluation of patients with suspected bleeding as it has been shown to be accurate in defining solid and hollow visceral injuries as well as associated vascular extravasation [1,2,3]. In particular, detection of the presence and source of active hemorrhage is important in assessing the need for optimal intervention – being medical, surgery or transcatheter embolization [4,5,6,7,8,9,10,11,12]. Active extravasation is best characterized with a Multi-phase CTA protocol [4]. A multiphase acquisition typically includes a non-enhanced, arterial, and venous phases with the possible addition of a delayed phase for trauma patients. However, there is no clear consensus regarding optimal CT protocols for patients with suspected active bleed specifically in trauma setting [5, 6].
The classic pattern of active extravasation is a jet or focal area of hyperattenuation on initial images, generally within a hematoma, that fades into an enlarged, enhanced area on more delayed images [4]. The timing of the arterial phase is very strictly tailored to bolus arrival in the anatomy of interest [13, 14]. A portal venous phase imaging is standard for evaluation after blunt trauma, providing optimal contrast enhancement of solid visceral parenchyma [15]. Some institutions use arterial phase scan followed by a portal venous phase scan in abdominal trauma [6]. It has also been suggested that the use of more delayed phase imaging allows for a better estimation of the enlargement of the area of active hemorrhage [16]. This led some authors to routinely acquire portal venous images followed by up to 5-min delayed scans [17,18,19]. Others implemented an arterial phase followed by a 5-min delayed phase [16].
While the need to minimize radiation dose requires restraint in the application of multiphase CT, these protocols have demonstrated utility in active bleeding and abdominal trauma imaging [6]. Unfortunately, the effective radiation doses of standard multi-phase abdominal pelvic CT scans can surpass 10-mSv, or in some cases, even 30-mSv at certain facilities [20, 21]. However, weighing the risk of radiation exposure against the advantage of a precise clinical diagnosis is crucial. A study that is inadequate or suboptimal might necessitate supplementary or repeated tests, thereby increasing the cumulative radiation dose [22,23,24].
As mentioned previously, despite the regular use of multiphase CT for suspected active bleeding, there is no universally agreed-upon optimal CT protocol. Our institution uses a multiphasic CT protocol that includes a pre-contrast phase, arterial phase, venous/portal-venous phase, and delayed phase to improve the detection of active bleeding. The theoretical advantage of more accurately identifying active hemorrhage with the addition of delayed phases must be measured against the added information and clinical relevance of detecting contrast extravasation in addition to the potential increase in radiation and procedural time.
This study aims to evaluate the diagnostic significance of delayed images of a multiphasic CT protocol in suspected active bleed by assessing the presence of additional findings on this phase in relation to the standard arterial and porto-venous phases protocols. This study also aims to assess the average increase in study duration and radiation dose when a delayed phase is added.
Materials and methods
Patient population
Our retrospective study was approved by the Institutional Review Board (IRB), which waived the requirement for informed consent. The picture archiving and communication system (PACS) at the American University of Beirut Medical Center was queried to identify patients who underwent multi-phasic CT angiography (CTA) of the extremities, chest, abdomen, and/or pelvis for suspected active bleeding between January 2019 and November 2021. PACS was searched for cases with the words “bleed”, “hemorrhage” and “hematoma” within the clinical indication section of CTA reports. The search was limited to the emergency department and inpatient cases. Patients were excluded if the CT protocol did not include non-enhanced, arterial, proto-venous, and delayed phases. Electronic record system was reviewed for the patient’s age, sex, and BMI in addition to the suspected bleeding etiology which was categorized into traumatic and non-traumatic.
CTA protocol
CTA was performed on one of three CT scanners: 256 slice, IQon Spectral CT, Philips Healthcare; 256 slice, Brilliance iCT, Philips Healthcare; or 128 slice, Incisive, Philips Healthcare. No oral contrast was administered. Following the acquisition of a non-contrast CT, 150 ml of low-osmolar iodinated contrast (350 mg/ml Iodine or higher concentration) was administered by power injector intravenously at 4 ml/ sec. Arterial-phase imaging was triggered using bolus tracking with a descending aorta threshold of 120 HU. Image acquisition is preformed 5 to 30 s from the time of the injection, depending on the region of interest. The portal/venous phase was acquired 80s to 100s from the time of the injection and a delayed phase was obtained at 3-min after the venous phase and 5-min from the beginning contrast injection. All CT scans were reconstructed and archived with contiguous thin sections of 1 mm thickness and routine acquisition and archiving of coronal and sagittal reconstructions.
