Abstract
Tetraploid fish are the key source of diploid gametes in polyploid breeding, and they can be induced by disrupting the first mitotic cell cleavage. In this study, the induction protocol of tetraploid by hydrostatic pressure shock and the viability of the tetraploid progenies were investigated in turbot, Scophthalmus maximus. Under water temperature of 14.5 ± 0.5 °C, fertilized diploid zygotes were treated by different combination of timing (65–90 min after fertilization, maf), pressure intensity (60–85 MPa), and duration (2–10 min), respectively. Ploidy level was determined by silver staining of NOR regions, karyotype analysis, and flow cytometry. The optimal protocol for the pressure shock induction was determined to be the combination of timing of 80 maf, 75 MPa of hydrostatic pressure, and 6 min of duration time. Two batches of tetraploid-induced progenies with a total of more than 73,000 morphologically normal larvae were produced. The hatching rates and tetraploidy rates of the two induction groups were 8.97% and 4.09% and 53.33% and 46.67% at 1 day after hatching (dah), respectively. At 150 dah, 1 out of 20 juveniles was identified as tetraploid by karyotype analysis. However, none of tetraploid juveniles was detected by the flow cytometry analysis among the 446 juveniles survived at 365 dah. This preliminary study provides the evidence supporting the large-scale production of tetraploid turbot progenies, thus encouraging further research on the subject.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetraploidy is considered to be beneficial and useful for further ploidy manipulation and improvement of aquaculture traits in teleost. Fertile tetraploids, which have four haploid sets of chromosomes, are expected to generate functional diploid gametes (Pandian and Koteeswaran, 1998; Piferrer et al. 2009). Mass production of sterile triploids and fertile auto- and allo-tetraploids is possible through simple interploidy crossing (Chourrout et al. 1986; Zhou et al. 2010). Gynogenetic and androgenetic diploids fertilized with genetically inactivated sperm or eggs can also be produced directly without any treatment to duplicate chromosomes (Piferrer et al. 2009). Thus, ploidy manipulation with diploid gametes from tetraploids appears to be more reliable and avoids the detrimental effects associated with conventional chromosome manipulation (Sakao et al. 2009; Li et al. 2013). Artificial tetraploidization of a diploid species can be theoretically induced by disrupting the first mitotic cleavage of normal fertilized eggs with hydrostatic pressure or thermal treatment (Chourrout, 1982; Lou and Purdom, 1984; Hartono et al. 2016). Pressure shocks seem to be more reliable and easier to apply in case of floating eggs and large volumes of eggs in a commercial setting (Piferrer et al. 2009; Li et al. 2012; Wang et al. 2020). However, tetraploid embryos exhibit extremely low survival in most cases and may even be unviable, thus limiting applications (Piferrer et al. 2009). Viable mature and fertile tetraploids have only been obtained in rainbow trout, Oncorhynchus mykiss (Chourrout et al. 1986); blunt snout bream, Megalobrama amblycephala (Zou et al. 2004); mud loach, Misgurnus mizolepis (Nam and Kim, 2004); and loach, Misgurnus anguillicaudatus (Yoshikawa et al. 2008). Tetraploid embryos were produced for some aquaculture marine teleost including European sea bass, Dicentrarchus labrax (Peruzzi and Chatain, 2003); yellow perch, Perca flavescens (Malison et al. 1993); olive flounder, Paralichthys olivaceus (Yi et al. 2012; Wang et al. 2020); and half-smooth tongue sole, Cynoglossus semilaevis (Li et al. 2012). Though most of the artificially induced tetraploid did not survive the fingerling stage or died later, the survival of juvenile tetraploids in olive flounder encouraged tetraploid induction in other flatfishes (Yi et al. 2012).
The turbot, Scophthalmus maximus, has become the main species of land-based tank cultured marine finfish in China since its introduction in 1992 (Lei et al. 2012). Owning to the well-developed market and industrial chain, turbot is now regarded as the best candidate species for the development of offshore marine farming. Culturing of triploid turbot is necessary to avoid alien species invasion and ecological and genetic pollution from accidental farmed fish escapes. Moreover, triploid turbot culture had been demonstrated to attain a higher biomass and better survival rate at a given age than diploid culture (Cal et al. 2006). Thus, mass production of triploid turbot would be beneficial in developing not only offshore marine farming but also land-based commercial farming. Tetraploid stock of turbot provides a practical and convenient method to produce sterile triploid population. Studies on the induction of tetraploidy in turbot have been reported (Wu et al. 2014, 2019; Zhu et al. 2017). The initiation time of tetraploid induction was expressed as percentage of the first cleavage interval (FCI) in these studies. However, the time of FCI was associated with water temperature and egg quality, which had not been well studied in turbot. Thus, induction parameters for hydrostatic treatments should be established more precisely under strict control of water temperature. The survival and growth performance of tetraploid progenies were not reported. In addition, no viable tetraploid juveniles have been produced until now. As a result, no method for mass production of triploid turbot offspring (with a 100% induction rate) has been established through cross-fertilization between gametes from tetraploid and diploid individuals. Further studies are needed to improve the efficiency of tetraploid induction by pressure shock and to evaluate the viability of tetraploid larvae beyond hatching.
The objectives of this study, therefore, were as follows: (1) to optimize the protocol for inducing tetraploidy in turbot, including the intensity, timing, and duration of hydrostatic pressure under strict controlled temperature; (2) to attempt to scale up the method for the mass production of tetraploid turbot using the best combination of the three parameters; and (3) to study the early viability of the resulting tetraploid turbot in comparison to their diploid counterparts.
