Abstract
Acute small bowel ischemia is a life-threatening condition with a high mortality rate due to its lack of specific symptoms and laboratory profile, which render difficulty in establishing early diagnosis. The etiology of acute small bowel ischemia includes occlusive forms (arterial embolism, arterial thrombosis, and venous thrombosis) and nonocclusive mesenteric ischemia, of which arterial causes are far more common than venous causes. CT, the mainstay of accurate diagnoses, allows the identification of the features of vascular abnormalities and intestinal ischemic injuries, and helps clinicians to restore intestinal blood flow. Without treatment, the prognosis for acute small bowel ischemia is poor. A high index of suspicion and familiarity with the CT spectral findings of bowel ischemia are required to ensure rapid recognition of this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
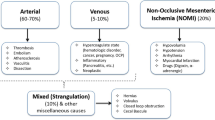
Acute small bowel ischemia (ASBI) is an abdominal emergency accounting for about 2% of gastrointestinal illness. Its incidence is increasing with the aging of the population and the consequential growing incidence of degenerative vascular diseases [1]. ASBI is a complicated disorder caused by interruption of the vascular flow to the small bowel, and it has occlusive and nonocclusive forms.
The mortality rate of ASBI is high. This is partly due to delays in its diagnosis, which is typically made at the irreversible stage of intestinal insult. Prompt diagnosis is critical when mesenteric ischemia is suspected as intestinal ischemia can progress to lethal infarction in the absence of timely diagnosis and treatment [2, 3].
Unlike chronic mesenteric ischemia, ASBI treatment is mainly surgical. This is because of the emergent need for revascularization combined with an assessment of bowel viability [1,2,3]. Before proceeding with surgical management, it is crucial to establish the correct etiology as ASBI comprises a complex of diseases, including acute arterial mesenteric ischemia, acute venous mesenteric ischemia, nonocclusive mesenteric ischemia (NOMI), and ischemia/reperfusion injury.
Although angiography is considered the gold standard investigation to directly assess mesenteric vascularity, it is invasive and often unavailable [1,2,3]. Computed tomography (CT) has become the mainstay for the establishment of diagnosis, with a high expectation of demonstrating early findings of bowel ischemia. CT imaging also provides a roadmap to aid clinicians with the restoration of intestinal blood flow as rapidly as possible.
The vascular and nonvascular CT findings of ASBI have been well documented, with the latter group comprising both bowel and mesenteric findings. However, pitfalls of diagnosis frequently occur as ASBI findings overlap with those of infection, inflammation, mechanical obstruction, and malignancy [1,2,3]. This article summarizes the vascular anatomy, classification of acute mesenteric ischemia, clinical manifestations, and CT protocol, with an emphasis on the CT findings, pearls, and pitfalls.
Response time is critical
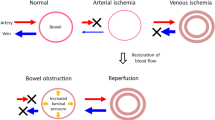
“Time to surgery” is the most important prognostic factor as mortality significantly increases with the duration of symptoms. Ritz et al. determined that the survival rate in the first 12 h was 84.3%, compared with only 11.6% after 24 h and 2% after 48 h [4]. In response to this serious emergency condition, all patients with a high index of clinical suspicion of ASBI should be assessed via contrast-enhanced CT. Close cooperation between the acute care medical teams, radiologists, and surgeons is of the utmost importance. The utilization of a “mesenteric ischemic stroke code” (MISC), similar to the coding used for neurological strokes, would assist in conveying the urgency attached to the condition and emphasizing the need for its emergency treatment. Radiologists play a vital role in establishing or confirming a diagnosis of ASBI (Fig. 1).
Clinical manifestation
ASBI frequently occurs in older patients (with a mean age of 70 years) and hospitalized patients with comorbidities [2]. In younger populations, it is secondary to underlying diseases such as vasculitis, collagen vascular disease, and hypercoagulability [3]. Elevation of serum lactase or serum amylase levels is also not specific, often arising late in the course of the disease. The clinical symptoms are nonspecific and include vague abdominal pain, nausea, vomiting, and diarrhea. Drastic or severe abdominal pain plus forceful bowel evacuation is suggestive of arterial occlusive disease [5]. The abdominal pain in venous mesenteric ischemia is more indolent (a few days to months) [1, 3]. Findings that suggest late or irreversible bowel ischemia include abdominal guarding and rigidity, absent bowel sounds, and hematochezia.
Pearls
-
There is a discrepancy between subjective pain and objective tenderness; this relates to the timeline of the ischemic process (Fig. 2a,b).
-
The nonspecific symptoms of ASBI often mislead clinicians to diagnose patients with other conditions, such as infection, inflammation, mechanical obstruction, and malignancy. Therefore, a high index of suspicion on the part of clinicians and radiologic findings are crucial to making a prompt diagnosis of mesenteric ischemia.