The scanning parameters were as follows: slice thickness, 1.5 mm; reconstruction increment, 1.5 mm; beam pitch, 0.8; gantry rotation time, 0.5 s; tube voltage, 120–140 kV (depending on the patient body habitus); and tube current between 60 and 180 mAs with automated tube current modulation. The maximum intensity projection and multiplanar reformation images were generated for all the patients.
CT image interpretation
CTA images were reviewed by an interventional radiologist, a body imaging radiologist, and a general radiologist independently (with 10 years, 7 years, and 20 years of experience respectively). The readers had no access to other images or clinical information. Image reviews were performed using the institutional PACS on a primary reporting workstation (IntelliSpace, Philips Healthcare, Netherlands) with a GSDF-calibrated 3-megapixel monitor. Illumination was adjusted at 25–32 lx, with a calibrated photometer (Chroma meter CL-200). Radiologists were blinded to the patient’s name, study report, and clinical information.
CT images were initially reviewed to identify the region of interest as follows: neck, chest, abdomen (including gastro-intestinal bleeds), pelvis, and extremities. The radiologists were asked to interpret five different sequences of image sets, each time in the following order: (1) First interpreting the non-enhanced phase, (2) second interpreting the arterial phase only, (3) third interpreting the venous phase only, (4) fourth interpreting the three phases, (5) and finally interpreting the three phases in addition to the delayed phase. Each radiologist independently reviewed CTA images looking for the presence of any additional finding of contrast extravasation on the delayed phase (step 5) which could not be seen on earlier phases. The three radiologists also assessed the images to diagnose whether there was a hematoma, the hematoma size, the presence of contrast extravasation, whether the extravasation is contained (pseudoaneurysm) or free, and the extravasation volume. Disagreement was solved by consensus. Findings on delayed phases were categorized into detection (i.e., confirming contrast extravasation not seen on prior phases) and characterization (i.e., identifying whether the extravasation is contained (pseudoaneurysm) or free). If the finding was only seen on delayed images, it was characterized as a free extravasation.
Radiation dose estimation
Radiation doses were analyzed independently from the body region. Mean Dose length product (DLP, measured in mGy ∗ cm) was used for the quantitative assessment of radiation exposure separately for the non-enhanced, arterial, porto-venous, and delayed phases. The mean CT dose index (CTDI) (measured in mGy) was only calculated for delayed phases.
Statistical analysis
Fisher exact test was used for categorical variables, and Wilcoxon rank-sum test for continuous variables. We used SPSS for analysis, and p-values of 0.5 were considered statistically significant. Reader’s agreement regarding the presence of additional findings on delayed phases and the type of additional findings on delayed phases were assessed using percentage agreement and Fleiss Kappa Value.
Results
The search of PACS system revealed 174 cases of body CTA with words “bleed”, “hemorrhage” and “hematoma” included in the clinical indication section of the final report. 70 cases were excluded because they did not include all 4 phases. All 4 phases were performed in 104 cases (60%) including the delayed phase.
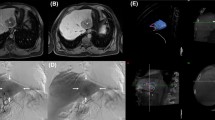
A total of 104 patients were included in the study (mean age 52 ± 22 years). The presence of additional findings on delayed phase images was observed in 4 cases (3.9%) with the rest 100 cases (96.1%) showed no additional findings on delayed phased images. All additional findings were categorized as “detection”, seen in the presence of a hematoma, and seen as free contrast extravasation (Figs. 1, 2, 3 and 4). The presence of additional findings was not significantly associated with any categorical variable evaluated (Table 1).
Regarding the effect of delayed images on procedural time, the mean time from venous phase to delayed phase was 3.6 min (SD = 3.43) with a median time of 3 min. The mean time from pre-contrast phase to delayed phase was 8.21 min (SD = 8.55) with a median time of 7 min. The mean DLP of delayed images was 847.75 mGy*cm (SD = 508.80) and the mean CTDI was 12.6 mGy (SD = 6.17) (Table 2).
