Abstract
Objectives
Urinary tract infection (UTI) is one of the most common extraintestinal infections, and uropathogenic Escherichia coli (UPEC) is the main cause of UTIs. However, the ability to treat UTI has been compromised by the increase in antimicrobial resistance, especially carbapenem resistance. Here, we aimed to characterize the antimicrobial resistance and molecular epidemiology of carbapenem-resistant UPEC isolated in Shandong, China.
Methods
In total, 17 carbapenem-resistant UPEC (CR-UPEC) isolates were collected from July 2017 to May 2020 in the Shandong Provincial Hospital. Whole-genome sequencing and bioinformatics analyses were performed to understand the molecular epidemiology of CR-UPEC. Phylogenetic groups, drug resistance genes, biofilm formation, and virulence-related gene profiles of the isolates were analyzed. Plasmid profiling and conjugation assay were performed to evaluate the ability to transfer carbapenem resistance-related genes to other E. coli isolates. Biofilm formation was also evaluated, as it is important for the persistence of infectious diseases.
Results
We observed that 15 out of 17 CR-UPEC strains were blaNDM producers, among which 4 isolates could transfer blaNDM to recipient cells. The predominant sequence type was ST167 (6/17), followed by ST410 (3/17). The most prevalent phylogenetic group was phylogenetic group A (10/17), followed by phylogenetic group C (3/17). One isolate was resistant to polymyxin, which was caused by the carriage of a transferable plasmid harboring mcr-1. Statistical analysis did not reveal any significant difference in the carriage rate of fimbriae-coding genes between strong and weak biofilm producers.
Conclusions
Our observations may assist in developing new therapeutic methods for drug-resistant organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infection (UTI) is one of the most common community-acquired and nosocomial infectious diseases in people of all ages. According to CHINET bacterial drug resistance monitoring in China (http://www.chinets.com/), the pathogens isolated from urine are only second to those isolated from the respiratory tract. Uropathogenic Escherichia coli (UPEC) is the major etiological factor of UTI (Kot 2019). Reports show that 40% of women and 12% of men will experience at least one symptomatic UTI episode in their lifetimes; in addition, 27 − 48% of the affected women will experience recurrent UTIs by the end of their lives (Zangane Matin et al. 2021). According to statistics, 150 million people worldwide have UTI every year, which directly leads to health care expenses exceeding 4 billion pounds. Of the affected women, 25–30% will continue to develop recurrent infections not associated with any functional or anatomical urinary tract abnormalities (Kucheria et al. 2005).
UPEC colonize the human gastrointestinal tract, can infect and colonize the urinogenital tract under suitable conditions. The virulence factors and host-related characteristics of UPEC promote establishment of UTI (Abd El Ghany et al. 2018). A range of virulence factors have been reported to contribute to UPEC pathogenesis by promoting colonization and infection of the urethra, which include fimbriae with adhesin tips, protectins, toxins, and iron-acquisition systems (Kucheria et al. 2005).
Owing to antibiotic misuse, antimicrobial resistance of clinical E. coli has considerably increased worldwide (Wang et al. 2020). The proportion of E. coli strains insensitive to third-generation cephalosporins and carbapenems isolated from 25 tertiary hospitals in Greece increased from 25.3% to 28.1% in the last decade (Polemis et al. 2020). Although carbapenems are the last line of defense against multidrug-resistant E. coli, the emergence of carbapenem-resistant E. coli (CR-ECO) worldwide has led to difficulties in treating infections caused by these bacteria (Zhang et al. 2021) owing to the lack of effective and safe alternatives, presenting an alarming picture of public health hazard (Doi 2019). In Hebei Province, China, CR-ECO accounted for 2.65% of all E. coli that caused clinical infections, and most CR-ECO from 2017 to 2019 were isolated from urine (Zhang et al. 2021).