Materials and methods
Broodstock maturation and gamete collection
A turbot broodstock of 25 females and 35 males (body weight 1.5–4.0 kg, 4 years old), which originated and mass selected from artificially reproduced juveniles, was held in two 36,000 L concrete tanks at Tianyuan Aquaculture Co., Ltd. (Yantai City, Shandong Province, China).
The induction of broodstock maturation, ovulation, and artificial fertilization were performed as described by Meng et al. (2016). In brief, the broodstock had been maintained under controlled conditions with a photoperiod of 16 h light/8 h dark and a water temperature of 12–14 °C for more than 2 months before the experiment. Ovulated eggs from each female by exerting gentle abdominal pressure were collected into a 1000 mL glass beaker, quality checked, and retained for fertilization in a water bath temperature of approximately 14 °C. Milt from 2–3 males was collected into a 2.5 mL polypropylene syringe, diluted 10 times with modified Ringer’s solution (4.33 g/L NaCl, 2.01 g/L KCl, 0.54 g/L CaCl2, 0.23 g/L MgCl2·6H2O, 0.28 g/L NaH2PO4, 0.20 g/L NaHCO3, and 1.00 g/L glucose), motility checked, and stored at 4 °C until later use.
Artificial fertilization and egg hatching
Eggs from each female were split into different groups in selected volumes, fertilized with the diluted sperm at a ratio of 1:20 (Vmilt/Veggs), and activated by the addition twice the volume of the gametes of seawater at 14.5 °C. The moment of activation was taken as time zero for the development of eggs. Fertilized eggs were incubated and left undisturbed in net cages (15 L or 100 L depending on the number of fertilized eggs), which were suspended inside 2500 L fiberglass reinforced plastic tanks with flow-through sea water at 14.5 ± 0.5 °C. Before the treatment, floating eggs were collected, rinsed, and placed into individual plastic vials with perforated mesh for shock treatment. After the treatment, the eggs were incubated as above.
Optimal parameters of pressure shock treatment
A hydrostatic pressure chamber (FH-200 M, Qingdao Starfish Instrument Co., Ltd., China) was used to inhibit the first cleavage. The vial holding the eggs for treatment was placed in a 1500 mL stainless steel cylinder filled completely with sea water at 14.5 °C, and the cylinder was sealed with a screw cap. The pressure inside the cylinder was elevated to the required level in less than 10 s and maintained stably at that level. Decompression was instantaneous at the end of the treatments, and the treated eggs were moved to the same incubation tank described above.
Three experiments were designed to determine the optima of three key parameters (timing, intensity, and duration) of hydrostatic pressure shock for the induction of tetraploidy in turbot. Based on the results of the mitogynogenetic induction in turbot (Meng et al. 2016), in each experiment, one parameter at a time was tested at various levels, while the other two were maintained at a fixed level (optimal level was chosen based on the related trial) as follows: timings of 65, 70, 75, 80, 85, and 90 min after fertilization (maf) with a fixed intensity of 65 MPa for 6 min; intensities of 60, 65, 70, 75, and 80 MPa with a fixed timing of 80 maf for 6 min; and durations of 2, 4, 6, 8, and 10 min with a fixed intensity of 75 MPa initiated at 80 maf. For each experiment, 60 mL of eggs from one female were divided into equal samples and placed individually into a 1000 mL beaker; one sample was left untreated and made up the control group, and the other samples were shocked with hydrostatic pressure and moved to a small net for hatching as described above. All experiments were repeated up to three times with egg batches derived from different females. Data on the fertilization rate and hatching rate of the eggs and the tetraploid rate of larvae in each experiment were collected and analyzed to determine the three optima.
Determination of induction rate and ploidy level
Under the incubation conditions described above, hatching typically took place over 115 h after fertilization (haf). The fertilization rate was recorded as the percentage of fertilized eggs at 4 haf (approximately 16 cells) out of the total number examined eggs (n ≥ 100 per group). The non-fertilized eggs, non-hatched eggs, and larvae were counted and added to determine the total number of eggs remaining after the fertilization rate examination in each group. The hatching rate was defined as the survival rate, according to the percentage of phenotypically normal swimming larvae at approximately 1 day after hatching (dah) out of the total number of fertilized eggs.
Ploidy level was determined by quantifying the maximum number of Ag-stained nucleolar organizer regions (NORs) in the nuclei of 50 cells from each larva (n = 30 larvae per group), as described by Piferrer et al. (2000). The larvae with a maximum number of NORs ≥ 4 were recognized as tetraploids, whereas those with a maximum number ≤ 3 were recognized as diploids. Ploidy was verified in 30 individuals randomly selected from the first timing experiment by flow cytometry analysis with a PARTEC cell counter analyzer (CCA-II; PARTEC, Germany). Ploidy status was determined based on the relative DNA content using diploid control larvae as a standard (Fig. 1A) (Nascimento et al. 2020). In this manner, a single larva was split into two parts. The anterior 2/3 (head) was thawed in 0.5 mL of 4′,6-diamidino-2-phenylindole (DAPI) (Solabrio, China) solution and rapidly desegregated with a pipette, vortexed and filtered through a 30 μm Partec CellTrics filter, and then tested in 30 min. The posterior 1/3 (tail) was used to quantify the Ag-stained NORs. The tetraploidy rate was recorded as the percentage of tetraploid larvae out of the total examined larvae. Tetraploid yield, the percentage of tetraploid larvae per total number of fertilized embryos, was calculated for each treatment as the product of survival and tetraploidy (%) divided by 100.
