Abstract
Tetraploid induction by inhibiting mitosis I with heat shock (32, 35, and 38°C), cold shock (1, 4, and 7°C), and nocodazole (0.02 to 1.6 mg/L) was investigated in the hard clam Mercenaria mercenaria. All treatments were applied to fertilized eggs about 5 min before the first cell division at 22 to 23°C, and lasted for 10, 15, and 20 min. Three replicates were produced for each treatment with different parents. The ploidy of resultant larvae and juveniles was determined with flow cytometry. Heat shock of 35 and 38°C was effective in inhibiting mitosis I, producing 54% to 89% tetraploid larvae. Heat shock of 32°C accelerated embryonic development without inhibiting mitosis or producing tetraploids. In all heat-shock groups, the survival to D-stage larvae was lower than in controls, suggesting that heat-shock treatments and tetraploidy were detrimental to larval development. At the juvenile stage, survivors from heat-shock groups contained no tetraploids. Cold shocks suspended the first cell division during the treatment, but produced no tetraploids in the 4°C and 7°C treatment groups. Cold shock of 1°C produced 31% tetraploid larvae in one replicate, with none surviving to juvenile stage. Nocodazole inhibited mitosis I at concentrations of 0.04 mg/L or higher, but did not produce tetraploids. This study indicates that heat shock is most effective in inducing tetraploids through mitosis I inhibition, although none of the induced tetraploids survived to juvenile stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triploids are useful in aquaculture because of their superior growth, sterility, and sometimes improved meat quality (Guo, 2004). Commercial production of triploids is often dependent on the successful development of tetraploids, which produce 100% triploids by mating with diploids. Despite strong interest and repeated attempts, the induction of tetraploids remains a challenge in most molluscan species. So far, tetraploid stocks have been established in only a few species of Crassostrea oysters, using a unique method developed in the Pacific oyster Crassostrea gigas by Guo and Allen (1994), in which tetraploids were produced using eggs from triploids fertilized with haploid sperm and followed by inhibiting polar body 1 (PB1). The Guo–Allen method has led to the production of viable tetraploids in several other bivalve species, including the pearl oyster Pinctada martensii Dunker (He et al., 2000), eastern oyster Crassostrea virginica (Guo et al., 2002), Suminoe oyster Crassostrea ariakensis (Allen et al., 2003), and Catarina scallop Argopecten ventricosus (Maldonado et al., 2003).
However, the application of the Guo–Allen method is limited by the availability of eggs from triploids. Triploids show highly retarded gametogenesis in many species of molluscs such as the soft-shell clam Mya arenaria (Allen et al., 1986), noble scallop Chlamys nobilis (Komaru et al., 1988), hard clam Mercenaria mercenaria (Eversole et al., 1996), Sydney rock oyster Saccostrea commercialis (Cox et al., 1996), and blue mussel Mytilus edulis (Brake et al., 2004). In those species, triploid females produce few eggs, making it impossible to produce tetraploids using the Guo–Allen method. Therefore, other approaches to tetraploid induction that do not require eggs from triploids are still needed.
Tetraploid induction using normal eggs from diploids is possible, and several methods have been tested in molluscs, including meiosis I inhibition, mitosis inhibition, cell fusion, gynogenesis with meiosis inhibition (Beaumont and Fairbrother, 1991; Guo, 1991). Meiosis I inhibition (blocking polar body I) has led to the production of a few viable tetraploids in several species, including the blue mussel Mytilus galloprovincialis (Scarpa et al., 1993), Manila clams Tapes philippinarum (Allen et al., 1994), zhikong scallop Chlamys farreri (Yang et al., 2000), and dwarf surfclam Mulinia lateralis (Peruzzi and Guo, 2002; Yang and Guo, 2004). In all of these studies, only a few tetraploids (fewer than 10) were obtained, and no breeding populations of tetraploids were established. Mitosis inhibition, an effective method for tetraploid induction in finfish (Chourrout, 1984; Myers, 1986; Hershberger and Hostuttler, 2005), has been attempted in several molluscs without producing any viable tetraploids (Guo et al., 1994; Yang et al., 1997, 1999; Jiang et al., 1998; Chang et al., 2002; Yang and Guo, 2006). The tetraploid induction efficiency was low in most of those studies, and further research is needed to develop effective treatments and determine if viable tetraploids can be produced by mitosis I inhibition.
In this study, we tested tetraploid induction by inhibiting mitosis I with heat shock, cold shock, and nocodazole (a microtubule inhibitor) in the hard clam Mercenaria mercenaria, a major aquaculture species in the United States. The hard clam is a slow-growing species, and hard clam aquaculture may benefit from the development of triploid-tetraploid technologies (Eversole et al., 1996; Guo et al., 2001; Scarpa and El-Wazzan, 2005).