The mechanism of resistance of E. coli to carbapenem antibiotics varies between countries and regions (Li et al. 2021). The Shandong Provincial Hospital receives a large population flow from within and outside the province, and the resistance type and mechanism of transmission of carbapenem-resistant uropathogenic E. coli (CR-UPEC) here are unknown. Hence, in this study, we analyzed 17 CR-UPEC strains for conventional carbapenemase phenotype, and the relevant drug resistance profile, biofilm formation ability, and molecular biological characteristics were analyzed to determine the genotypic and phenotypic features of CR-UPEC in Shandong.
Methods
Bacterial strains
A total of 1578 non-duplicate strains of Escherichia coli were isolated in Shandong Province hospital from July 2017 to May 2020, of which 39 strains were CR-ECO (2.47%,39/1578). Among the 39 CR-ECO strains, 22 isolates were from urine. This retrospective study was conductd on the 17 CR-UPEC strains which were preserve in the -80 oC refrigerator. All the strains were identified as E. coli using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (BioMérieux, Marcyl’Étoile, France). The carbapenemase-producing phenotype of bacterial strains was detected was screened using the carbapenem inactivation method (CIM) and EDTA-modified CIM (eCIM) (CLSI 2020). E. coli ATCC25922 was used for quality control.
Antibiotic susceptibility assay
Antibiotic susceptibility was analyzed using a VITEK-2 compact system (BioMérieux, France) for ampicillin (AMP), ampicillin/sulbactam (SAM), piperacillin-tazobactam (TZP), ceftazidime (CAZ), ceftriaxone (CRO), cefepime (FEP), aztreonam (ATM), ertapenem (ETP), imipenem (IMP), meropenem (MEM), amikacin (AMK), gentamicin (GEN), trimethoprim-sulfamethoxazole (SXT), ciproflfloxacin (CIP), and levoflfloxacin (LVX). Susceptibility assay was performed using the broth microdilution method for polymyxin B (POL) and tigecycline (TGC), and the agar dilution method with Mueller–Hinton agar supplemented with 25 µg/mL glucose-6-phosphate (G6P) for fosfomycin (FOS). The results of the susceptibility assay were interpreted using the guidelines of the Clinical and Laboratory Standards Institute 2020(Clinical and M100, 2017), with the exception of tigecycline and polymyxin B, for which the breakpoints from the European Committee on Antimicrobial Susceptibility Testing guidelines (EUCAST 2020) were used (Chen et al. 2021).
In vitro biofilm formation assay
The clinical strains were grown overnight at 37 °C in Luria Bertani (LB) broth. The cultures were then diluted 1:100 in fresh LB, 200 µL of which was added to each well of a sterile 96-well flat-bottomed plastic tissue culture plate and incubated at 37 °C under static conditions for 36 h. Then, the wells were washed thrice with 200 μL phosphate buffered saline. The adherent bacterial biofilms were fixed using 100 μL methanol per well and incubated for 15 min. After the methanol was poured out, the wells were dried at room temperature, followed by the addition of 100 µL 0.1% crystal violet solution and incubation for 5 min at 37 °C; excess dye was washed with flowing water. After air drying at room temperature for 2 h, 100 μL of 30% glacial acetic acid was added to each well to extract the dye bound to the adherent cells. The optical density (OD) was measured at 570 nm. This procedure was repeated thrice for all the 17 isolates and the results were presented as mean ± standard error of the mean. Biofilm formation was scored as follows: strong biofilm (S) = OD ≥ 0.3, moderate biofilm (M) = 0.2 ≤ OD ≤ 0.299, weak biofilm (W) = 0.1 ≤ OD ≤ 0.199, negative (N) = OD < 0.1. The negative control wells contained only LB broth (Zamani and Salehzadeh 2018).
Genotyping using pulsed field gel electrophoresis
The homology of the strains was analyzed using pulse-field gel electrophoresis (PFGE) and multisite sequence analysis. Genomic DNA from clinical strains embedded in low melting point (LMP) agarose blocks was digested using QuickCut XbaI (Takara, Shiga, Japan). The CHEF Mapper apparatus (Bio-Rad, Hercules, CA, USA) was used to separate the restriction fragments for 19 h and the pulse time was switched from 2.16 s to 63.8 s. The PFGE patterns were compared using the Gel-J software, version 2.0 (Heras et al. 2015). Pulsotypes with 80% similarity were assigned to one cluster.