Mass production of tetraploid juveniles
Approximately 580 mL eggs pooled from two females were divided into two groups: one group contained 60 mL eggs that were not shocked and composed the control group, and another group contained 520 mL eggs that were shocked with pressure under the optimal conditions determined in the above experiment (“Optimal parameters of pressure shock treatment” section) to compose a tetraploid group (1st). An additional 690 mL eggs from the other two females were mixed to compose another tetraploid group (2nd). After fertilization and shock, the eggs in both groups were hatched in large nets according to the method described above (“Artificial fertilization and egg hatching” section). The embryonic development and external appearance were investigated in the 1st induced group and the diploid control. The hatched larvae were reared with the protocol for turbot developed by Person-Le Ruyet et al. (1991). Juveniles were cultured in indoor tanks with sea water flow-through at a temperature of 18–21 °C and fed commercial dry feed. The larvae from each group were reared separately from 1 to 60 dah. Then, juveniles from the 1st and 2nd shock group were mixed and reared together.
Examination of tetraploid viability
Survival rates and ploidy levels were determined at different developmental stages: newly hatching larvae (1 dah), 40 dah juveniles, 60 dah juveniles, and 365 dah juveniles. Ploidy levels were analyzed individually with NORs-Ag staining at 1 dah (n = 30 per group), direct chromosome counting at 150 dah (n = 20), and flow cytometry at 365 dah (n = 465). Chromosomes were prepared according to Dong et al (2013). In brief, 5-month-old juveniles from the control and induction group were intraperitoneally injected with 2.5 μg/g colchicine 3 h before sacrifice. The head-kidney tissues were removed, minced finely, and suspended in 0.075 M KCl hypotonic solution for 40–60 min at room temperature. The cells were fixed with chilled Carnoy’s solution (3:1 Vmethanol:Vacetic acid) and stored at 4 °C. Karyotypes were prepared on the chilled slides and Giemsa stained. Flow cytometry analysis was performed with red blood cells collected by caudal vein puncture and stained with propidium iodide (PI, Solabrio, China). The stained blood cell suspensions were kept at 4 °C in the dark and analyzed within 2 h (Zhou et al. 2010).
Statistical analysis of data
Data on the fertilization rates of the eggs, survival rates, tetraploidy rates, and tetraploid yields of larvae in the experiments in the “Determination of induction rate and ploidy level” section were arcsin transformed before analysis with Tukey’s honest significant difference test (ANOVA). The hypothesis test of two sample frequencies was performed on the hatching rates and survival rates of the control group and the tetraploid induction group in the “Mass production of tetraploid juveniles” and “Examination of tetraploid viability” sections. All differences analyzed were assessed at a significance level of 0.05. SPSS 17.0 software was used for data analysis.
Results
Optimal parameters of pressure shock treatment
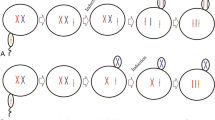
Flow cytometry analysis was used to verify the validity of maximum NOR numbers in tetraploid identification. Typical diploids and tetraploids were detected in 30 selected larvae with normal appearance from the treatment group, whereas the larvae from the control group were used as a diploid standard (Fig. 1A, B). Examination of Ag-stained slides revealed that the maximum number of active NORs per nucleus in diploid cells was two to three (Fig. 2A), whereas it was four to five in verified tetraploids (Fig. 2B, C). In this manner, the maximum number of NORs per larva was correlated with the true ploidy level, as assessed by flow cytometry analysis. No mosaic, hypo-, or hyper-tetraploidy was detected by flow cytometry analysis from the 30 larvae of the treatment group in the present study.
Ploidy identification of the newly hatched swimming larvae of diploidy (A) and tetraploidy (B and C) by counting the maximus number of NORs in the nuclei of 100 cells from each larvae (n = 30). The larvae with the maximus numbers of NORs ≥ 4 (B, n = 4; C, n = 5) were recognized as tetraploidy, while larvae with the maximus numbers ≤ 3 (A, n = 2) were recognized as diploidy. Black arrows indicate the maximus numbers of NORs. Ploidy level in the three parameters experiments was determined by quantifying the maximum number of Ag-stained NORs
There were no significant differences in fertilization rates among the control groups in the experiments of the three key parameters (Fig. 3). This result indicated that the qualities of eggs and sperm were good, owing to the gamete quality control measures. In addition, the shock timing, pressure intensities, and duration did not significantly affect the fertilization rates (which ranged from 68.1 to 91.2%) in each experiment. The pressure shock after fertilization did not affect the fertilization rate.
Fertilization rates of the control and treated groups in the three key parameters experiments. Data are presented as means and standard errors of the raw data from three replicated experiments. Ctr-T, control groups in the timing parameter experiment; Ctr-P, control groups in the pressure intensity parameter experiment; Ctr-D, control groups in the duration parameter experiment; 65–90 min, timing of the pressure shock; 60–80 MPa, pressure intensity; 2–10 min, duration of the pressure shock
The hatching rates and tetraploidy rates in the three experiments (timing, intensity, and duration) are shown in Fig. 4. When initiated at 65, 70, 75, 80, 85, and 90 maf, pressure shocks with an intensity of 65 MPa for 6 min produced viable tetraploid larvae (Fig. 4-A). However, the groups initiated at 75 and 80 maf had significantly higher hatching rates and tetraploidy rates than the other four groups (65, 70, 85, and 95 maf) (F = 20.72 and 18.82, p < 0.05), among which no clear differences in these two rates were observed. Nevertheless, 80 maf was found to be the optimal timing, given the higher rate of tetraploid yield, which was 6.41% ± 1.15%, compared with 4.64% ± 1.12% at 75 maf (F = 30.83, p < 0.05).