a and b Nonspecific symptom of ASBI. a In early or non-transmural small bowel ischemia, the inner mucosa is affected first. While subjective pain can be intense, tenderness upon palpation is minimal because the outer layer obscures or masks palpation of the ischemic mucosa. b In transmural small bowel ischemia, all layers are involved; therefore, there is concordance between the subjective pain and the marked pain at palpation. Dashed circle, shaded area, inner solid circle, and the big outer circle refer to inner mucosa, submucosa, serosa, and abdominal wall, respectively. The arrows mean radiating pain. c and d Location of arterial obstruction. c Drawing shows a clot (arrowhead) proximal to MCA and RCA; there is a right-sided colonic involvement in addition to a small bowel ischemia. d Drawing of a clot (arrowhead) distal to MCA and RCA, showing a small bowel ischemia and no colonic involvement. Darker bowel segment represents bowel ischemia. Abbreviations: SMA, superior mesenteric artery; RCA, right colic artery; MCA, middle colic artery
Vascular anatomy of small bowel
The major splanchnic arteries originating from the abdominal aorta are the celiac artery, superior mesenteric artery (SMA), and inferior mesenteric artery. The SMA arises from the abdominal aorta and courses downward to the right lower abdomen, ending as the ileocolic artery. The multiple branches of the SMA comprise the inferior pancreaticoduodenal artery, the jejunal and ileal arteries, and the right and middle colic arteries. In the case of arterial flow reduction, flow compensation occurs via different pathways [6]. Collateralization between the celiac artery and the SMA is primarily via the pancreaticoduodenal and gastroduodenal arteries, and less commonly via the arc of Barkow (an anastomosis between the right and left gastroepiploic arteries) or the arc of Buhler (a persistent communication between the embryonic ventral segmental arteries) [5,6,7]. The superior mesenteric vein (SMV) and inferior mesenteric vein drain blood from the veins within their corresponding arterial territories.
Pearls
-
Involvement of the middle colic artery and vein, and of the right colic artery and vein, are hallmarks to determine the extent of involvement in arterial and venous small bowel ischemia. This explains why there is right-sided colonic involvement in ASBI (Fig. 2c, d).
Bowel wall thickness (through thick and thin)
The normal thickness of the bowel wall ranges from 3 to 5 mm, depending on the degree of bowel distension. The small bowel involvement in the ischemic process is typically segmental, and it is either short (about 10–30 cm) or long (more than 30 cm) [4, 8, 9].
Bowel wall thickening is the commonest finding of small bowel ischemia. It is more common in venous ischemia than arterial ischemia due to venous stasis resulting in edema and hemorrhage [8]. The thickening is typically circumferential, homogeneous, and uniform, and it is depicted as a halo or target sign (increased enhancement of the mucosa and serosa surrounding a submucosal, low-attenuating, edematous layer). Bowel wall thickening can be observed in patients with ischemia caused by NOMI, a strangulating obstruction, and arterial ischemia with a reperfusion injury. The presence and degree of bowel wall thickening does not correlate with the severity of ischemia [10].
Bowel wall thinning is a common feature of small bowel arterial ischemia. The bowel wall can become markedly thin or severely thin (a paper-thin appearance or a vanishing bowel wall). This finding results from a loss of arterial blood flow to the bowel wall mucosa, which can progress to transmural infarction.
The use of oral or rectal water, or a neutral contrast, is frequently avoided due to the extra time involved and the urgency associated with an acute bowel ischemia. An under-distended bowel is the most challenging situation when evaluating bowel wall thickening as it may lead to false-positive cases. Occasionally, normal peristalsis can mimic bowel wall thickening. Assessing the bowel wall enhancement can overcome this situation because an abnormal enhancement is associated with an abnormal bowel wall thickness in almost all cases of small bowel ischemia. The abnormal enhancement can be increased, decreased, or absent. Due to the decreased vascular flow of the bowel, a thin bowel wall is commonly associated with a decreased or absent bowel wall enhancement [4, 9,10,11].
One of the key features of the occlusive form of ASBI is that the affected segments of the bowel correspond with its vascular distribution. An abrupt interface between the normal and involved bowel segment may be seen (Fig. 3a).
a A 56-year-old man with severe abdominal pain. An axial contrast-enhanced CT shows intimal flap in abdominal aorta (open arrowheads) extending into proximal superior mesenteric artery, causing luminal narrowing. Dilated jejunal loop with air fluid level (star), absent bowel wall enhancement, mural wall thinning, and pneumatosis intestinalis (arrow) are consistent with transmural small bowel necrosis. There is clear demarcation (solid arrowhead) between the infarcted bowel and normal bowel wall enhancement. b A 61-year-old man with sudden abdominal pain. Coronal contrast-enhanced CT shows the cut off sign, seen as complete segmental arterial occlusion by emboli (white arrowheads). Dilated ileal loop with bowel wall mural thinning and marked decreased wall enhancement (arrows). Normal enhancement of the adjacent small bowel loop is shown (black arrowheads). Surgical embolectomy of the SMA and intestinal resection were performed
Imaging modalities
As patient management is imaging-based, prompt imaging diagnosis of ASBI is required for treatment planning. The gold standard of investigation is catheterization angiography, but it is invasive. Plain radiographs have limited diagnostic value in the evaluation of ASBI, although signs of intestinal perforation may be detected. Ultrasonography is operator-dependent and difficult to perform in patients with ASBI due to abundant intestinal meteorism and bowel gas artifacts. Moreover, magnetic resonance imaging is not suitable in emergency conditions owing to its long scan time. Contrast-enhanced CT is the mainstay of diagnosis, having excellent reported sensitivity and specificity [12], and it should be utilized as the first-line imaging technique. A CT scan should be performed as swiftly as possible after the onset of symptoms. Compared to ultrasound and magnetic resonance imaging, CT is particularly advantageous because its rapid scan time obviates bowel motion artifacts, it provides an excellent field of view and optimal patient tolerance, and there are few gas artifacts [7, 12].
The disadvantages of CT include radiation exposure, nephrotoxicity, and adverse reactions to intravenous contrast material. CT imaging of ASBI changes is widely dependent upon numerous factors such as underlying physiopathology; the severity of the ischemia (i.e., superficial mucosal or transmural bowel wall necrosis); and the duration and severity of the ischemic event (i.e., only the small bowel, or both the small and large bowel) [13]. Details of the CT protocol are presented in Table 1.