Materials and Methods
Parents and gametes
Parental hard clams were obtained from Dry Bay in Tuckerton, NJ. Before spawning the clams were maintained indoors at 22 to 23°C. To induce spawning, the clams were kept damp at 6 to 8°C overnight, and then individually placed in beakers with 28°C seawater and single-cell algae. Most clams spawned within 1 to 2 h. After spawning for 20 to 30 min, the clams were removed from beakers, and eggs were collected using a 25-μm screen and then resuspended in fresh seawater (22 to 23°C). Eggs were checked through a microscope for sperm contamination. Sperm were filtered through a 25-μm screen to remove tissue debris and feces, and checked through a microscope for motility before use.
Fertilization and Treatment
Eggs were fertilized by adding sperm into the egg suspension, and the time was recorded as the fertilization time (0 min post-fertilization, MPF). Sperm density was controlled and adjusted to about five to seven per egg. Fertilized eggs were allowed to develop in one container until the first polar body was released. To inhibit mitosis I, all treatments were applied when the majority of the eggs (about 60% to 70%) released both polar bodies and about to enter mitosis I, usually around 50 to 55 MPF at 23°C.
For heat and cold shocks, the fertilized eggs were separated into four groups before treatments; one group was used as control (at 23°C), and the other three for different temperature treatments: 32, 35, and 38°C for heat shocks and 1, 4, and 7°C for cold shocks. The heat and cold shocks were performed by collecting fertilized eggs on a 25-μm screen and immersing them in either preheated or cooled seawater. Each temperature treatment was applied for three durations: 10, 15, and 20 min. Treatments were ended by returning the fertilized eggs to fresh seawater at 23°C. Three replicates of the heat- and cold-shock experiments were produced using different sets of parents. One female and one male were used in each replicate.
Nocodazole treatment was applied to the fertilized eggs at final concentrations of 0.02, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 mg/L in the first trial, and 0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, and 0.18 mg/L in the second trial. Treatments were terminated by rinsing out nocodazole with a 25-μm screen, when 80% of fertilized eggs in control groups completed the first mitosis, and the fertilized eggs after treatment were returned to fresh seawater (23°C) for culturing.
Ploidy Determination
The ploidy of larvae and juvenile were determined using flow cytometry (FCM) with 4′,6-diamidino-2-phenylindole (DAPI) staining (10 μg/ml, dissolved in Tris buffer: 10 mM Tris, 146 mM NaCl, 2 mM CaCl2, 22 mM MgCl2, 0.005% bovine serum albumin, 0.1% Triton-X and 10% dimethyl sulfoxide). Larvae (200 to 300) were pooled for FCM analysis, and juveniles were analyzed individually. Before FCM analysis, samples in DAPI staining solution were vortex-mixed for 10 s and syringed with 25-gauge needles three times to dissociate cells, and filtered through a 25-μm screen.
Data Collection and Analysis
The percentage of divided eggs was recorded at 2 h post-fertilization. Survival was calculated as the percentage of survivors over the initial divided eggs. Data were analyzed using SYSTAT 11. Effects of treatment (or group) were tested by ANOVA. Percentage data were arcsine-transformed before analysis. Significance level was set at P < 0.05 unless otherwise noted.
Results
Heat Shock
Heat-shock treatments had no significant effects on zygote development, as measured by the percentage of eggs that divided after heat shock treatments (Table 1). ANOVA of transformed egg division data revealed no significant effects from treatment (32, 35, and 38°C, P = 0.092), duration (10, 15, and 20 min, P = 0.620) or their interaction (P = 0.625). One-way ANOVA of treatments including the control group also indicated that treatments did not affect the egg’s ability to resume cell division (P = 0.090). Overall, the percentage of eggs divided was 98.3% in the control, 96.4% in the 32°C treatment, 97.1% in the 35°C treatment, and 88.6% in the 38°C treatment. The slightly lower level of egg division in the 38°C treatment was mostly caused by one replicate, where only 50% of the eggs divided for unknown reasons.