Effects of timing, intensity, and duration of the pressure shock on the hatching rate of eggs and the tetraploidy rate of larvae of the tetraploidy induction in turbot. The fertilized eggs were pressure shocked at different timings of 65, 70, 75, 80, 85, and 90 min after fertilization (maf) with a fixed intensity of 65 MPa for 6 min (Fig. 1A); different intensities of 60, 65, 70, 75, 80, and 85 MPa with a fixed timing of 80 maf for 6 min (Fig. 1B); and different durations of 2, 4, 6, 8, and 10 min with a fixed intensity of 75 MPa initiated at 80 maf (Fig. 1C). Data are presented as means and standard errors of the raw data from three replicated experiments. Different lowercase letters above the column indicate significant differences in the hatching rate between groups with Tukey’s honest significant difference test (ANOVA) (p < 0.05). Different uppercase letters above the line indicate significant differences in tetraploid rate between groups (p < 0.05)
When treatment was initiated at 80 maf and lasting for 6 min, the maximum hatching rate was recorded at an intensity of 60 MPa, whereas the maximum tetraploidy rate was obtained at an intensity of 75 MPa (F = 71.33, p < 0.05) (Fig. 4-B). The highest rate of tetraploid yield was recorded at an intensity of 75 MPa, with an average of 8.19% ± 2.14% (F = 4.21, p < 0.05). Therefore, 75 MPa was considered the optimal intensity for the pressure treatment.
When treatment was initiated at 80 maf with an intensity of 75 MPa, the two groups receiving shocks lasting 4 and 6 min had significantly higher hatching rates than the other three groups receiving shocks lasting 2, 8, and 10 min (Fig. 4-C, F = 7.53, p < 0.05). The tetraploidy rates in groups receiving shocks lasting 6 and 8 min were significantly higher (F = 93.46, p < 0.05). Therefore, 6 min was found to be the optimal shock duration because it produced the highest tetraploid yield (18.38% ± 1.50%) (F = 14.09, p < 0.05).
Mass production of tetraploid juveniles
Large-scale production of tetraploid larvae
With the optimal protocol of pressure shock (with an intensity of 75 MPa, initiated at 80 maf, and lasting for 6 min), we attempted to scale up the method for the mass production of tetraploid turbot with a total of approximately 1.45 million eggs and obtained about 73 thousand hatched larvae in two experimental series (Table 1). The fertilization rates in the shock groups (1st and 2nd) and the control group were 81.47%, 79.92%, and 83.52%, respectively. In the diploid control group, embryos showed normal morphology of cell division during the cleavage stage (Fig. 5A), normal blastodisc and embryonic body during the gastrula stage (Fig. 5B), and normal appearance of embryos and larvae during the hatching stage (Fig. 5C, D). However, a portion of embryos showed asymmetrical and unsynchronized cell division during the cleavage stage in tetraploid group, whereas the remaining embryos showed similar morphology to that of the diploid control (Fig. 5E). Many embryos with deformed or fuzzy embryonic bodies stopped developing during the gastrula stage in the tetraploid group (Fig. 5F). The rest of embryos showed no difference in morphology to that of diploid embryos but took about 5 h longer than diploid for hatching. The hatching rates were much lower (8.97% and 4.09%) than that of the control group (68.37%), whereas the rates of abnormalities were the direct opposite. The malformed larvae generally showed thick, short, or curved bodies and died sooner after hatching, and the remaining larvae showed a normal appearance similar to that of the diploid control (Fig. 5G, H).
Comparison of the embryonic development between diploid control (A–D) and the 1st tetraploid induction group (E–H). Diploid control embryos showed normal morphology of cell divisions during the cleavage stage (A), normal blastodisc and embryoid body during the gastrula stage (B), and normal appearance of larvae during the hatching stage (C and D). A proportion of tetraploid embryos showed asymmetrical and unsynchronized cell division during the cleavage stage (E), deformed or fuzzy embryoid body during the gastrula stage (F), and normal or abnormal appearance of larvae after hatching (G and H)
Examination of tetraploid viability
The survival rates in both shock groups during 1–40 dah (0.82% and 0.22%) were much lower than that in the control group (21.36%), slightly lower during 40–60 dah (52.55%, 74.56%, and 88.34%, respectively), and similar during 60–360 dah (95.48% in the shock group and 95.43% in the control group).
At 1 dah, 30 newly hatched larvae were randomly selected from the two shock groups respectively, and the maximum number of NORs of each individual was observed with Ag staining method. The tetraploidy rates were determined to be 53.33% in the 1st shock group and 46.67% in the 2nd shock group (Table 1).
At 150 dah, a total of 487 juveniles survived out of 1210 mL of eggs pooled from four females in the two shock groups. Karyological analysis showed that all metaphase spreads of juveniles from the diploid control group had 44 chromosomes (Fig. 6A). Almost all 20 juveniles randomly selected from the shock group had an equal number of chromosomes to that of the diploid control, except for one individual that exhibited two times the number of chromosomes (Fig. 6B), thus indicating the tetraploidy level.