Table 3 demonstrates the agreement between the three readers regarding the presence of additional findings and the type of findings. Studies rated with absent additional findings on delayed images were 102 (98.1%) by the interventional radiologist, 101 (97.1%) by the body imaging radiologist, and 100 (96.1%) by the general radiologist. There was agreement between the 3 readers regarding the absence and presence of additional findings with a Kappa value of 0.5424 (p < 0.001). All the additional findings were categorized as detection with none categorized as characterization.
Discussion
Although it is suggested that using late delayed images (up to 5-min) might be useful to further characterize areas of focal extravasation [16, 17] we found no study that investigated the merit of such a claim. Performing additional delayed phase body CTA is a common practice in our hospital. Of the 104 cases that underwent multiphasic CTA with non-enhanced, arterial, porto-venous, and delayed phases for suspected active bleeding, only 4 cases of contrast extravasation were only detected on delayed images. These four additional findings were unlikely to be clinically significant (Figs. 1, 2, 3 and 4). For the four cases, two were treated conservatively (Figs. 1 and 4), one might represent redistribution of contrast from another bleeding foci and was reported as an additional finding only by one radiologist (Fig. 2), and one was present in a setting of multiple active arterial bleeds making its contribution to the general prognosis of the patient questionable (Fig. 3).
Case 2 a 20-year-old male with blunt abdominal trauma. There is contrast extravasation along the lateral aspect of the spleen seen only on the delayed phase image. Only the general radiologist reader reported this finding. This patient had other foci of contrast extravasation in the spleen and left kidney, thus redistribution of contrast in the peri-splenic space is a possibility
Case 3 is a 43-year-old female with sigmoid colon cancer following peritonectomy. There is perihepatic contrast extravasation on the delayed phase image. Only the general radiologist and abdominal radiologist readers reported this finding. There may be a faint focus of extravasation at that site on the venous phase. This patient had other foci of contrast extravasation and underwent endovascular embolization
A study by Kim SJ et al. found that the diagnostic performance of combined arterial and portal venous phase CT, with regards to detecting active bleeding in patients with traumatic abdominal injury, did not show a significant advantage compared to diagnosis using single arterial or single portal venous phase CT protocol with no significant difference in diagnostic performance between two CT protocol sets (arterial vs. portal venous, arterial vs. combined, portal venous vs. combined). In the pooled analysis, arterial and combined CT phases were slightly superior for the identification of contained vascular injuries, compared to portal venous phase CT; however, generalization was limited due to a small sample size [25].
On the other hand, Pouw ME et al. showed that specificity increased with additional contrast phases without changes in sensitivity [26]. Overall agreement among readers similarly increased. However, Kim JW et al. observed no significant difference in sensitivity or specificity when diagnosing gastrointestinal bleeding using biphasic protocols compared to a triphasic one [27].
Previous studies have shown that contained vascular injuries are identified more accurately at the arterial phase of image acquisition compared to the portal-venous phase, in more than half of these cases [5, 6]. Boscak AR et al. demonstrated that for CT evaluation of blunt splenic injury, the arterial phase is superior to portal venous phase imaging for pseudoaneurysm but inferior for active bleeding and parenchymal disruption. Dual-phase review was equivalent to or better than single-phase review for all injuries [6]. Compared to active extravasation, an isolated pseudoaneurysm is contained by connective tissue or the vessel wall (i.e. the adventitia). Therefore, a pseudoaneurysm is likely to be adjacent to a vessel and does not enlarge or increase in attenuation as the contrast material washes out of the arterial system on 5-min delayed images [16]. In our study, arterial portal/venous phases acquired 80 to 100s after contrast injection were sufficient to characterize bleed as free or contained (i.e. pseudoaneurysm) with the late 5-min delayed phases adding no significant additional information.