Conjugation assay
Carbapenemase-producing isolates were conjugated with sodium azide-resistant E. coli J53AziR. The donors and recipient bacteria were cultured in LB broth for 10 h, following which 200 μL donor and 100 μL recipient cells were mixed in 10 mL LB broth. After co-incubation for 48 h at 37 °C, the transconjugants harboring carbapenemase resistance genes were screened on Mueller–Hinton agar plates containing 100 mg/mL sodium azide (100 µg/mL) and ceftriaxone (100 µg/mL). Antibiotic susceptibility test and polymerase chain reaction (PCR) were performed to confirm carbapenemase gene transfer (Chen et al. 2019).
Whole genome sequencing and analysis
Genomic DNA was extracted and sequenced using an Illumina Hiseq platform at Novogene Co. Ltd. (Beijing, China). The Illumina sequences were assembled de novo using SPAdes v3.10 (20). Antimicrobial resistance genes, multilocus sequence type (MLST), and virulence genes were analyzed in silico using the Abricate software (https://github.com/tseemann/abricate). CR-UPEC phylogroups were determined using clermontyping (http://clermontyping.iame-research.center/). Serotype and fimH typing were performed using the Bacterial Analysis Pipeline (BAP) on Center for Genomic Epidemiology website tool (http://www.genomicepidemiology.org/). Mutations in fluoroquinolone resistance gene were identified using https://cge.cbs.dtu.dk/services/ResFinder-4.0/. Single nucleotide polymorphism (SNP) calling was performed using Snippy 3.1 (https://github.com/tseemann/snippy), and recombinant variants were excluded using ClonalFrameML 1.0. Maximum likelihood phylogenetic trees were constructed using RAxML (https://github.com/stamatak/standard-RAxML) and the recombination-free SNPs. The tree file was visualized using iTOLV.5 (https://itol.embl.de), and annotated information was edited using iTOL editor v1_1.
For our dataset, core-genome MLST (cgMLST) analysis was performed using the SeqSphere + software (8.0.2 version Ridom, Germany). The resulting set of target genes was then used for interpreting the clonal relationship displayed in a minimum spanning tree using the "pairwise ignoring missing values" parameter during distance calculations.
Statistical analysis
Data were analyzed using SPSS version 26.0. Fisher’s exact test was used to establish.the association between biofilm formation ability and adhesion factor genes in CR-UPEC isolates.
P < 0.05 was considered statistically significant.
Results
Antimicrobial susceptibility
All 17 CR-UPEC isolates were resistant to MEM, AMP, SAM, TZP, CAZ, CRO, FEP, CTT, and SXT, and only one isolate showed resistance to POL (minimal inhibitory concentration or MIC ≥ 8). The highest resistance (> 94%) was observed against ETP, IMP, GN, CIP, and LEV. In the antimicrobial susceptibility test, all strains, except SLDY04, showed resistance to fluoroquinolone. All the strains showed 100% susceptibility to TGC. The results of the antibiotic susceptibility test are shown in Table 1.
Molecular typing and genotyping of phylogenetic groups
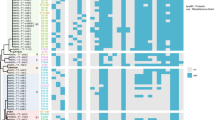
The 17 CR-UPEC strains had 8 sequence types (STs), with ST167 being the most common (n = 6, 35%) (Fig. 1), followed by ST410 (n = 3), ST617 (n = 2), ST405 (n = 1), ST641 (n = 1), ST1771 (n = 1), ST156 (n = 1), and ST361 (n = 1). Nine serotypes were detected and O8:H9 was found to be the predominant serotype (7/17), with ST167-O8:H9 being the most common clone. Eight fimH types were detected, with fimH24 (3/17) and fimH54 (3/17) being the superior fimH types. Five isolates could not be identified with known fimH type according to the Center for Genomic Epidemiology database (Fig. 1).