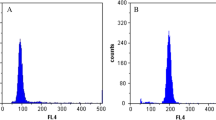
On the other hand, heat-shock treatments significantly changed the ploidy composition of resultant larvae as measured by the percentage of diploid larvae at 48 h post-fertilization. One-way ANOVA of transformed diploid percentages suggested that effects due to treatment (control, 32, 35, and 38°C) were highly significant (P < 0.001). When the three heat-shock treatments (32, 35 and 38°C) and durations (10, 15, and 20 min) were analyzed in a two-way ANOVA, different heat shocks differed significantly (P < 0.001) in their effects on ploidy composition, while effects due to duration and treatment × duration interaction were not significant (P = 0.323 and 0.823, respectively). No tetraploids were produced by the 32°C heat shock, and all resultant larvae remained as diploids. Actually, the 32°C treatment did not stop the first mitotic division; rather, it speeded up embryonic development that appeared to be normal. During the 35°C treatment, the fertilized eggs did not enter mitosis I, and their membranes shrank. On returning to 23°C seawater, the treated eggs became round and smooth again, and then entered mitosis I. The 38°C treatment also suspended the first cell division, and the fertilized eggs shrank more severely than that in the 35°C treatment. After the 38°C treatment, most fertilized eggs appeared to be abnormal: some eggs were deformed or broken, and some divided into unequal blastomeres. On average, the 35°C treatment produced 77.3% tetraploids, the 38°C treatment produced 67.2% tetraploids, and the difference was not significant (Table 1). The highest level of tetraploid larvae (88.8%) was observed in a replicate treated with 35°C for 20 min. Representative FCM graphs used for ploidy analysis are given in Figure 1.
Flow cytometry analysis of 2-day-old larvae resulting from mitosis I inhibition with heat shock in fertilized eggs of hard clam Mercenaria mercenaria. (A) Control group; (B) 32°C heat shock; (C) 35°C heat shock; (D) 38°C heat shock. The x-axis represents the channel number (or DNA content) and the y-axis represents cell count.
Although the heat shocks did not affect the egg's ability to resume mitosis, they seriously affected later development and survival to D-stage. ANOVA of survival data was not possible because many groups had zero survival to D-stage, but the negative effects of heat shocks on survival were strong and clear, as shown in Table 1. Even for the 32°C treatment that did not inhibit mitosis I, average survival to D-stage was reduced to 6.6%, compared with 49.7% for the control. Many groups treated with 35 and 38°C heat shocks did not produce any D-stage larvae (Table 1). The average survival to D-stage was only 0.7% for the 35°C treatment and 0.1% for the 38°C treatment. At day 2, most of the larvae in the treated groups remained as trochophores that appeared abnormal. The abnormal trochophores were smaller than normal and covered with cilia all over their body, with no or sometimes deformed shells. These abnormal larvae died gradually during the first week post-fertilization.
Larvae from the 35°C treatment were combined and cultured to juvenile stage. At day 24 (2 weeks post-metamorphosis), 210 juveniles were harvested and ploidy was individually determined by FCM: 6 (3%) were triploid and the others (97%) were all diploids. No tetraploids were detected at the juvenile stage. All juveniles (n = 50) from the control groups were diploid.
Cold Shock
Cold-shock treatments did not affect the proportion of eggs divided after treatment, as indicated by one-way ANOVA of transformed data using treatment (control, 1, 4 and 7°C) as a factor. Two-way ANOVA with treatment (1, 4, and 7°C) and duration (10, 15, and 20 min) as main factors detected no significant effects from the two main factors or their interaction (P > 0.609). The average percentage of divided eggs was 98.0% for the control, 98.1% for the 1°C treatment, 98.5% for the 4°C treatment, and 97.7% for the 7°C treatment (Table 2).
Similarly, ANOVA analyses showed that cold-shock treatments and their durations had no effects on survival to D-stage (P > 0.150) or on the proportion of diploid larvae produced (P > 0.173). On average, survival of divided eggs to D-stage was 28.1% for the control, 15.7% for the 1°C treatment, 21.3% for the 4°C treatment, and 28.8% for the 7°C treatment (Table 2). The proportion of diploid larvae was 100% for the control, 82.6% for the 1°C, 91.3% for the 4°C, and 96.3% for the 7°C treatment. Tetraploid larvae were observed only in some replicates of the 1°C treatment, ranging from 22.6% to 31.4%. Triploid larvae, up to 23.8%, were detected in some cold-shock treatment (Table 2). Representative FCM graphs used for ploidy determination are presented in Figure 2.
Flow cytometry analysis of 2-day-old larvae resulting from mitosis I inhibition with cold shock in fertilized eggs of hard clam Mercenaria mercenaria. (A) Control group; (B) 1°C cold shock; (C) 4°C cold shock; (D) 7°C cold shock. The x-axis represents the channel number (or DNA content) and the y-axis represents cell count.
Fertilized eggs under all cold-shock treatments stopped developing during the treatments. The membrane of fertilized eggs also shrank, similar to those under the 35 and 38°C treatments. Membrane shrinking is more pronounced in the 1°C than in the 7°C treatment. The treated eggs regained their normal appearance on returning to normal temperature, and subsequent cell divisions appeared to be normal.