Regarding the effect on radiation exposure, in our study, the addition of late delayed images led to a mean increase of DLP by 847.75 mGy*cm (SD = 508.80) and a delayed phase CTDI of 12.6 mGy (SD = 6.17) while adding a median of 3-min of scanning time. The increase in radiation exposure is critical as single-phase CT, exposure was estimated to result in a lifetime excess risk of 34 cancers per 100,000 patients, and for the biphasic CT, the estimated risks were 59 cancers per 100,000 patients [28]. Kim SJ et al. demonstrated that the cumulative effective dose (cED) was significantly reduced with single-phase CT used alone compared to multiple phase CT. Projected cancer incidence and mortality were also significantly lower with single-phase protocol [25]. Yaniv et al. reported that revised single-phase body CT showed better vascular and abdominal parenchymal imaging with a reduction in radiation dose, compared with conventional protocol in patients with trauma with the single-phase protocol decreased mean effective radiation dose from 18.2 ± 8.2 mSv to 12.4 ± 4.4 mSv [24].
Moreover, decreasing the relatively high radiation dose of multiphase CT can be achieved with the implementation of low-radiation protocols. Alagic et al. demonstrated that multiphase low-radiation protocol improved diagnostic accuracy of arterial injuries with reduced radiation in this study, low-radiation protocol decreased mean (± SD) effective radiation dose from 1932 ± 247 mGy ∗ cm to 1681 ± 183 mGy ∗ cm, compared with conventional single-phase CT [23].
Our study has some limitations. The small sample size limits the conclusions that may be drawn from this study and reduces the statistical power of our results. We calculated the dose using DLP and not effective dose while not differentiating between the various imaged regions. Using the effective dose would be a better estimate of the increased risk of radiation exposure. Moreover, hemodynamic and laboratory variables, such as blood pressure and heart rate during imaging, were not evaluated in this study.
Conclusion
In conclusion, although a common practice, delayed phase CTA showed no significant diagnostic value when assessing for active bleeding while adding unnecessary increase in radiation dose and procedural time. The findings detected on delayed images were unlikely to be of clinical significance.
References
Novelline RA, Rhea JT, Rao PM, Stuk JL (1999) Helical CT in emergency radiology. Radiology 213:321–339
Shuman WP (1997) CT of blunt abdominal trauma in adults. Radiology 205:297–306
Novelline RA, Rhea JT, Bell T (1999) Helical CT of abdominal trauma. Radiol Clin North Am 37:591–612
Ryan MF, Hamilton PA, Chu P, Hanaghan J. ;55(3):160–169., Uyeda JW, LeBedis CA, Penn DR, Soto JA, Anderson SW (2004) Active extravasation of arterial contrast agent on post-traumatic abdominal computed tomography. Can Assoc Radiol J. Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology 2014;270:99–106
Uyeda JW, LeBedis CA, Penn DR, Soto JA, Anderson SW (2004) Active extravasation of arterial contrast agent on post-traumatic abdominal computed tomography. Can Assoc Radiol J. Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology 2014;270:99–106
Boscak AR, Shanmuganathan K, Mirvis SE, Fleiter TR, Miller LA, Sliker CW et al (2013) Optimizing trauma multidetector CT protocol for blunt splenic injury: need for arterial and portal venous phase scans. Radiology 268:79–88
Hagiwara A, Yukioka T, Ohta S, Nitatori T, Matsuda H, Shimazaki S (1996) Nonsurgical management of patients with blunt splenic injury: efficacy of transcatheter arterial embolization. AJR Am J Roentgenol 167:159–166
Marmery H, Shanmuganathan K, Alexander MT, Mirvis SE (2007) Optimization of selection for nonoperative management of blunt splenic injury: comparison of MDCT grading systems. AJR Am J Roentgenol 189:1421–1427
Marmery H, Shanmuganathan K, Mirvis SE, Richard H, Sliker C, Miller LA et al (2008) Correlation of multidetector CT findings with splenic arteriography and surgery: prospective study in 392 patients. J Am Coll Surg 206:685–693
Fang JF, Wong YC, Lin BC, Hsu YP, Chen MF (2006) The CT risk factors for the need of operative treatment in initially hemodynamically stable patients after blunt hepatic trauma. J Trauma 61:547–553 discussion 553–554