Based on the PFGE profile, four different clusters were identified as A–D clone groups, along with five singletons. The ST410 and ST167 isolates clustered into two distinct clades and sub-clades (B, C, D clades and sub-clades). Genetic relatedness and phylogenetic analysis showed that the same ST isolates had a high degree of homology (Fig. 1). Phylogenetic group A (58.82%) was predominant, followed by phylogenetic group C (17.65%), phylogenetic group B1 (11.76%), and phylogenetic group B2 (5.88%) (Fig. 1).
The 17 CR-UPEC strains were assigned to different branches, indicating distant homology between strains, and, overall, the strains showed diversity in the SNPs present. Based on the branch length, which reflects evolutionary variability, SLDY04 and SLDY12 were found to share the closest evolutionary relationship (Fig. 2).The predominant plasmid type was Col (n = 16) followed by IncFII (n = 10) (Fig. 2).
A minimum spanning tree of the 17 CR-UPEC isolates was constructed based on cgMLST allelic profiles, which showed the presence of two cluster types (≤ 15 allele differences). The ST167 isolates mainly belonged to cluster 1 (n = 5), while the ST410 isolates belonged to cluster 2 (n = 3) (Fig. 3).
Minimum spanning tree showing core genome multilocus sequence type (cgMLST) of the 17 CRKP isolates, showing 2 cluster types that are numbered consecutively. Each circle represents an allelic profile. Colors of circles indicate the different sequence types (STs). Cluster types consist of closely related genotypes (≤ 15 allele differences). The numbers on the connecting lines illustrate the numbers of target genes with different alleles
Characterization of drug-resistance genes
After the genomes of the 17 CR-UPEC strains were annotated with drug resistance genes, multiple resistance mechanism-associated genes were found in all the strains (Fig. 4). In the 17 CR-UPEC, 15 CR-UPEC strains were blaNDM producers (88.24%, 15/17). Isolates SLDY04 and SLDY16 did not carry any carbapenemase-coding genes, which might be due to over-expression of extended spectrum β-lactamases (ESBLs), AmpC enzymes, and efflux pumps, combined with porin loss (Fig. 4). As expected, all the ST167 strains harbored the blaNDM-5 gene, and it was the most common clinical strain type in this study, which was coincident with the results of previous studies. These strains harbohhred ESBLs and efflux pump genes, as well as deletion in the membrane porin gene. CTX-M was the most common ESBL gene, with CTX-M-15 detected in four isolates, CTX-M-65 in two isolates, CTX-M-55 in two isolates, and CTX-M-199 in one isolate. The plasmid-borne fosfomycin resistance gene, fosA3, was detected in six isolates. In addition, one NDM-5-producing isolate also harbored mcr-1.
Regarding fluoroquinolone resistance, three isolates harbored acc (6’) Ib-cr. Mutations in gyrA (n = 17), encoding DNA gyrase, parC (n = 16) and parE (n = 12), and encoding subunits of topoisomerase IV were found in the FQ-R isolates. In this study, all the gyrA mutations resulted in the S83L substitution, while the D87N substitution was detected in 15 isolates other than S83L substitution. The mutations in parC resulted in S80I substitution in 16 isolates and in E84G substitution in one isolate, while the mutations in parE resulted in S458A substitution (Fig. 4 and Table S3).
Distribution of virulence genes
Figure 5 shows the distribution of virulence genes among the CR-UPEC isolates. The 12 CR-UPEC isolates identified were enriched with virulence genes such as fimA-I, hlyE, iutA, sitA, iroN, and fyuA. SLDY03, SLDY04, SLDY06, SLDY08, and SLDY09 were enriched with siderophores, which is indicative of higher invasive virulence. fimH was detected in 12 UPEC isolates, indicating higher expression of potential adhesins. In addition, four isolates belonging to the ST167 type (66.67%, 4/6) harbored genes encoding adhesins (fimF and fimG) and siderophores (sitA and iroN) simultaneously.