The larvae from the 1°C treated groups were pooled and cultured to juvenile stage. FCM analysis of juveniles (n = 100) found only diploids.
Nocodazole Treatment
Two trials of nocodazole treatment were conducted. The first trial was designed to test a wide range of nocodazole concentrations, and the second to evaluate selected concentrations at a finer scale based on results from the first trial. In the first trial, 10 concentrations ranging from 0.02 to 1.6 mg/L were tested (Table 3). Treatment with 0.02 mg/L did not inhibit mitosis I, and the treated eggs continued normal development during the treatment. Treatments with 0.1 to 1.6 mg/L nocodazole effectively stopped the first mitosis during the treatment; however, only in groups treated with the 0.1, 0.2, and 0.4 mg/L, some treated eggs resumed development after the treatment, although none developed to D-stage larvae. In groups treated with 0.6 to 1.6 mg/L nocodazole, mitosis I was irreversibly inhibited, and fertilized eggs did not divide after the removal of nocodazole.
In the second trial, nine concentrations ranging from 0.02 to 0.18 mg/L were tested (Table 3). Again, 0.02 mg/L nocodazole did not inhibit or delay mitosis I. Under treatments with 0.04 to 0.1 mg/L nocodazole, about 1% to 5% of the treated eggs were not affected and proceeded to the first mitosis division without delay, but mitosis I in the majority of the eggs was inhibited or suspended. Nocodazole at concentrations between 0.10 and 0.18 mg/L blocked mitosis I in all treated eggs.
Mitosis blocking by nocodazole was reversible at concentrations between 0.04 and 0.16, where virtually all fertilized eggs resumed development and divided after the removal of nocodazole (Table 3). After the 0.18 mg/L treatment, only 38.6% of the treated eggs divided, compared with 87% in the controls. Although mitosis inhibition by nocodazole was reversible at 0.04 to 0.16 mg/L and the majority of the treated eggs resumed development, the treatments sharply reduced survival to D-stage larvae. At day 2, only a small fraction (0.6% to 2.8%) of divided eggs developed into D-stage larvae, while the majority of larvae remained as abnormal trochophores (Table 3).
Ploidy analysis at day 2 showed that all resultant larvae were diploid (FCM histograms not shown).
Discussion
Mitosis I inhibition is the most widely used method for tetraploid induction. It has led to the successful production of tetraploids in fish and amphibians (Fischberg, 1958; Reinschmidt et al., 1979; Chourrout, 1984; Myers, 1986; Hershberger and Hostuttler, 2005). Mitosis I can be inhibited by a number of physical and chemical treatments, some of which have been tested for tetraploid induction in molluscs including heat shock, cold shock, pressure shock, and chemicals such as cytochalasin B, 6-DMAP (dimethylaminopurine), caffeine, colchicine, and nocodazole (Guo, 1991; Guo et al., 1994; Yang et al., 1997, 1999; Jiang et al., 1998; Chang et al., 2002; Yang and Guo, 2006). While most of the treatments tested so far produced some tetraploid embryos, the induction efficiency was low in most studies, and no viable tetraploids have been obtained from mitosis I inhibition. In a recent study on the dwarf surfclam (Mulinia lateralis), heat shock-induced mitosis I inhibition produced high levels of tetraploids (up to 82.8%), although none survived beyond metamorphosis (Yang and Guo, 2006). The Mulinia study raises the hope that, with the development of highly effective methods, the production of viable tetraploids through mitosis I inhibition may be possible.
Comparison of Treatments
In this study, we compared heat shock with cold shock and nocodazole for mitosis I inhibition and tetraploid induction in the hard clam. This is the first reported attempt on tetraploid induction in this species. Cold shock was included in this study with the expectation that it may be less harmful than heat shock (based on past experience). Nocodazole is a microtubule inhibitor with effects that are more specific and reversible than those of colchicine (Guo, 1991). Results of this study demonstrate that heat shock is more effective than cold shock and nocodazole in inducing tetraploids through mitosis I inhibition. Although there is no direct comparison with other methods, the level of tetraploids produced by heat shock in this study, up to 88.8%, is the highest among all studies reported so far. Levels of tetraploids produced by other methods typically ranged from 10% to 45%. Results of this and other studies suggest that, among all methods tested so far for tetraploid induction by mitosis I inhibition in molluscs, heat shock is the most effective.