Pearls
-
CT is a powerful imaging tool that provides direct visualization of the mesenteric vasculature, intestines, and mesentery.
-
CT outperforms laboratory and physical examination findings in the detection of bowel ischemia [8].
-
Use of the portal venous phase alone is not recommended due to a lower rate of diagnosis [9].
Subtypes of ASBI
The subtypes of ASBI are arterial occlusive disease (mesenteric arterial embolism and thrombosis), venoocclusive disease, and strangulating bowel obstruction and nonocclusive mesenteric ischemia; each may illustrate specific imaging features.
Acute arterial mesenteric ischemia
Mesenteric arterial embolism
Mesenteric arterial emboli usually originate in the heart or aorta and then float before lodging to form an arterial occlusion. The SMA is the most affected artery because of its more vulnerable takeoff angle from the aorta, compared with the celiac and inferior mesenteric arteries [14]. The two vascular signs depend on the extent of the occlusion [2]. The cut-off sign refers to the abrupt termination of contrast in the vessel due to acute occlusive emboli in the distal branch (Fig. 3b), while the ring sign refers to an eccentric filling defect in the vascular lumen due to nonocclusive emboli. Typically, no collateral vessels are associated with either sign due to the acute status of the condition. SMA enlargement proximal to the emboli and a decreased SMV diameter that is secondary to reduced venous return can be observed (SMV/SMA diameter ratio < 1) [2]. Due to the sudden nature of the condition and the lack of collateral pathways, patients tend to have severe abdominal pain that is worse than that experienced with an arterial thrombosis [15].
Vascular imaging findings
-
Location of emboli: usually 6–10 cm beyond the SMA ostium, near the origin of the middle colic artery [16].
-
The proximal branches (such as the inferior pancreaticoduodenal artery) and the jejunal arteries are preserved in most patients [14, 15].
-
Smaller emboli may affect only the small distal branches. Small showering emboli in the small distal branches are difficult to identify. In this setting, helpful clues—such as infarction of solid organs like the spleen and kidneys—may be suggestive of small, nonocclusive emboli [10, 11].
Mesenteric arterial thrombosis
The most common cause is rupture of an unstable atherosclerotic plaque. Therefore, the typical location of an SMA thrombosis is proximal, near the ostium [16, 17]. It is the leading cause of ASBI in patients older than 70 years [18]. Unlike arterial embolic ischemia, collateral pathways can be observed in these patients because they have underlying atherosclerotic disease, and previous symptoms and signs of chronic mesenteric ischemia (for instance, weight loss, food fear, and postpandrial pain [abdominal angina]). An uncommon cause of arterial thrombosis is SMA dissection, which can arise from a continuation of aortic dissection or in isolation. Bowel ischemia may develop if the SMA arises from the thrombosed false lumen of an aortic dissection [19].
Vascular imaging findings
-
The severity and extent of the ischemic injury are determined by the degree of collateral pathway, systemic blood pressure, and degree of arterial narrowing.
-
The cross-sectional area of the artery is usually narrowed by 50–80% before symptoms appear [20].
-
If collateral circulation is sufficient, transmural bowel necrosis or infarction may not develop, even with occlusion of the main SMA [21].
-
Apart from atherosclerotic disease, a patient may have a history of coronary, cerebrovascular, or peripheral arterial disease.
Chronological bowel findings in arterial mesenteric ischemia
Early phase
The affected small bowel loop is contracted or collapsed owing to spastic reflex ileus in response to the ischemic damage, with diminished or poor bowel enhancement (pale ischemia) [22].
Intermediate phase
The small bowel evolves from spastic reflex ileus into hypotonic ileus. The small bowel loops appear distended and are gas-filled [22, 23]. Blood and fluid continue to drain via the venous system, and the bowel wall becomes thin [22,23,24]. Peritoneal fluid is identified due to the increased hydrostatic pressure inside the bowel loops, which allows extravasation of plasma, and to the peritoneal reaction to the ischemic injury [23]. Diminished or poor bowel enhancement can be identified [23, 24].
Late phase
Hypotonic ileus evolves into paralytic ileus with more distended loops and air fluid levels [25]. The paper-thin wall appearance which is typically found is caused by extreme thinning of the bowel wall, the inadequate vascular flow, and decreased muscular tone [22, 25]. The presence of segmental mesenteric fat stranding and free fluid in the leaves of the mesenteric folds is associated with either poor or absent enhancement of the bowel wall; it is highly suggestive of transmural infarction [21,22,23,24]. Without appropriate management, the ischemia rapidly changes into infarction. Pneumatosis intestinalis and portomesenteric venous gas can be observed in transmural infarction. Perforation and peritonitis are high-mortality complications of bowel infarction [21,22,23,24].
Pitfalls
-
It is sometimes difficult to differentiate between a thinned bowel wall and a decreased/absent bowel wall enhancement. In practice, bowel wall thinning is frequently associated with decreased/absent bowel wall enhancement. Occasionally, a bowel loop is difficult to distinguish, blending into the adjacent mesenteric fluid [26].
-
Bowel wall thickening (up to 15 mm) is most frequently observed in reversible mesenteric arterial ischemia due to intramural hemorrhage, edema, or superimposed infection [27].
-
Paradoxical hyperenhancement of the bowel wall is a finding in the reperfusion phase after a bowel ischemic injury [21,22,23] resembling shock bowel.
-
A stratified bowel wall enhancement, or a target sign implying enhancement of the mucosa and serosa with central nonenhancing low edematous submucosa, can also be seen in arterial occlusion with reperfusion. However, this finding can be identified in NOMI and venous ischemia [22, 25,26,27].