Association between adhesion factor genes and biofilm formation ability
The biofilm formation ability of the UPEC isolates was investigated using the crystal violet staining method. Our results showed that seven isolates were strong biofilm producers, while four were moderate and two were weak biofilm producers. In addition, four UPEC isolates did not show any biofilm formation ability.
To evaluate the association between biofilm formation ability and the presence of adhesion factor genes in the UPEC isolates, the isolates were classified into two groups. Group Ι included no or weak biofilm producers and group II included moderate to strong biofilm producers. Statistical analysis did not reveal any significant difference in the carriage rate of fimbriae genes between the groups [fimA, P = 0.395; fimB, P = 0.395; fimC, P = 0.395; fimD, P = 0.395; fimE, P = 0.261; fimH, P = 0.395; fimI, P = 0.395] (Table S4).
Conjugal transfer
The conjugation assay showed that the blaNDM-harboring plasmids of four CR-UPEC isolates, namely SLDY03, SLDY20, SLDY21, and SLDY27, were successfully transferred into the recipient strain, E. coli J53AziR; therefore, these strains were capable of disseminating the carbapenemase genes horizontally. The antimicrobial resistance profiles of the transconjugants are shown in Table S1. Genomic DNA of all the strains were processed using next-generation sequencing, which revealed that all the strains harbored NDM-5. The plasmid type of transconjugant J-SLDY03 contained IncI1_1, IncFII, and IncFIA_1. The plasmid type of transconjugant J-SLDY20 and J-SLDY21 harbored IncFII. The plasmid type of transconjugant J-SLDY27 harbored Col(MG828)_1 and IncX3_1. All the antimicrobial resistance genes are shown in Table S2.
Nucleotide sequence accession numbers
The raw reads of all 17 isolates have been deposited in GenBank (BioProject PRJNA779169).
Discussion
According to CHINET bacterial drug resistance monitoring report in China (http://www.chinets.com/), the detection rate of CR-ECO was 1.8% from 2017 to 2020 in Shandong Provincial,China.However,the detection rate of CR-ECO(2.47%,39/1578) unpublished data in Shandong Provincial Hospital, China, was higher than the detection rate of CR-ECO in Shandong Province. The drug resistance to commonly used antibiotics in the clinic was high, especially the drug resistance rate to ETP, IMP, GN, CIP, and LEV, which was above 90%.
Recently, the rate of resistance to antimicrobial agents, especially the carbapenem class of drugs, in E. coli has increased (Wu et al. 2020). This poses an obstacle in the treatment of UTI.Li et al. showed that ST167, carrying blaNDM-5 was the most common clinical strain type (Li et al. 2021). Zhang et al. showed that KPC and NDM, mainly NDM-5, were the genesis types of CR-ECO in 25 hospitals in 14 provinces and cities in China (Zhang et al. 2018). However, the resistance mechanisms and genotypic characteristics of CR-ECO, especially the CR-UPEC strains, might differ with regions. In this study, the molecular biological and molecular epidemiological information, such as serological type, MLST, drug resistance genes, and virulence factors, were analyzed using whole-genome sequencing and bioinformatics analyses. Among the 17 CR-UPEC strains, 15 strains were carbapenemase-producers, all of which were due to NDM. ST167 was the most common ST of NDM-producing CR-ECO, which was consistent with the observations of a previous report on the distribution of NDM-producing E. coli most prevalent in Asia, especially in China and India (Dadashi et al. 2019). NDM-5 producing E. coli has emerged as the second-most common NDM variant after NDM-1 in the world (Sun et al. 2019). In this study,( 88.24%,15/17)of the CR-UPEC isolates produced NDMs, among which, the NDM-5-producing strains were dominant, accounting for( 82.35%,14/17)of all the carbapenemase producers. All of the NDM genes are located on the plasmid,therefore, the probability of transmission is relatively high among the strains of this region.