Although the mechanism by which heat shock inhibits cell division is poorly understood, it has been widely used for inhibiting mitosis or meiosis for ploidy manipulation. Heat shock is highly effective in inhibiting mitosis I in shrimp (Li et al., 2003) and polar body release in salmonids (Thorgaard, 1983; Guo et al., 1990). In molluscs, heat shock has been used primarily for polyploid induction by meiosis inhibition (Arai et al., 1986; Quillet and Panelay, 1986; Yamamoto and Sugawara, 1988; Gosling and Nolan, 1989; Scarpa et al., 1994), and mitosis I inhibition by heat shock has been reported in only two studies (Guo et al., 1994; Yang and Guo, 2006). One of the two studies on mitosis I inhibition (Yang and Guo, 2006) produced high levels of tetraploids that are similar to what we observed here.
Effective temperature for heat shock varies greatly among species. In fish, for example, the effective heat shock temperature is around 27°C for rainbow trout, a coldwater species (Guo et al., 1990), but 40 to 42°C for warm water species, such as carps and tilapia (Pandian and Koteeswaran, 1998). In the Pacific abalone Haliotis discus hannai Ino, heat shock of 26°C in combination with caffeine produced 70% triploids by meiosis inhibition (Mao et al., 2000). In the Pacific oyster, heat shocks of 35 to 40°C were effective in blocking polar body release (Quillet and Panelay, 1986) and mitosis I (Guo et al., 1994). In the dwarf surfclam Mulinia lateralis Say, 35 and 38°C heat shocks effectively inhibited meiosis or mitosis, producing high levels of triploid juveniles (up to 98.5%) or tetraploid larvae (up to 82.8%), respectively (Yang and Guo, 2006). This study shows that the effective heat-shock temperature for the hard clam is also about 35 to 38°C, similar to results from the Pacific oyster and the dwarf surfclam but very different from the 26°C reported for the Pacific abalone. Heat shock with 32°C was clearly ineffective in inhibiting mitosis I; it actually speeded up embryonic development without inducing any changes in ploidy.
Heat shocks are clearly deleterious as treatments, as all three temperatures significantly reduced survival to D-stage. The 38°C heat shock was apparently more harmful than the 35°C treatment. Even the 32°C heat shock, which did not inhibit mitosis I, sharply reduced survival to D-stage compared with the control groups (Table 1). Interestingly, treatment duration has no clear effects on early development or ploidy composition. Longer treatments appear to negatively affect survival to D-stage (Table 1), although the differences were difficult to measure because many treatments produced zero survival to D-stage. Nevertheless, results of this study suggest that shorter treatments tend to offer better survival without significantly reducing the level of tetraploids induced.
Cold shock has been used for meiosis inhibiting in several molluscs (Yamamoto and Sugawara, 1988; Wada et al., 1989; Yang et al., 1998), which is effective in producing triploids. Cold shock was tested for mitosis I inhibition in only one study, producing 2% to 7% tetraploids (Yang et al., 1999). Results of this study also indicate that cold shock is not an effective method for tetraploid induction by mitosis I inhibition. Although cold shocks stopped mitosis I during the treatment, it produced no tetraploid embryos at temperatures of 4 and 7°C. Only the 1°C cold shock produced low levels of tetraploids (up to 31%). Interestingly, cold shock induced low levels of triploids, while heat shocks did not. It is possible that, immediately on application, heat shock speeded up polar body release before having a chance to inhibit it, while cold shocks slowed down polar body release and subsequently inhibited its release.
Nocodazole is an inhibitor of microtubule assembly, and widely used for preparing cultured cells synchronized at the state of mitosis. It has been used to induce tetraploids in mammalian cell culture (Zieve, 1984), and also in p53- deficient hamster embryos (Cross et al., 1995). Nocodazole has been tested once for mitosis I inhibition in the Pacific oyster without producing significant levels of tetraploids (Guo, 1991). In this study, nocodazole was effective in inhibiting the first mitosis division, but not in producing tetraploid embryos. The effects of nocodazole were highly dependent on concentration, ranging from no inhibition at 0.02 mg/L or lower, reversible inhibition at 0.04 to 0.16 mg/L, and irreversible inhibition at 0.6 mg/L or higher. However, the nocodazole treatments tested here produced no tetraploids.