Acute mesenteric venous ischemia
Venous occlusion accounts for 5–15% of ASBI cases and frequently occurs in younger patients [14]. The symptoms of mesenteric venous ischemia secondary to impaired venous drainage are less acute than those of mesenteric arterial ischemia, but the CT findings are more conspicuous and striking [28]. Mesenteric venous occlusion can be seen in patients with a mechanical obstruction and venous thrombosis [28, 29]. Venous circulation can be compromised in association with bowel strangulation, which is typically identified in a volvulus, intussusception, or a closed loop obstruction. Venous thrombosis may be associated with many conditions. They include hypercoagulability (such as antiphospholipid antibody syndrome); protein C/S deficiency; oral contraceptive consumption; and vasculitis (like systemic lupus erythematous) resulting in occlusion or thrombosis of the small, intramural veins [14, 29]. The mesenteric venous ischemia is not frequently associated with postpandrial syndrome, despite the presence of abdominal distension, bloating, fever, and positive stool occult blood [29]. Thrombosis from other local conditions—for example, inflammatory or infectious bowel disease, pancreatitis, and portal hypertension [30]—may uncommonly cause mesenteric ischemia secondary to a slow retrograde distal progression that allows collateral veins to form [29, 30].
Vascular imaging findings
-
Acute thrombus may show high attenuation in noncontrast-enhanced CT. It is associated with an expansion, or increased caliber, of the mesenteric vein (Fig. 4a).
-
Thrombus is seen as a rounded or tubular, low-attenuating, filling defect in contrast-enhanced CT. This feature is highly specific (94–100%) [31, 32].
-
An isolated SMV or proximal mesenteric venous thrombosis is generally not sufficient to cause bowel ischemia as there are collateral pathways between the mesenteric and systemic veins.
-
The more distal the thrombosis is, the fewer the collateral venous channels are, and the greater the risk of ischemia is [33].
-
Progression of the thrombosis and insufficient collateral circulation result in infarction of the small bowel.
-
Mesenteric venous engorgement is typically shown due to venous outlet obstruction.
a and b A 37-year-old woman with venous small bowel ischemia. a Axial noncontrast-enhanced CT shows fresh thrombus in the mesenteric vein at jejunal mesentery (arrows) and mesenteric fat streakiness (arrowheads). b Axial contrast-enhanced CT shows occlusive mesenteric venous thrombosis (arrowheads). Variable thickening of jejunal loops (arrows) are detected. c and d A 62-year-old man with transmesenteric hernia. c Axial noncontrast-enhanced CT shows dilated cluster small bowel loops at the right-side abdominal cavity with a C-shaped distribution (arrowheads). An area of intramural hemorrhage with intraluminal hemorrhage and mesenteric stranding are identified (arrow). d Coronal-enhanced CT shows defect at small bowel mesentery (black arrowheads) and cluster of ileal loops (white arrowhead). Proximal small bowel dilatation is seen (arrow). A strangulated bowel obstruction and ischemia from a transmesenteric hernia were found during the surgery
Chronological bowel findings in venous mesenteric ischemia
-
Early phase: In contrast to the paper-thin appearance of the bowel wall in the case of arterial ischemia, venous occlusive disease frequently results in circumferential bowel wall thickening (up to 1.5 cm) [33]. Submucosal edema between the enhancing mucosa and serosa gives rise to a target or halo appearance. Mesenteric stranding and fluid are more often seen in patients with venous ischemia than arterial ischemia due to extravasation of the fluid into the mesentery (Fig. 4b).
-
Late phase: Prolonged impaired venous drainage ultimately results in a loss of incoming arterial flow, causing bowel ischemia or infarction with a diminished or absent bowel wall enhancement that resembles arterial ischemia [32, 33].
-
The enhancement of the bowel is related to the duration of the venous obstruction. In early obstruction, small bowel wall hyper-enhancement is evident because only the venous flow is compromised. By comparison, the arterial flow in late obstruction is affected, causing a diminished or absent wall enhancement [32, 33].
Strangulating bowel obstruction
Strangulation is encountered in patients with a closed-loop obstruction, which is defined as a segment of bowel that obstructs proximally and distally at a single point. This condition occurs in approximately 10% of small bowel obstructions [34, 35]. It is most frequently caused by adhesive bands and, occasionally, by internal or external hernia [34] (Fig. 4c,d). It is considered a venous form of acute mesenteric ischemia. Closed loop obstruction impairs venous outflow first followed by arterial inflow because of arterial pressure is higher than venous pressure.
Initially, there is venous return compromise that raise the intravascular pressure [34,35,36]. With the continuing influx of arterial blood, there is engorgement and distension of the mural vessels. This can cause hemorrhage into the bowel wall and bowel lumen as well as fluid shifting across the serosa, leading to mesenteric fluid and ascites. The lack or absence of vascular flow is the final event, which is the result of increased pressure from interstitial hemorrhage, anoxia, and capillary stasis under pressure from the venous side [35, 36].
Closed loop obstruction appears in CT as C- or U-shaped, fluid-filled, distended loops (balloons with a string appearance), with fan-shaped mesentery and mesenteric vessels converging toward the site of the obstruction.