Thompson BE, Munera F, Cohn SM, MacLean AA, Cameron J, Rivas L, Bajayo D (2006) Novel computed tomography scan scoring system predicts the need for intervention after splenic injury. J Trauma 60:1083–1086
Rhodes CA, Dinan D, Jafri SZ, Howells G, McCarroll K (2005) Clinical outcome of active extravasation in splenic trauma. Emerg Radiol 11:348–352
Murphy DJ, Aghayev A, Steigner ML (2018) Vascular CT and MRI: a practical guide to imaging protocols. Insights Imaging 9:215–236
Baliyan V, Verdini D, Meyersohn NM (2018) Noninvasive aortic imaging. Cardiovasc Diagn Ther 8:S3–18
Shyu JY, Khurana B, Soto JA, Biffl WL, Camacho MA, Diercks DB, Glanc P, Kalva SP, Khosa F, Meyer BJ, Ptak T (2020) ACR appropriateness criteria® major blunt trauma. J Am Coll Radiol 17(5):S160–S174
Hamilton JD, Kumaravel M, Censullo ML, Cohen AM, Kievlan DS, West OC (2008) Multidetector CT evaluation of active extravasation in blunt abdominal and pelvic trauma patients. Radiographics 28(6):1603–1616
Anderson SW, Lucey BC, Rhea JT, Soto JA (2007) 64 MDCT in multiple trauma patients: imaging manifestations and clinical implications of active extravasation. Emerg Radiol 14:151–159
Stuhlfaut JW, Lucey BC, Varghese JC, Soto JA (2006) Blunt abdominal trauma: utility of 5-minute delayed CT with a reduced radiation dose. Radiology 238(2):473–479
Anderson SW, Varghese JC, Lucey BC, Burke PA, Hirsch EF, Soto JA. ;243(1):88–95. Fingar SC, Weiss, Steiner AJ (2007) Blunt splenic trauma: delayed-phase CT for differentiation of active hemorrhage from contained vascular injury in patients. Radiology CA. Most frequent operating room procedures performed in U.S. hospitals, 2003–2012: statistical brief #186. Healthcare cost and utilization project (HCUP) statistical briefs Rockville (MD): agency for healthcare research and quality (US); 2006
Fingar SC, Weiss, Steiner AJ (2007) Blunt splenic trauma: delayed-phase CT for differentiation of active hemorrhage from contained vascular injury in patients. Radiology CA. Most frequent operating room procedures performed in U.S. hospitals, 2003–2012: statistical brief #186. Healthcare cost and utilization project (HCUP) statistical briefs Rockville (MD): agency for healthcare research and quality (US); 2006
Park JH (2014) Diagnostic imaging utilization in cases of acute appendicitis: multi-center experience. J Korean Med Sci 29:1308–1316
Soto JA, Anderson SW (2012) Multidetector CT of blunt abdominal trauma. Radiology 265:678–693
Alagic Z, Eriksson A, Drageryd E, Motamed SR, Wick MC (2017) A new low-dose multiphase trauma CT protocol and its impact on diagnostic assessment and radiation dose in multi-trauma patients. Emerg Radiol 24:509–518
Yaniv G, Portnoy O, Simon D, Bader S, Konen E, Guranda L (2013) Revised protocol for whole-body CT for multi-trauma patients applying triphasic injection followed by a single-pass scan on a 64-MDCT. Clin Radiol 68:668–675
Kim SJ, Ahn SJ, Choi SJ, Park DH, Kim HS, Kim JH (2019) Optimal CT protocol for the diagnosis of active bleeding in abdominal trauma patients. Am J Emerg Med 37(7):1331–1335
Pouw ME, Albright JW, Kozhimala MJ, Baird GL, Nguyen VT, Prince EA, Scappaticci AA, Ahn SH (2022) Adding non-contrast and delayed phases increases the diagnostic performance of arterial CTA for suspected active lower gastrointestinal bleeding. Eur Radiol 32(7):4638–4646
Kim JW, Shin SS, Yoon W et al (2011) Diagnosis of acute gastrointestinal bleeding: comparison of the arterial, the portal, and the combined set using 64-section computed tomography. J Comput Assist Tomogr 35:206–211
National Research Council of the National Academies (2006) Health risks from exposure to low levels of ionizing radiation-BEIR VII phase 2. Committee to assess health risks from exposure to low levels of ionizing radiation. National Academies, Washington, DC
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khdhir, M., Ghosn, Y., Jabbour, Y. et al. Does delayed phase imaging in CT angiography provide additional information in patients with suspected active bleeding?. Emerg Radiol 31, 439–446 (2024). https://doi.org/10.1007/s10140-024-02239-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-024-02239-9