Viability of Tetraploids
Even though high percentages of tetraploid were produced by heat shocks of 35°C and 38°C, these tetraploid larvae were mostly trochophores and did not survive beyond metamorphosis. The viability of tetraploid embryos is low in all molluscs studied so far (Beaumont and Fairbrother, 1991; Guo et al., 1994). Several factors can potentially affect the viability of induced tetraploids. First, certain treatments may be lethal to embryos. Extreme temperatures are known to be detrimental to normal development. The fact that the 32°C heat shock reduced survival without producing any tetraploids suggests that heat-shock treatment alone can explain some of larval mortality. However, heat shock per se may not be the fundamental cause of the reduced survival of tetraploids. Triploids induced by similar heat shocks are viable in this and several other studies (Yamamoto and Sugawara, 1988; Gosling and Nolan, 1989; Wada et al., 1989; Davis, 1997; Yang and Guo, 2006). It seems that the heat shocks are lethal only when applied to inhibit mitosis I, regardless of whether the inhibition was effective or produced tetraploidy. Mitosis I in molluscs is sensitive to stress, probably owing to unique features of molluscan development. Embryonic development in molluscs is mosaic and programmed by the segregation of morphogenic determinants (Gilbert, 1988). Any disruption in the segregation and final distribution of morphogenic determinants during mitosis I would cause problems in differentiation and morphogenesis. Heat shocks applied during mitosis may disrupt the segregation of morphogenic determinants and cause mortality of tetraploids.
In addition to effects of heat shock and disruption of mitosis I, tetraploidy itself may have serious consequences in embryonic development. Tetraploid nuclei contain twice as much DNA as diploid ones and require more cytoplasm to maintain the proper cytoplasm/nucleus ratio and function. The cleavage of an egg with a fixed volume by a large tetraploid nucleus would lead to either a reduction in the number of blastomeres or blastomeres with inadequate amounts of cytoplasm. The deficiency in cytoplasm or cells may prevent tetraploid embryos from developing normally (Guo et al., 1994). Triploid embryos are mostly viable probably because a 50% increase in nucleus size is tolerated while a 100% increase in tetraploids is not. Supporting the cytoplasm-deficiency hypothesis, tetraploids produced using large eggs from triploids are viable (Guo and Allen, 1994). Arguing against the cytoplasm-deficiency hypothesis, viable tetraploids have been produced using eggs from diploids in several species (see Introduction and McCombie et al., 2005), but the survival is extremely low. It is also possible that tetraploidy creates imbalance in gene expression, which in turn affects larval development.
In conclusion, heat shock is highly effective in producing tetraploid embryos by inhibiting mitosis I in the hard clam. Nocodazole and cold shocks are effective in stopping mitosis I, but not in producing tetraploids. Although high levels of tetraploid embryos were produced by heat shock, none of them survived to the juvenile stage. The poor survival of induced tetraploids may be caused by a combination of factors including heat shock, disruption of mitosis I, and problems associated with tetraploidy. Further studies are needed to determine if heat shocks can be improved to allow better survival of induced tetraploids. Heat-shock treatments developed here can be used to test hypotheses such as whether differences in egg size or genetic background affect the viability of tetraploids. Heat shocks may also be effective in other molluscs and should be evaluated.
References
SK Allen SuffixJr H Hidu JG Stanley (1986) ArticleTitleAbnormal gametogenesis and sex ratio in triploid soft-shell clam Mya arenaria Biol Bull 170 198–210
SK Allen SuffixJr M Shpigel S Utting B Spencer (1994) ArticleTitleIncidental production of tetraploid Manila clams, Tapes philippinarum (Adams and Reeve) Aquaculture 128 13–19 Occurrence Handle10.1016/0044-8486(94)90097-3
SK Allen SuffixJr AJ Erskine E Walker R Zebal (2003) ArticleTitleProduction of tetraploid Suminoe oysters Crassostrea ariakensis J Shellfish Res 22 317