Nonocclusive mesenteric ischemia
NOMI is the form of mesenteric ischemia without mesenteric artery and venous occlusion that develops in the area of a bowel necrosis [36]. It accounts for 20–30% of all cases of mesenteric ischemia [36, 37]. Regarding its pathogenesis, intestinal vasospasm due to consistently low perfusion is believed to be the cause of the ischemic disorder [37]. It commonly occurs in patients older than 50 years who have conditions that decrease cardiac output, for example, myocardial infarction, congestive heart failure, aortic insufficiency, hepatic or renal diseases, and septic or hemorrhagic shock, a profound decrease in systemic blood pressure, or severe volume depletion [36, 37]. NOMI is frequently associated with worsened outcomes and high mortality rates, which reach 58–70% [1]. From a pathological point of view, the ischemic injury may range from reversible superficial damage localized to the watershed areas, to a more severe form that extends to the entire bowel [36, 37]. Recognition of NOMI is important as the treatment approach may differ from that for occlusive ischemia. Medical treatment by vasoactive substances has gained a role, whereas surgery is only performed for advanced forms of NOMI with necrosis of the involved tract due to an ineffective reperfusion event [36, 37].
Vascular imaging findings
-
Because the reduction of cardiac output can affect the SMA and the inferior mesenteric artery, all collateral vascular channels are ineffective.
-
The diagnostic criteria include an absence of vascular occlusion in both the mesenteric artery and the venous system.
-
There is a patent SMA trunk with diffuse, small-sided branches which cannot be seen within 3 cm distal of the mesentery (Fig. 5) [38].
-
With advancements in multidetector-row CT imaging, findings previously described on angiography can also be demonstrated on CT. These include the “string-of-sausages” sign, which refers to an alternating stenosis and dilatation of the SMA branches; intestinal marginal artery spasm; and a reduction of the veins in the muscular layer [38].
A 77-year-old man with septic shock with NOMI. Coronal contrast-enhanced CT shows a dilated small bowel loop with luminal fluid with interrupted bowel wall enhancement (stars) and markedly decreased bowel wall enhancement (thick arrow), mesenteric fluid, nicely shown portomesenteric venous gas (thin arrows), and small-sized or constricted mesenteric arterial branches (arrowheads)
Chronological bowel findings in NOMI
-
The evolution of the bowel findings is similar to those for mesenteric arterial ischemia (if the systemic blood pressure is not restored, or if there is a persistently inadequate cardiac output).
-
The bowel wall shows a reduction of enhancement. Thinning of both the small and large bowel is a significant clue (the decreased flow affects both the SMA and the inferior mesenteric artery).
-
In cases of restored blood pressure, bowel wall hemorrhage and bowel wall edema may be observed. The two conditions result from extravasation into the damaged bowel wall of red blood cells and plasma, respectively.
Pitfalls
-
CT findings of NOMI may overlap with other small bowel diseases, such as infectious or inflammatory enteritis.
-
The bowel wall enhancing pattern in NOMI is quite variable, comprising absent, decreased, or increased enhancements (Fig. 6) [39].
A 68-year-old man with septic shock and acute small bowel ischemia. a Axial contrast-enhanced CT shows multiple patterns of enhancement at small bowel. Areas of absent enhancement (arrows) and decreased enhancement (white arrowheads), and a few areas of increased enhancement (black arrowhead). Ascites is seen. b A difference in the extent of the small bowel ischemia from a mild (arrowhead) to a severe degree (arrow) was found in the surgical theater
Important factors associated with prognosis
Ischemia/reperfusion injury
The primary insult resulting from an ischemic injury can be repaired by reperfusion, meaning the re-establishment of the normal mesenteric blood supply after an ischemic attack.
If the process which initiated the ischemia is corrected before irreversible alterations occur, the oxygen return allows for the reestablishment of energetic metabolism, the removal of toxic products, and the progressive return of normal cell functions [40].
However, the ischemic injury may be worsened in the event of an ineffective reperfusion [40]. This entity is known as an ischemia/reperfusion injury (IRI) [41, 42]. An IRI of the intestine is associated with a high morbidity and mortality. It is more often seen in cases of NOMI than occlusive arterial or venous mesenteric ischemic conditions. It may be the consequence of the restored blood pressure after the low-flow state, or the result of revascularization after occlusive mesenteric ischemia (Fig. 7).
Tissue damage due to alterations in the mesenteric blood flow frequently results from cellular injuries. These injuries are associated with reperfusion: the tissue lesions produced during the reperfusion were greater than those produced during the ischemia [41, 42].
Although cell structures are progressively damaged during ischemia, the lesions caused by the ischemia are, paradoxically, intensified by the restoration of the blood flow. The mechanisms of ischemia injury and reperfusion have not been completely defined. A cascade of injuries may occur, such as an acute inflammatory reaction due to the production of reactive metabolites [43] and active neutrophils. Numerous studies have been undertaken in an effort to find an ideal therapy for IRI. Nevertheless, many cases still progress to shock, multiple organ failure, and death [44].
The CT finding of reperfusion is similar to those detected for venous ischemia [43]. The bowel affected by the reperfusion may have a different pattern, depending on the degree of microvascular wall damage. Extravasation of plasma, the contrast medium, and the presence of red blood cells in the bowel wall due to disrupted microvessels cause bowel wall thickening, increased bowel wall enhancement, and intramural hemorrhage, respectively (Fig. 8) [44]. Red blood cells and fluid may leak into the bowel lumen [43, 44]; this could even progress to necrosis of the entire bowel wall.