According to a previous study, the dissemination of NDM-5 among CR-ECO was mainly related to the presence of IncX3 type of plasmids. Based on the results of plasmid analysis of transconjugants, transfer of NDM-5 was mainly due to IncF type plasmids (3/4) in this study, which was different from those reported by other groups.
According to previous studies, strains can combine NDM-5 production with other resistance mechanisms to mediate increased resistance to cephalosporins, quinolones, and aminoglycosides (Sun et al. 2019). In our study, seven isolates simultaneously harbored NDM-5 and CTX-M. Fosfomycin, the “old” antibiotic agent, is being reconsidered as a possible auxiliary drug due to limitations in treatment options for carbapenem-resistant organisms. In this study, 41.2% E. coli was resistant to fosfomycin, which was considerably higher than that reported by Kansak et al. (15.8%). Furthermore, fosfomycin resistance was mostly because of the plasmid-borne fosfomycin resistance gene, fosA3 (6/7). Therefore, fosfomycin should be cautiously used for treating UTIs caused by NDM-5-producing UPEC.
Homology analysis showed that ST167, ST617, and ST410 caused clonal expansion in our hospital, which indicated that our region has endemic potential and warrants extensive surveillance. A recent study reported that ST167 and ST410 were the dominant types of CR-ECO; they are widely disseminated in China, and are also the major blaNDM-bearing strains that may become a significant problem in clinical settings in China (Zhang et al. 2017). We observed that 35.29% (6/17) of the CR-UPEC isolates belonged to ST167, followed by 17.65% (3/17) of ST410. Therefore, these sequence types are rapidly spreading among the strains of this region. In addition, homology analysis showed that these high-risk clones are distributed in different branches and that they belong to different phylogroups. All the ST167 strains belong to phylogroup A, while all the ST410 strains belong to phylogroup C. Furthermore, ST1771 was first reported as an NDM producer, indicating the potential horizontal transfer of blaNDM-bearing plasmids among different clones of E. coli strains.
Biofilm formation by UPEC is considered a determinant of the long-term persistence of bacterial cells in the urinary tract, which causes inflammatory reactions associated with UTIs. According to a previous report, phenotypic expression of type 1 fimbriae was associated with biofilm formation. However, we did not observed any relationship with biofilm formation ability and carriage of type 1 fimbriae.
In this study, we detected co-existence of blaNDM-5 and mcr-1 genes in the SLDY13 isolate. The conjugation experiments revealed a carbapenem-susceptible phenotype, which suggested that blaNDM-5 and mcr-1 were located on different plasmids. The emergence of such superbugs is of considerable concern for clinical therapy, and efforts to control their dissemination should be increased.
Conclusions
UPEC is the main etiological factor of UTI. Treatment of UTI is difficult owing to the high antibiotic resistance rate of the pathogen and easy recurrence rate of the infection. Here, we demonstrated prevalence of blaNDM-5 among CR-UPEC in the Shandong Province of China.
Data availability
The data presented in this study are available on reasonable request from the corresponding author.