K Arai F Naito K Fujino (1986) ArticleTitleGenetic-studies on the Pacific abalone. 12. Triploidization of the Pacific abalone with temperature and pressure treatments Bull Jpn Soc Sci Fish 52 417–422
AR Beaumont JE Fairbrother (1991) ArticleTitlePloidy manipulation in molluscan shellfish: a review J Shellfish Res 10 1–18
J Brake J Davidson J Davis (2004) ArticleTitleField observation on growth, gametogenesis and sex ratio of triploid and diploid Mytilus edulis Aquaculture 236 179–191 Occurrence Handle10.1016/j.aquaculture.2003.09.016
Y Chang J Xiang Z Wang J Ding C Yang (2002) ArticleTitleTetraploid induction in Patinopecten yessoensis with chemicals Oceanol Limnol Sinica 1 105–112
D Chourrout (1984) ArticleTitlePressure-induced retention of second polar body and suppression of first cleavage in rainbow trout: production of all-triploid, all-tetraploid, heterozygous and homozygous diploid gynogenetics Aquaculture 36 111–126 Occurrence Handle10.1016/0044-8486(84)90058-9
ES Cox MSR Smith JA Nell GB Maguire (1996) ArticleTitleStudies on triploid oysters in Australia. 6. Gonad development in diploid and triploid Sydney rock oysters Saccostrea commercialis (Iredale and Roughley) J Exp Mar Biol Ecol 197 101–120 Occurrence Handle10.1016/0022-0981(95)00147-6
SM Cross CA Sanchez CA Morgan MK Schimke S Ramel RL Idzerda WH Raskind BJ Ried (1995) ArticleTitleA p-53-dependent mouse spindle checkpoint Science 267 1353–1356
JP Davis (1997) ArticleTitleOptimizing triploid production techniques and comparative field performance of Mediterranean mussels (Mytilus galloprovincialis) in Puget Sound J Shellfish Res 16 311–312
AG Eversole CJ Kempton NH Hadley WR Buzzi (1996) ArticleTitleComparison of growth, survival, and reproductive success of diploid and triploid Mercenaria mercenaria J Shellfish Res 15 689–694
M Fischberg (1958) ArticleTitleExperimental tetraploidy in newts J Embryol Exp Morphol 6 393–402
SF Gilbert (1988) Developmental Biology EditionNumber2nd ed. Sinauer Associates Sunderland, MA
EM Gosling A Nolan (1989) ArticleTitleTriploidy induction by thermal shock in the manila clam, Tapes semidecussatus Aquaculture 78 223–228 Occurrence Handle10.1016/0044-8486(89)90100-2
Guo X (1991) Studies on tetraploid induction in the Pacific oyster, Crassostrea gigas (Thunberg). PhD thesis, University of Washington, Seattle, WA, USA
X Guo (2004) ArticleTitleOyster breeding and the use of biotechnology Bull Aquacult Assoc Canada 104 26–33
X Guo SK Allen SuffixJr (1994) ArticleTitleViable tetraploids in the Pacific oyster Crassostrea gigas (Thunberg), produced by inhibiting polar body 1 in eggs from triploids Mol Mar Biol Biotechnol 3 42–50
X Guo WK Hershberger J Myers (1990) ArticleTitleSurvival and growth of intrastrain and interstrain rainbow trout (Onchorynchus mykiss) triploids J World Aqua Soc 21 250–256
X Guo WK Hershberger K Cooper KK Chew (1994) ArticleTitleTetraploid induction with mitosis inhibition and cell fusion in the pacific oyster Crassostrea gigas (Thunberg) J Shellfish Res 13 193–198
X Guo H Yang J Kraeuter (2001) ArticleTitleTriploid and tetraploid technology for hard clam aquaculture The Jersey Shoreline 20 6–9
X Guo J Wang BJ Landau L Li GA DeBrosse KD Krista (2002) ArticleTitleThe successful production of tetraploid eastern oyster Crassostrea virginica Gmelin J Shellfish Res 21 380–381
M He Y Lin Q Shen J Hu W Jiang (2000) ArticleTitleProduction of tetraploid pearl oyster Pinctada martensii (Dunker) by inhibiting the first polar body in eggs from triploids J Shellfish Res 19 147–151
Hershberger WK, Hostuttler MA (2005) Development of tetraploid rainbow trout strains for production of improved triploid. Aquaculture America 2005, January 17–20, New Orleans, Louisiana, USA, p. 182 (Abstract)
W Jiang Y Lin M He (1998) ArticleTitleA study on induction of tetraploid in pearl oyster, Pinctada martensii (D.) Trop Oceanol 2 45–51
A Komaru Y Uchimura H Ieyama KT Wada (1988) ArticleTitleDetection of induced triploid scallop Chlamys nobilis by DNA microfluorometry with DAPI staining Aquaculture 69 201–209 Occurrence Handle10.1016/0044-8486(88)90329-8
F Li J Xiang X Zhang C Wu C Zhang L Zhou K Yu (2003) ArticleTitleTetraploid induction by heat shocks in Chinese shrimp Fenneropenaeus chinensis J Shellfish Res 22 541–545
R Maldonado AM Ibarra JL Ramirez (2003) ArticleTitleInduction to tetraploidy in catarina scallop, Argopecten ventricosus (Sowerby II, 1842) Cienc Mar 29 229–238