A 69-year-old man with acute arterial small bowel ischemia. a Axial noncontrast-enhanced CT shows segmental area of submucosal hemorrhage of ileal loop (arrowhead), with evidence of minimal hemoperitoneum due to red blood cell leakage from the ischemic bowel into the peritoneal cavity (arrow); band-like and cystic-like pneumatosis intestinalis (stars); and vascular calcifications. b Axial contrast-enhanced CT shows viable perfusion in the hemorrhagic bowel segment (arrowhead); normal segment of bowel (thin arrow); and dead bowel segment with no enhancement (thick arrow) (vanishing bowel wall). c Photograph of the gross surgical specimen after ileal resection shows transmural infarction with dark discoloration or hemorrhage of all layers
The consequences of intestinal IRI are many. One is an alteration of the absorptive function of the intestine, which is associated with significant morbidity [45]. There is also bacterial translocation, caused by the intestinal hyperpermeability; subsequently, bacteria disseminate throughout the body, producing sepsis, shock, or multiple organ failure. Moreover, there is injury to distant organs because the IRI of the intestines results in the production of molecules such as hydrogen peroxide, superoxides, and inflammatory cytokines, leading to the development of a systemic inflammatory response which can progress to multiple organ failure [44, 45]. Intestinal IRI also causes pulmonary infiltration of neutrophils, which contributes to the development of acute respiratory distress syndrome. Hence, the gut has been referred to as “the motor” of multiple organ failure [45].
Pearls
Paradoxically, restoration of blood flow to the ischemic tissue initiates a cascade of events that may lead to additional cell injury known as reperfusion injury. This reperfusion damage frequently exceeds the original ischemic insult [43,44,45]. On restoration of the blood supply, the molecular and biochemical changes that occur during ischemia predispose to free radical-mediated damage [45].
Pneumatosis intestinalis and portomesenteric venous gas
ASBI is considered an emergency condition if it is related to portomesenteric venous gas or pneumatosis intestinalis. Bowel infarction can cause bowel wall gas infiltration. CT has been shown to be more sensitive than plain radiography in detecting pneumatosis intestinalis and portomesenteric venous gas [44, 45]. Pneumatosis intestinalis is a frequent radiological sign of ASBI. Its early recognition is of high value because it permits immediate surgical treatment and appropriate medical supportive care, with the consequent prevention of necrosis [41, 43]. In ASBI, pneumatosis intestinalis usually appears as a linear or bubble pattern of intramural gas that is associated with other vascular, bowel, or mesenteric findings, for instance, decreased or absent bowel wall enhancement, thin bowel wall, dilated small bowel, ascites, and vascular occlusion. A linear or band-like pattern is frequently seen in transmural infarction, whereas a bubble-like pattern is more commonly observed in cases of partial mural ischemia. The bubbles are sometimes localized in the central part of the bowel, taking on a ray-like appearance termed the “kiwi sign” (Fig. 9).
a and b A 74-year-old man with acute small bowel ischemia. a Coronal contrast-enhanced CT shows a long segment of small bowel transmural infarction (arrows), portal venous gas (black arrowhead), and hepatic infarction (white arrowhead). The inset image nicely shows the kiwi sign. b A specimen from the surgical exploration confirmed an extensive small bowel infarction (arrows), with redness of the small bowel mesentery (arrowheads). c A 85-year-old man with severe abdominal pain. Axial contrast-enhanced CT shows dilatation with fluid-filled jejunum and evidence of pneumatosis intestinalis (white arrowhead), an area of decreased bowel wall enhancement (black arrowhead), stranding around the jejunum (thin arrow), and fluid. Mesenteric venous gas with air-contrast level is seen (thick arrow). Jejunal infarction was seen and resected in the surgical theater
Pneumatosis intestinalis is sometimes associated with portomesenteric venous gas. Portomesenteric venous gas is differentiated from aerobilia by its characteristic tubular branching lucencies that extend to the periphery of the liver, whereas biliary air is more central [43,44,45]. Pneumatosis intestinalis in ASBI is considered an indicator of a poor prognosis when related to advanced necrosis, particularly if it is associated with portomesenteric venous gas. Air-contrast level may be observed in the mesenteric vein (Fig. 9). Having analyzed 23 patients, Weisner et al. found that pneumatosis intestinalis and portomesenteric venous gas had mortality rates of 44 and 56%, respectively; however, when pneumatosis intestinalis and portomesenteric venous gas were present concurrently, the mortality rate climbed to 72% (15).
Pitfalls
-
Pneumatosis intestinalis can be seen in many other clinical conditions with both local bowel and systemic pathologies (Table 2; Fig. 10a–c).
-
There is a need to differentiate between portomesenteric venous gas and aerobilia (Fig. 10d).
-
Pneumatosis intestinalis may be overlooked when CT images are viewed at the standard abdominal window-level setting.
-
Gas is trapped between the bowel wall and the residual content in the lumen, and it is commonly seen at the dependent aspect of the bowel lumen. When in doubt, rescanning the patient in the prone position can be of use as pneumatosis intestinalis will not change position whereas debris in the bowel will. Occasionally, sagittal or coronal reformation may be helpful.
a and b Pneumatosis cystoides intestinalis. a Plain radiograph shows multiple gas pockets or round lucencies in the large bowel wall (arrows). b Lung window-setting CT abdomen shows multiple circular-form gas-filled cysts in the submucosa of the large bowel wall (arrowheads). The patient had no abnormal symptoms. c Pneumatosis intestinalis. Coronal contrast-enhanced CT shows hidebound sign (arrow) and bubbly bowel gas in the distal ileal wall (arrowheads). Minute pneumoperitoneum beneath the right hemidiaphragm. All were considered to be abdominal manifestations of scleroderma. d Aerobilia and portal venous gas. Axial contrast-enhanced CT shows portal venous gas (white arrowheads) and aerobilia (arrow). The two have different causes and implications, and they need to be differentiated via imaging. An area of hepatic infarction (black arrowhead) and pneumoperitoneum (asterisk) are also detected
Intestinal perforation
It is imperative that radiologists not only recognize the finding of bowel ischemia, but also differentiate it from transmural bowel necrosis with perforation, which requires emergency surgical treatment. The pathognomonic finding of bowel perforation is free intraperitoneal gas. In patients with intestinal perforation, patients need to be diagnosed and treated immediately.