At 365 dah, a total of 446 juveniles in the shock group survived. However, all juveniles were identified as diploids, with a value of nuclear DNA content in erythrocytes equal to that of the diploid nuclear DNA content. No tetraploid was identified.
Discussion
In the present study, a protocol for the induction of tetraploidy in turbot through hydrostatic pressure shock under strict control of water temperature before treatment was developed. Mass production of tetraploid was carried out in two batches using the best combination of the three parameters. The tetraploid progenies were shown to survive up to 150 dah and were not detected on 365 dah.
In practice, induction of tetraploid requires the precise timing of the application of the shock treatment, usually a pressure shock. The timing was species specific and related to the water temperature (Flajšhans et al. 1993; Malison et al. 1993; Váradi et al. 1999; Nam et al. 2001). To avoid the water temperature effect, strict control at a steady temperature before treatment (Peruzzi and Chatain, 2003; Francescon et al. 2004; Farahmand et al. 2007; Sakao et al. 2009) or relative units of embryological age (τ0 and FCI) (Flajšhans et al. 1993; Váradi et al. 1999; Zou et al. 2004; Weber and Hostuttler, 2012; Fujimoto et al. 2013) are often used to standardize the optimal timing in tetraploid induction. In this study, the optimal timing for tetraploidy induction was determined to be 80 maf under strictly controlled temperature (14.5 ± 0.5 °C), which is slightly earlier than that for mitogynogenetic induction in turbot under the same conditions (85–90 maf) (Meng et al. 2016). A similar delay in gynogenetic embryos has also been reported in amago salmon (Kobayashi, 1997) and masu salmon (Sakao et al. 2006). The cytological characteristics of a heterogeneous or dense chromatin body in genetically inactivated sperm in mitogynogenetic embryos might be associated with the delay.
The optimal timing had been standardized as percentage of the FCI and proved to be highly correlated with FCI in turbot, which is the time span between insemination and first cleavage of the zygote (Wu et al. 2014 and 2019). However, the first cleavage takes more than 2 h under normal hatching conditions in turbot, and the time is affected by water temperature (Sun et al., 2005). Since the equation between FCI and water temperature had not been fully established, the FCI of different batches of eggs under the current water temperature should be determined firstly. This process, in turn, can lead to overripening of the eggs or a decline in the quality of the eggs. Therefore, unless an equation between FCI and water temperature has been well established, the optimal timing defined as time after fertilization under strictly controlled water temperature conditions is more conducive to the repetition of tetraploid induction in turbot.
Moreover, the timing is considered to be effective in the time from prometaphase to metaphase of the first cell division (approximately 50–75% of the first division) in tetraploidization by the pressure shock treatment (Malison et al. 1993; Váradi et al. 1999; Sakao et al. 2003; Weber and Hostuttler, 2012). Late prometaphase is sometimes determined to be the optimal timing (Zhang and Onozato, 2004; Sakao et al. 2006). The time from prometaphase to metaphase in turbot has been identified as 74–82 maf if eggs are hatched at 15.5 °C (Sun et al. 2005) and will be slightly later if eggs are hatched at a lower temperature of 14.5 °C that is used in our study. Thus, the optimal timing of 80 maf was in accordance with the time of prometaphase to late prometaphase during the first division of turbot embryos.
The effect of pressure intensity and duration on tetraploid induction is less significant than that of timing. Despite the enormous differences in the diameters of fish eggs across species, ranging from approximately 1 to 7 mm, the optimal pressure intensity to induce chromosome duplication typically varied from 58 to 85 MPa (Komen and Thorgaard, 2007; Piferrer et al. 2009). The optimal pressure intensity of 75 MPa determined in this study was in agreement with that of the mitogynogenesis induction described in a previous study (Meng et al. 2016) and was higher than the 67.5 MPa reported by Wu et al. (2014 and 2019). The reason for this difference remains unclear. The different statistical methods of hatching rate and induction rate may potentially cause the difference. However, the maximum tetraploidy rate and the highest rate of tetraploid yield were both obtained at an intensity of 75 MPa, even though the hatching rate was lower than that of the lower pressure intensities (60 and 65 MPa). The optimal pressure intensity of 75 MPa determined in this study was similar with that determined in European sea bass (81–91 MPa) (Francescon et al. 2004), which may be related to the similar egg size of the two species. With regard to the duration, 4–10 min duration was used in most of the hydrostatic shock-induced teleost tetraploid operations (Piferrer et al. 2009). The duration was mainly associated with the embryonic development rate, particularly the time for the formation and depolymerization of the microtubules of the spindle (Sakao et al. 2006; Zhu et al. 2017). Thus, tetraploid induction using a combination of pressure and cold treatments, which could theoretically delay the cytological development and increase the synchronicity of division of embryos, may be a beneficial trial in improving the induction rate in future studies (Myers, 1986).