L Mao Z Wang X Liu Y Li Y Gao (2000) ArticleTitleInduction of polyploid in the Pacific abalone by caffeine-heat shock treatment Acta Genetica Sinica 27 956–965
H McCombie C Ledu P Phelipot S Lapegue P Boudry A Gerard (2005) ArticleTitleA complementary method for production of tetraploid Crassostrea gigas usingcrosses between diploids and tetraploids with cytochalasin b treatments Mar Biotechnol 7 318–330 Occurrence Handle10.1007/s10126-004-0440-2
JM Myers (1986) ArticleTitleTetraploid induction in Oreochromis spp Aquaculture 57 281–287 Occurrence Handle10.1016/0044-8486(86)90206-1
TJ Pandian R Koteeswaran (1998) ArticleTitlePloidy induction and sex control in fish Hydrobiologia 384 167–243 Occurrence Handle10.1023/A:1003332526659
S Peruzzi X Guo (2002) ArticleTitleTetraploid induction by meiosis inhibition with cytochalasin B in the dwarf surfclam Mulinia lateralis Say: effects of temperature J Shellfish Res 21 677–684
E Quillet PJ Panelay (1986) ArticleTitleTriploid induction by thermal shock in the Japanese oyster Crassostrea gigas Aquaculture 57 271–279 Occurrence Handle10.1016/0044-8486(86)90205-X
DC Reinschmidt SJ Simon EP Volpe R Tompkins (1979) ArticleTitleProduction of tetraploid and homozygous diploid amphibians by suppression of first cleavage J Exp Zool 210 137–143 Occurrence Handle10.1002/jez.1402100115
Scarpa J, El-Wazzan E (2005) Cytological evaluation of triploid induction in the hard clam Mercenaria mercenaria. Aquaculture America 2005, January 17–20, New Orleans, LA, pp. 399 (Abstract)
J Scarpa KT Wada A Komaru (1993) ArticleTitleInduction of tetraploid in mussels by suppression of polar body formation Bull Jpn Soc Sci Fish 59 2017–2023
J Scarpa JE Toro KT Wada (1994) ArticleTitleDirect comparison of six methods to induce triploidy in bivalves Aquaculture 119 119–133 Occurrence Handle10.1016/0044-8486(94)90169-4
GH Thorgaard (1983) Chromosome set manipulation and sex control in fish WS Hoar DJ Randall EM Donaldson (Eds) Fish Physiology, Vol. 9 (B) Academic Press London 405–434
KT Wada A Komaru Y Uchimura (1989) ArticleTitleTriploid production in the Japanese pearl oyster, Pinctada fucata martensii Aquaculture 76 11–19 Occurrence Handle10.1016/0044-8486(89)90247-0
S Yamamoto Y Sugawara (1988) ArticleTitleInduced triploidy in the mussel, Mytilus edulis, by temperature shock Aquaculture 72 21–29 Occurrence Handle10.1016/0044-8486(88)90143-3
H Yang X Guo (2004) ArticleTitleTetraploid induction by meiosis inhibition in the dwarf surfclam Mulinia lateralis: effects of cytochalasin B duration Aquacult Res 35 1187–1194 Occurrence Handle10.1111/j.1365-2109.2004.01118.x
H Yang X Guo (2006) ArticleTitlePolyploid induction by heat shock-induced meiosis and mitosis inhibition in the dwarf surfclam, Mulinia xlateralis Say Aquaculture 252 171–182 Occurrence Handle10.1016/j.aquaculture.2005.07.017
H Yang R Wang X Guo Z Yu (1997) ArticleTitleTetraploid induction by blocking polar body 1 and mitosis 1 in fertilized eggs of the scallop Chlamys (Azumapecten) farreri with cytochalasin B J Ocean Univ Qingdao 27 166–172
HS Yang HC Chen YY Ting (1998) ArticleTitleInduction of polyploidy and embryonic development of the abalone, Haliotis diversicolor, with temperature treatment Am Malacol Bull 14 139–147
H Yang X Guo Z Chen Y Wang (1999) ArticleTitleTetraploid induction by inhibiting mitosis I in scallop Chlamys farreri Chin J Oceanol Limnol l7 350–358
H Yang F Zhang X Guo (2000) ArticleTitleTriploid and tetraploid zhikong scallop Chlamys farreri (Jones et Preston) produced by inhibiting polar body I Mar Biotechnol 2 466–475
GW Zieve (1984) ArticleTitleNocodazole and cytochalasin D induce tetraploidy in mammalian cells Am J Physiol 246 c154–c156
Acknowledgments
The authors thank Biosphere Inc. (Tuckerton, NJ) for providing broodstock clams used in this study. This study is partly supported by grants from the New Jersey Commission on Science and Technology (Award No 00-2042-007-20) and New Jersey Sea Grant Consortium (Award R/BT-2001). This is publication IMCS-2006-1 and NJSG-06-626.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Guo, X. Tetraploid Induction by Inhibiting Mitosis I with Heat Shock, Cold Shock, and Nocodazole in the Hard Clam Mercenaria mercenaria (Linnaeus, 1758). Mar Biotechnol 8, 501–510 (2006). https://doi.org/10.1007/s10126-005-6183-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-005-6183-x