Conclusions
The clinical and laboratory evaluations of ASBI are nonspecific. Understanding CT findings of ASBI including the related pearls and pitfalls will aid the formulation of definitive and timely diagnoses.
References
Gore RM, Thakrar KH, Mehta UK, Berlin J, Yaghmai V, Newmark GM (2008) Imaging in intestinal ischemic disorders. Clin Gastroenterol Hepatol 6:849–858. https://doi.org/10.1016/j.cgh.2008.05.007
Dhatt HS, Behr SC, Miracle A, Wang ZJ, Yeh BM (2015) Radiological evaluation of bowel ischemia. Radiol Clin North Am 53(6):1241–1254. https://doi.org/10.1016/j.rcl.2015.06.009
Costa AF, Chidambaram V, Lee JJ (2014) Multidetector computed tomography of mesenteric ischemia. Insights Imaging 5(6):657–666. https://doi.org/10.1007/s13244-014-0361-1
Ritz JP, Germer CT, Buhr HJ (2005) Prognostic factors for mesenteric infarction: multivariate analysis of 187 patients with regard to patient age. Ann Vasc Surg 19:328–334. https://doi.org/10.1007/s10016-005-0005-5
Martinez JP, Hogan GJ (2004) Mesenteric ischemia. Emerg Med Clin North Am 22:909–928. https://doi.org/10.1016/j.emc.2004.05.002
Rosenblum JD, Boyle CM, Schwartz LB (1997) The mesenteric circulation. Anat Physiol Surg Clin North Am 77(2):289–306. https://doi.org/10.1016/s0039-6109(05)70549-1
DiPoce J, Jimenez G, Weintraub J (2014) Historical perspective: eponyms of vascular radiology. Radiographics 34(4):1120–1140. https://doi.org/10.1148/rg.344130125
Schieda N, Fasih N, Shabana W (2013) Triphasic CT in the diagnosis of acute mesenteric ischaemia. Eur Radiol 23:1891–1900. https://doi.org/10.1007/s00330-013-2797-y
Hellinger JC (2004) Evaluating mesenteric ischemia with multidetector-row CT angiography. Tech Vasc Interv Radiol 7(3):160–166. https://doi.org/10.1053/j.tvir.2005.02.002
Jo PC, Cabral FC, Sahin A, Camacho A, Brook A, Brook OR (2018) Split-bolus single scan CTA for evaluation of mesenteric ischemia. Abdom Radiol (NY) 43:1368–1378. https://doi.org/10.1007/s00261-017-1333-y
Ha HK, Rha SE, Kim AY, Auh YH (2000) CT and MR diagnoses of intestinal ischemia. Semin Ultrasound CT MR 21:40–5. https://doi.org/10.1016/s0887-2171(00)90012-x
Menke J (2010) Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 256:93–101. https://doi.org/10.1148/radiol.10091938
Kim AY, Ha HK (2003) Evaluation of suspected mesenteric ischemia. Efficacy of radiologic studies. Radiol Clin N Am 41:327–342. https://doi.org/10.1016/s0033-8389(02)00075-1
Mazzei MA, Volterrani L (2015) Nonocclusive mesenteric ischaemia: think about it. Radiol Med 120(1):85–95. https://doi.org/10.1007/s11547-014-0460-6
Wiesner W, Khurana B, Ji H (2003) Ros PRCT of acute bowel ischemia. Radiology 226:635–650. https://doi.org/10.1148/radiol.2263011540
Horton KM, Fishman EK (2010) CT angiography of the mesenteric circulation. Radiol Clin North Am 48:331–345. https://doi.org/10.1016/j.rcl.2010.02.004
Wasnik A, Kaza RK, Al-Hawary MM, Liu PS, Platt JF (2011) Multidetector CT imaging in mesenteric ischemia–pearls and pitfalls. Emerg Radiol 18:145–156. https://doi.org/10.1007/s10140-010-0921-8
Furukawa A, Kanasaki S (2009) Kono N CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol 192(2):408–416. https://doi.org/10.2214/AJR.08.1138
Horton KM, Fishman EK (2007) Multidetector CT angiography in the diagnosis of mesenteric ischemia. Radiol Clin North Am 45(2):275–288. https://doi.org/10.1016/j.rcl.2007.03.010
Catalini R, Alborino S, Giovagnoli A, Zingaretti O (2010) Color Duplex evaluation of the mesenteric artery. J Ultrasound 13(3):118–122. https://doi.org/10.1016/j.jus.2010.09.002
Chou CK, Mak CW, Tzeng WS, Chang JM (2004) CT of small bowel ischemia. Abdom Imaging 29(1):18–22. https://doi.org/10.1007/s00261-003-0073-3
Mazzei MA, Mazzei FG, Marrelli D, Imbriaco G, Guerrini S, Vindigni C et al (2012) Computed tomographic evaluation of mesentery: diagnostic value in acute mesenteric ischemia. J Comput Assist Tomogr 36(1):1–7. https://doi.org/10.1097/RCT.0b013e31823b4465
Romano S, Niola R, Maglione F, Romano L (2008) Small bowel vascular disorders from arterial etiology and impaired venous drainage. Radiol Clin North Am 46(5):891–908. https://doi.org/10.1016/j.rcl.2008.07.003
Hou CK, Mak CW, Tzeng WS, Chang JM (2004) CT of small bowel ischemia. Abdom imaging 29(1):18–22. https://doi.org/10.1007/s00261-003-0073-3
Romano S, Lassandro F, Scaglione M, Romano L, Rotondo A, Grassi R (2006) Ischemia and infarction of the small bowel and colon: spectrum of imaging findings. Abdom imaging 31(3):277–292. https://doi.org/10.1007/s00261-005-0376-7