The ploidy level was determined with methods of silver staining of NOR regions, karyotype analysis, and flow cytometry in this study. Karyotype analysis and flow cytometry are accurate, but the former is laborious, and the latter is costly (Bencsik et al. 2012; Zhao et al. 2004). The analysis of the number of nucleoli per nucleus is a straightforward and simple technique to determine the level of ploidy (Flajšhans et al., 1993; Váradi et al. 1999; Mustami, 2017) and had been demonstrated to be effective in triploidy identification in turbot (Piferrer et al. 2000, 2003). Polymorphisms in the number of NORs have been discovered in diploid turbot, which have a maximum number of two or three nucleoli in most instances (Pardo et al. 1998). The tetraploid individuals, whose ploidy level was verified with method of flow cytometry, exhibited a maximum of four or five nucleoli. Therefore, despite the NORs polymorphisms, analysis of the maximum NORs number is effective in distinguishing the tetraploid from the diploid in turbot.
In many previous studies, tetraploid embryos had been successfully induced, but in most cases, the tetraploid progenies had no survival potential and died after hatching, feeding, or growing to adult or near-adult size (Sakao et al. 2006; Gil et al. 2016; Christopher et al. 2019; Wang et al. 2020). For example, the tetraploidy rates ranged from 75 to 94% in 9–11-day-old larvae of European sea bass but were lower in 46-day-old fry (4%) and 50-day-old fry (0%) (Peruzzi and Chatain, 2003). Similar to this, the rate of tetraploid progenies was about 50% at 1 dah and decreased to 5% at 150 dah and further to zero at 365 dah in this study. These results suggested that the mortality of tetraploid larvae is not only caused by the adverse effect of physical treatment, but also is a result of the elevated of ploidy status, from diploidy to tetraploidy (Arai, 2001; Zou et al. 2004; Piferrer et al., 2009; Zhou and Gui, 2017). The known and assumed reasons for tetraploid mortality are mosaicism, aneuploidy, wrong cytological events, and high homozygosity. Frequent occurrence of 2n/4n mosaics associated with thermal induction and hydrostatic pressure treatment have already recorded (Cassani et al. 1990; Sakao et al. 2006; Fujimoto et al. 2013). Another possible cause of tetraploid mortality may be the decreased cell surface area corresponding to the elevation of ploidy, which might cause deleterious effects on cellular metabolism (Pandian and Koteeswaran, 1998). Using a Coulter counter, Cassani et al. (1990) estimated that the cell number present in tetraploid grass carp, Ctenopharyngodon idella, was only 54% in comparison to diploids. Such a decrease in cell number and the corresponding increase in cell volume would decrease the cumulative cell surface available for physiological reactions. A third hypothesis is that after chromosome polyploidization, teleosts often undergo explosive multiple allelic divergence (gene divergence) to restore the diploid chromosomes (Amores et al. 1998; Zou et al. 2005). Thus, the ploidy level of dead individuals and the changes in the ploidy level of tetraploid individuals must be tracked and detected regularly in future studies to reveal the reasons for the mortality of autotetraploid turbot.
In conclusion, this study demonstrated that tetraploidy can be induced by pressure shock initiated at 80 maf for 6 min with an intensity of 75 MPa under strict control of water temperature at 14.5 °C and confirms the possible use of NORs as a faster and cheaper option to other techniques commonly employed for ploidy verification, namely karyotyping and FCM. Further studies are needed to clarify the relationship between the elevation of ploidy toward tetraploidy and the physiological and genetic mechanisms of high mortality. It will be better to treat eggs from different females separately in mass production of tetraploids, in order to avoid the asynchronization of the egg development and improve the induction rate and tetraploid rate. In addition, novel methods for tetraploidy induction, such as gyno-tetraploidy (Peruzzi and Chatain, 2003; Wang et al. 2020), allotetraploidy, and combined application of pressure shock and cold shock, should be investigated as a possible means of producing the viable tetraploid progenies.
Data availability
All relevant data are within the paper, and those are available from the corresponding author.
Code availability
Not applicable.
References
Amores A, Force A, Yan Y, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang Y, Westerfield M, Ekker M, Postlethwait JH (1998) Zebrafish hox clusters and vertebrate genome evolution. Science 282:1711–1714
Arai K (2001) Genetic improvement of aquaculture finfish species by chromosome manipulation techniques in Japan. Aquaculture 197:205–228
Bencsik I, Pacala N, Dumitrescu G, Dronca D, Stanculet J, Petculescu-Ciochina L, Boca L (2012) Tetraploidy determination in rainbow trout (Oncorhynchus mykiss) Based on erythrocytes dimensions. Animal Sci Biotechnol 45:111–114