References
Abd El Ghany M et al (2018) Genomic characterization of NDM-1 and 5, and OXA-181 carbapenemases in uropathogenic Escherichia coli isolates from Riyadh. Saudi Arabia Plos One 13:e0201613
Chen J et al (2019) Molecular Epidemiology of Plasmid-Mediated Fosfomycin Resistance Gene Determinants in Klebsiella pneumoniae Carbapenemase-Producing Klebsiella pneumoniae Isolates in China. Microb Drug Resist 25:251–257
Chen F et al (2021) Clinical Characteristics of Patients and Whole Genome Sequencing-Based Surveillance of Escherichia coli Community-Onset Bloodstream Infections at a Non-tertiary Hospital in CHINA. Front Microbiol 12:748471
CLSI (2020) Performance standards for antimicrobial susceptibility testing document M100, 30th edn. Clinical and Laboratory Standards Institute, Wayne, PA
Dadashi M et al (2019) Frequency distribution, genotypes and prevalent sequence types of New Delhi metallo-beta-lactamase-producing Escherichia coli among clinical isolates around the world: A review. J Glob Antimicrob Resist 19:284–293
Doi Y (2019) Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin Infect Dis 69:S565–S575
European Committee on Antimicrobial Susceptibility Testing (2020) Breakpoint tables for interpretation of MICs and zone diameters version 10.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf
Heras J et al (2015) GelJ–a tool for analyzing DNA fingerprint gel images. BMC Bioinformatics 16:270
Kot B (2019) Antibiotic Resistance Among Uropathogenic Escherichia coli. Pol J Microbiol 68:403–415
Kucheria R et al (2005) Urinary tract infections: new insights into a common problem. Postgrad Med J 81:83–86
Li F et al (2021) Genetic characterization of Carbapenem-Resistant Escherichia coli from China, 2015–2017. BMC Microbiol 21:248
Polemis M, Tryfinopoulou K, Giakkoupi P, Vatopoulos A (2020) Eight-year trends in the relative isolation frequency and antimicrobial susceptibility among bloodstream isolates from Greek hospitals: data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance - WHONET-Greece, 2010 to 2017. [Journal Article; Observational Study]. Euro Surveill 25(34). https://doi.org/10.2807/1560-7917.ES.2020.25.34.1900516
Sun P et al (2019) Characterization Of bla (NDM-5)-Positive Escherichia coli Prevalent In A University Hospital In Eastern China. Infect Drug Resist 12:3029–3038
Wang M et al (2020) A Clinical Extensively-Drug Resistant (XDR) Escherichia coli and Role of Its beta-Lactamase Genes. Front Microbiol 11:590357
Wu B et al (2020) Detection of microbial aerosols in hospital wards and molecular identification and dissemination of drug resistance of Escherichia coli. Environ Int 137:105479
Zamani H, Salehzadeh A (2018) Biofilm formation in uropathogenic Escherichia coli: association with adhesion factor genes. Turk J Med Sci 48:162–167
Zangane Matin F et al (2021) Virulence characterization and clonal analysis of uropathogenic Escherichia coli metallo-beta-lactamase-producing isolates. Ann Clin Microbiol Antimicrob 20:50
Zhang R et al (2017) Nationwide Surveillance of Clinical Carbapenem-resistant Enterobacteriaceae (CRE) Strains in China. EBioMedicine 19:98–106
Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, ... Wang H (2018) Epidemiology of carbapenem-resistant enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother 62(2). https://doi.org/10.1128/AAC.01882-17
Zhang W et al (2021) Clinical Distribution Characteristics of 1439 Carbapenem-Resistant Escherichia coli Strains in China: Drug Resistance, Geographical Distribution, Antibiotic MIC50/90. Infect Drug Resist 14:4717–4725
Acknowledgements
We thank Zhiming Lu for the help and support.
Funding
The study was supported by grants from National Natural Science Foundation of China (81902119) and Shandong Province Natural Science Fundation (ZR2020MH306).
Author information
Authors and Affiliations
Contributions
Yingying Hao contributed to experiment conception and design. Ran Chen and Guili Wang conducted bioinformatics analysis and the wrote the paper.Yueling Wang,Yuanyuan Bai and Zhen Song performed data analysis. Zhongkun Wan and Meng Zhang carried out the bacteria identification. Zaigeng Si,Xinglun Lu and Qian Wang prepared the Tables and figures. The corresponding author is responsible for submitting a competing interests statement on behalf of all authors of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Conflict of interest
the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, R., Wang, G., Wang, Q. et al. Antimicrobial resistance and molecular epidemiology of carbapenem-resistant Escherichia coli from urinary tract infections in Shandong, China. Int Microbiol 26, 1157–1166 (2023). https://doi.org/10.1007/s10123-023-00369-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-023-00369-7