Barrett T, Upponi S, Benaglia T, Tasker AD (2013) Multidetector CT findings in patients with mesenteric ischaemia following cardiopulmonary bypass surgery. Br J Radiol 86:20130277. https://doi.org/10.1259/bjr.20130277
Florim S, Almeida A, Rocha D, Portugal P (2018) Acute mesenteric ischaemia: a pictorial review. Insights Imaging 9(5):673–682. https://doi.org/10.1007/s13244-018-0641-2
Somma F, Berritto D, Iacobellis F, Landi N, Cavaliere C, Corona M et al (2013) 7T μMRI of mesenteric venous ischemia in a rat model: timing of the appearance of findings. Magn Reson Imaging 31(3):408–413. https://doi.org/10.2214/AJR.08.1138
Ozturk G, Aydinli B, Atamanalp SS, Yildirgan MI, Ozoğul B, Kısaoğlu A (2012) Acute mesenteric ischemia in young adults. Wien Med Wochenschr 162(15–16):349–353. https://doi.org/10.1007/s10354-012-0120-1
Kumar S, Sarr MG, Kamath PS (2001) Mesenteric venous thrombosis. N Engl J Med 345:1683–1688. https://doi.org/10.1056/NEJMra010076
Duran R, Denys AL, Letovanec I, Meuli RA, Schmidt S (2012) Multidetector CT features of mesenteric vein thrombosis. Radiographics 32:1503–1522. https://doi.org/10.1007/s13244-014-0361-1
Yikilmaz A, Karahan OI, Senol S, Tuna IS, Akyildiz HY (2011) Value of multislice computed tomography in the diagnosis of acute mesenteric ischemia. Eur J Radiol 80(297):302. https://doi.org/10.4329/wjr.v6.i5.130
Kirkpatrick ID, Kroeker MA, Greenberg HM (2003) Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology 229:91–98. https://doi.org/10.1148/radiol.2291020991
Furukawa A, Kanasaki S, Kono N, Wakamiya M, Tanaka T, Takahashi M, Murata K (2009) CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol 192(2):408–16. https://doi.org/10.2214/ajr.142.3.555
Chou CK (2016) CT manifestations of small bowel ischemia due to impaired venous drainage-with a correlation of pathologic findings. Indian J Radiol Imaging 26(3):342–351. https://doi.org/10.4103/0971-3026.190426
Woodhams R, Nishimaki H, Fujii K, Kakita S, Hayakawa K (2010) Usefulness of multidetector-row CT (MDCT) for the diagnosis of non-occlusive mesenteric ischemia (NOMI): assessment of morphology and diameter of the superior mesenteric artery (SMA) on multi-planar reconstructed (MPR) images. Eur J Radiol 76(1):96–102. https://doi.org/10.1016/j.ejrad.2009.05.012
Wiesner W, Mortele KJ, Glickman JN, Glickman JN, Ji H, Ros PR (2001) Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol 177(6):1319–1323. https://doi.org/10.2214/ajr.177.6.1771319
Wildermuth S, Leschka S, Alkadhi H (2005) Marinecek B Multislice CT in the pre- and postinterventional evaluation of mesenteric perfusion. Eur Radiol 15:1203–1210. https://doi.org/10.1007/s00330-005-2654-8
Chou CK (2002) CT manifestations of bowel ischemia. AJR Am J Roentgenol 178(1):87–91. https://doi.org/10.2214/ajr.178.1.1780087
Kernagis LY, Levine MS, Jacobs JE (2003) Pneumatosis intestinalis in patients with ischemia: correlation of CT findings with viability of the bowel. AJR Am J Roentgenol 180(3):733–736. https://doi.org/10.2214/ajr.180.3.1800733
Mallick IH, Yang W, Winslet MC (2004) Seifalian AM Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci 49:1359–1377. https://doi.org/10.1023/b:ddas.0000042232.98927.91
Cerqueira NF, Hussni CA, Yoshida WB (2005) Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras 20:336–343. https://doi.org/10.1590/s0102-86502005000400013
Ho LM, Paulson EK, Thompson WM (2007) Pneumatosis intestinalis in the adult: benign to life- threatening causes. AJR Am J Roentgenol 188(6):1604–1613. https://doi.org/10.2214/AJR.06.1309
Pierro A (2004) Eaton S Intestinal ischemia reperfusion injury and multisystem organ failure. Semin Pediatr Surg 13:11–17. https://doi.org/10.1053/j.sempedsurg.2003.09.003
Na SY, Kim KJ, Yang DH (2011) Pneumoperitoneum in a patient with ulcerative colitis after sigmoidoscopy: is this always an indication for surgery? Infamm Bowel Dis 17(6):E54-56. https://doi.org/10.1002/ibd.21704
Acknowledgements
The authors gratefully acknowledge the Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, for the contribution to this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srisajjakul, S., Prapaisilp, P. & Bangchokdee, S. Comprehensive review of acute small bowel ischemia: CT imaging findings, pearls, and pitfalls. Emerg Radiol 29, 531–544 (2022). https://doi.org/10.1007/s10140-022-02028-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-022-02028-2