Cal RM, Vidal S, Gómez C, Álvarez-Blázquez B, Martínez P, Piferrer F (2006) Growth and gonadal development in diploid and triploid turbot (Scophthalmus maximus). Aquaculture 251:99–108
Cassani JR, Maloney DR, Allaire HP, Kerby JH (1990) Problems associated with tetraploid induction and survival in grass carp, Ctenopharyngodon idella. Aquaculture 88:273–284
Chourrout D (1982) Tetraploidy induced by heat shocks in the rainbow trout (Salmo gairdneri R.). Reprod Nutr Dev 22:569–574
Chourrout D, Chevassus B, Krieg F, Happe A, Burger G, Renard P (1986) Production of second generation triploid and tetraploid rainbow trout by mating tetraploid males and diploid females - potential of tetraploid fish. Theor Appl Genet 72:193–206
Christopher JG, Siva R, Ezilrani P (2019) The effect of heat shock and timing on the induction of tetraploidy in catfish, Heteropnestus fossilis. J Appl Aquac 30:186–192
Dong J, Murakami M, Fujimoto T, Yamaha E, Arai K (2013) Genetic characterization of the progeny of a pair of the tetraploid silver crucian carp Carassius auratus langsdorfii. Fish Sci 79:935–941
Farahmand H, Razak SHA, Hwang G, Maclean N, Rahman MA (2007) Induction of tetraploidy in transgenic tilapia (Oreochromis niloticus) using physical shocks. Iran J Fish Sci 7:27–46
Flajšhans M, Linhart O, Kvasnička P (1993) Genetic studies of tench (Tinca tinca L.): induced triploidy and tetraploidy and first performance data. Aquaculture 113:301–312
Francescon A, Libertini A, Bertotto D, Barbaro A (2004) Shock timing in mitogynogenesis and tetraploidization of the European sea bass Dicentrarchus labrax. Aquaculture 236:201–209
Fujimoto T, Sakao S, Oshima K, Yamaha E, Arai K (2013) Heat-shock-induced tetraploid and diploid/tetraploid mosaic in pond loach, Misgurnus anguillicaudatus. Aquacult Int 21:769–781
Gil HW, Kong HJ, An CM, Kim B, Lim S, Park I (2016) Cytogenetic study of diploid and induced tetraploid in Korean rose bitterling, Rhodeus uyekii. SpringerPlus 5:186
Hartono DP, Witoko P, Purbosari N (2016) The effect of heat shock on the tetraploidy of catfish, Pangasius hypophthalmus. AACL Bioflux 9:597–603
Kobayashi T (1997) Survival and cytological observations on early development of normal, hybrid, and gynogenetic embryos of Amago Salmon. Fish Sci 63:33–36
Komen H, Thorgaard GH (2007) Androgenesis, gynogenesis and the production of clones in fishes: a review. Aquaculture 269:150–173
Lei J, Liu X, Guan C (2012) Turbot culture in China for two decades: achievements and prospect. Prog Fish Sci 33:123–130 ((in Chinese with English abstract))
Li W, Chen S, Ji X, Xie M, Xu Y, Deng H (2012) Induction and identification of tetraploid fry in Cynoglossus smilaevis. J Fish Sci China 19:196–201 ((in Chinese with English abstract))
Li Y, Yu Z, Zhang M, Qian C, Abe S, Arai K (2013) Induction of viable gynogenetic progeny using eggs and UV-irradiated sperm from the Chinese tetraploid loach, Misgurnus anguillicaudatus. Aquacult Int 21:759–768
Lou YD, Purdom CE (1984) Polyploidy induced by hydrostatic pressure in rainbow trout, Salmo gairdneri Richardson. J Fish Biol 25:345–351
Malison JA, Kayes TB, Held JA, Barry TP, Amundson CH (1993) Manipulation of ploidy in yellow perch (Perca flavescens) by heat shock, hydrostatic pressure shock, and spermatozoa inactivation. Aquaculture 110:229–242
Meng Z, Liu X, Liu B, Hu P, Jia Y, Yang Z, Zhang H, Liu X, Lei J (2016) Induction of mitotic gynogenesis in turbot Scophthalmus maximus. Aquaculture 451:429–435
Mustami MK (2017) The formation of polyploidy on Cyprinus carpio Linn Punten Race by heat shocking temperature. J Aquacult Res Dev 8(503):1–4
Myers JM (1986) Tetraploid induction in Oreochromis spp. Aquaculture 57:281–287
Nascimento NF, Pereira-Santos M, Levy-Pereira N, Monzani PS, Niedzielski D, Fujimoto T, Senhorini JA, Nakaghi LSO, Yasui GS (2020) High percentages of larval tetraploids in the yellowtail tetra Astyanax altiparanae induced by heat-shock: the first case in Neotropical characins. Aquaculture 520(734938):1–6
Nam YK, Choi GC, Park DJ, Kim DS (2001) Survival and growth of induced tetraploid mud loach. Aquacult Int 9:61–71
Nam YK, Kim DS (2004) Ploidy status of progeny from the crosses between tetraploid males and diploid females in mud loach (Misgurnus mizolepis). Aquaculture 236:575–582
Pandian TJ, Koteeswaran R (1998) Ploidy induction and sex control in fish. Hydrobiologia 384:167–243
Pardo BG, Bouza C, Castro J, Gullón J, Martı́nez P, Sánchez L (1998) Molecular cytogenetics of flatfishes (Pleuronectidae and Scophthalmidae). In: Proceedings of the 13th European Colloquium on Cytogenetics of Domestic Animals. Budapest, p 41 (Abstract)
Person-Le Ruyet J, Baudin-Laurencin F, Devauchelle N, Métailler R, Nicolas J, Robin J, Guillaume J (1991) Culture of turbot (Scophthalmus maximus). CRC handbook of mariculture 2, pp 21–41
Peruzzi S, Chatain B (2003) Induction of tetraploid gynogenesis in the European sea bass (Dicentrarchus labrax L.). Genetica 119:225–228
Piferrer F, Cal RM, Blázquez BA, Sánchez L, Martinez P (2000) Induction of triploidy in the turbot (Scophthalmus maximus). Ploidy determination and the effects of cold shocks. Aquaculture 188:79–90
Piferrer F, Cal RM, Gómez C, Bouza C, Martínez P (2003) Induction of triploidy in the turbot (Scophthalmus maximus) II. Effects of cold shock timing and induction of triploidy in a large volume of eggs. Aquaculture 220:821–831
Piferrer F, Beaumont A, Falguière J, Flajšhans M, Haffraye P, Colombo L (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293:125–156
Sakao S, Fujimoto T, Tanaka M, Yamaha E, Arai K (2003) Aberrant and arrested embryos from masu salmon eggs treated for tetraploidization by inhibition of the first cleavage. Nippon Suisan Gakkaishi 69:738–748 ((in Japanese with English abstract))
Sakao S, Fujimoto T, Kimura S, Yamaha E, Arai K (2006) Drastic mortality in tetraploid induction results from the elevation of ploidy in masu salmon Oncorhynchus masou. Aquaculture 252:147–160
Sakao S, Fujimoto T, Kobayashi T, Yoshizaki G, Yamaha E, Arai K (2009) Artificially induced tetraploid masu salmon have the ability to form primordial germ cells. Fish Sci 75:993–1000
Sun W, You F, Zhang P, Xu J (2005) Studies on fertilization biology of turbot (Psetta maxima). Mar Sci 29:75–80 ((in Chinese with English abstract))
Váradi L, Benkó I, Varga J, Horváth L (1999) Induction of diploid gynogenesis using interspecific sperm and production of tetraploids in African catfish, Clarias gariepinus Burchell (1822). Aquaculture 173:401–411
Wang G, Zhang X, Sun Z, Zhao Y, Du W, Cui J, Hou J, Wang Y (2020) Induction of gyno-tetraploidy in Japanese flounder Paralichthys olivaceus. J Ocean Limnol 38:288–293
Weber GM, Hostuttler MA (2012) Factors affecting the first cleavage interval and effects of parental generation on tetraploid production in rainbow trout (Oncorhynchus mykiss). Aquaculture 344–349:231–238
Wu Z, You F, Song Z, Hu J, Wang L, Zhu X, Tan X, Li J (2014) Induction of tetraploid in turbot Scophthalmus maximus. Oceanologia Et Limnologia Sinica 45:657–662 ((in Chinese with English abstract))
Wu Z, Wang L, Lu Y, Zhu X, Yue X, You F (2019) Artificial induction and genetic structure analysis of tetraploid turbot Scophthalmus maximus. Front Mar Sci 6(637):1–12
Yi Q, Yu H, Wang X, Wang Z, Wang X, Qi J, Zhang Q (2012) Production of viable tetraploid olive flounder (Paralichthys olivaceus) by hydrostatic pressure. Oceanologia Et Limnologia Sinica 43:382–388 ((in Chinese with English abstract))
Yoshikawa H, Morishima K, Fujimoto T, Rodriguez LA, Yamaha E, Arai K (2008) Ploidy manipulation using diploid sperm in the loach, Misgurnus anguillicaudatus: a review. J Appl Ichthyol 24:410–414
Zhang X, Onozato H (2004) Hydrostatic pressure treatment during the first mitosis does not suppress the first cleavage but the second one. Aquaculture 240:101–113
Zhao J, Liu L, Chen X, Qing N, Dong C (2004) Karyotypic analysis of the multiple tetraploid allogynogenetic pengze crucian carp and its parents. Aquaculture 237:117–129
Zhou L, Gui J (2017) Natural and artificial polyploids in aquaculture. Aquacult Fish 2:103–111
Zhou X, Abbas K, Li M, Fang L, Li S, Wang W (2010) Comparative studies on survival and growth performance among diploid, triploid and tetraploid dojo loach Misgurnus anguillicaudatus. Aquacult Int 18:349–359
Zhu X, Lin Z, Wu Z, Li J, You F (2017) Effect of initiation time of hydrostatic pressure shock on chromosome set doubling of tetraploidization in turbot Scophthalmus maximus. Mar Biotechnol 19:528–540
Zou S, Li S, Cai W, Zhao J, Yang H (2004) Establishment of fertile tetraploid population of blunt snout bream (Megalobrama amblycephala). Aquaculture 238:155–164
Zou S, Li S, Cai W, Yang H (2005) Characters of karyotype and morphology in auto-4n, 4n–F1 and reciprocal interploid 3n cross of blunt snout bream Megalobrama amblycephala. Acta Zoologica Sinica 51:455–461 ((in Chinese with English abstract))
Acknowledgements
We thank Jiangbo Qu, Tao Sun, and Wenlei Gao et al. of Tianyuan Aquaculture Co., Ltd. of Yantai Economic Development Zone and Ke Su and Lujun Li et al. of Qingdao General Aquaculture Co., Ltd. for assistance in fish culture.
Funding
This research was supported by funding from the Key R&D Project of Shandong Province (2019GHY112023), Agricultural Variety Improvement Project of Shandong Province (2019LZGC013), National Natural Science Foundation of China (31402284), and China Agriculture Research System (CARS-47).
Author information
Authors and Affiliations
Contributions
Zhen Meng and Xinfu Liu provided the experimental ideas. Zhen Meng conducted experiments, analyzed data, and wrote manuscript. Yudong Jia and Bin Liu participated in the experiments. Zhi Yang and Hesen Zhang took charge in broodstock and larval culture. Xinfu Liu revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable or not required for this study.
Consent to publication
All authors agree to submit the paper for publication in Aquaculture International.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, Z., Liu, X., Jia, Y. et al. Optimization of hydrostatic pressure, timing, and duration parameters for the induction of tetraploidy in turbot, Scophthalmus maximus. Aquacult Int 29, 2575–2589 (2021). https://doi.org/10.1007/s10499-021-00766-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00766-7