Abstract
Background
Invasive micropapillary carcinoma has been recognized as a rare disease entity with aggressive tumor behavior. However, few reports have described invasive micropapillary carcinoma in the gastrointestinal tract, particularly its involvement in gastric cancer.
Methods
We retrospectively analyzed 930 patients diagnosed with gastric cancer who underwent gastrectomy, and we then histopathologically evaluated the existence of a regional invasive micropapillary component. Clinicopathological features were investigated in patients with an invasive micropapillary component and compared with such features in 100 patients with gastric adenocarcinoma, selected as stage-matched controls, who underwent gastrectomy during the same period.
Results
Of the 930 patients, 14 were histopathologically diagnosed with gastric cancer with a regional invasive micropapillary component. There were no significant differences in age, gender, tumor location, macroscopic type, or type of surgery between patients with an invasive micropapillary component and the pT-matched controls. Histopathologically, significant differences were observed in lymphatic infiltration, venous invasion, the percentage of cases with lymph node metastasis, and the median number of metastatic lymph nodes. The three-year disease-free and overall survival rates of patients with an invasive micropapillary component were 40.5 and 59.3%, respectively, compared with those for the stage-matched controls, which were 72.6 and 80.6%, respectively (p = 0.02 and 0.07).
Conclusions
Patients with gastric cancer with a regional invasive micropapillary component showed marked cancer infiltration in the lymphatic pathway and poor prognosis after gastrectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive micropapillary (IMP) carcinoma was first reported as a rare subtype of invasive ductal carcinoma of the breast [1], defined as a carcinoma composed of small clusters of tumor cells lying within clear spaces simulating vascular channels. This rare histological type frequently shows aggressive tumor behavior with marked lymph-vascular invasion, resulting in poor prognosis [2, 3]. Recently, carcinomas demonstrating histological findings similar to IMP carcinoma of the breast have been reported to occur in various organs, including the urinary bladder [4], ureter [5], lung [6], and parotid gland [7]. However, few reports have discussed such cancers originating from the gastrointestinal tract [8–10]. In particular, study of the IMP component in cancer of the stomach has been limited and has not been well addressed [8]. Previous investigation of primary IMP carcinoma of the stomach showed downregulation of E-cadherin expression [8], implying that the presence of an IMP component in stomach cancer is a poor prognostic factor, as with other organs. Nevertheless, because previous study has been confined to the immunohistochemical analysis of a single case of IMP carcinoma, the clinical behavior of gastric cancer with an IMP component has not been clarified. Moreover, it is unclear whether gastric cancer with an IMP component shares common features with IMP carcinoma of the breast, such as aggressive behavior with marked lymph-vascular invasion.
Therefore, in the present study, to reveal the clinical features of gastric cancer with an IMP component, we compared such cases of gastric adenocarcinoma with randomly assigned stage-matched controls who underwent gastrectomy during the same period, and here we discuss the prognosis of this unique histopathological entity.
Patients and methods
Patients
Patients who underwent surgery for gastric cancer from January 2005 to July 2009 were identified from the Division of Pathology database at the National Cancer Center Hospital East; their data were retrospectively analyzed following approval from The Investigational Review Board at the National Cancer Center. Preoperative diagnosis was based on preoperative imaging studies, including upper gastrointestinal studies, endoscopy, and conventional cross-sectional imaging studies (computed tomography). Histological evaluation of endoscope-guided biopsy specimens was performed in all cases. The patients’ medical records were reviewed to determine the preclinical stage of the disease, surgical procedures employed, histopathological findings of the lesions, and the outcomes.
In all cases, gastrectomy was performed in the usual manner under the direction of the regular attending surgeons. In distal gastrectomy, resection of about 2/3 of the stomach with D2 regional lymph node dissection was performed, regardless of the size of the tumor. In total gastrectomy, resection of the whole stomach with D2 regional lymph node dissection was performed. Splenectomy was performed in cases where the tumor invasion was found to extend further than the subserosal layer of the stomach, and when the tumor was located on the greater curvature of the stomach. Reconstruction of the stomach was performed mostly using the Billroth 1 procedure for distal gastrectomy and the Roux-en-Y procedure for total gastrectomy.
Histopathological and immunohistochemical analyses
The surgically resected stomachs were processed in the usual manner. In brief, the resected stomachs were opened along the greater curvature, placed on a wooden board with the mucosa facing up, and fixed with a 10% formalin solution for at least 24 h. Several portions, including the distal and proximal stump, as well as both main and sub-lesions, were sliced to a thickness of 5 mm and histologically examined. For the histopathological evaluation, at least 2 pathologists who specialized in the field of gastrointestinal tract evaluated all stained slides of the lesions. An IMP component was determined to exist if the component was found to be present in a macroscopic regional manner. In brief, we confirmed the micropapillary component by immunohistochemical staining for epithelial membrane antigen (EMA), and if the component was present in more than 10% of each tumor the diagnosis was gastric cancer with an IMP component.
The gastric cancers were evaluated according to the Japanese Gastric Cancer Association, Japanese classification of gastric carcinoma [11]. The macroscopic pattern of early gastric cancers was classified according to the Japanese Society for Gastroenterology endoscopic criteria as type 0-I (protruded), type 0-IIa (elevated), type 0-IIb (flat), type 0-IIc (depressed), type 0-III (excavated), and type 1-4 (Bormann 1-4). Histological grading of the gastric cancers was divided into 3 types; well, moderately, and poorly differentiated adenocarcinoma [12]. The IMP component was diagnosed by at least two pathologists who specialized in the field of gastrointestinal tract. To rule out adenocarcinoma with extensive lymphatic infiltration, or mucinous carcinoma (which could potentially mimic the IMP component), histopathological examination was performed using D2-40 and periodic acid-Schiff (PAS) stain.
Statistical analysis
Statistically significant differences were analyzed using the χ2 test and the Mann–Whitney U-test. Univariate analysis and multivariate analysis with the Cox proportional hazards model were performed to evaluate the significance of the clinical and histopathological parameters. A value of p < 0.05 was considered statistically significant.
Results
Incidence and clinical manifestations of gastric cancers with an IMP component
From January 2005 to July 2009, 930 patients with gastric cancers underwent gastrectomy at the National Cancer Center Hospital East. Of these, 14 patients (1.5%) histologically showed a regional IMP component. Representative images of the IMP component are shown in Fig. 1; no patients showed a pure form of IMP carcinoma.
In order to evaluate the biological characteristics of the tumor itself in patients with gastric cancer with an IMP component, the clinical manifestations of these 14 patients were compared with those of randomly assigned pT factor-matched controls (pT-matched controls) whose data were extracted from the data of the initial 930 patients and who had gastrectomies during the same period as the study subjects (see Table 1). The median ages of the patients with gastric cancer with an IMP component and the pT-matched controls were 62.1 years (range 43–75 years) and 60.4 years (range 38–82 years), respectively (p = 0.37). No significant difference was found in the gender distribution (IMP component, M:F = 2.5:1; pT-matched controls, M:F = 1.7:1), in the distribution of tumor location in the stomach (upper third of the stomach:middle third of the stomach:lower third of the stomach—IMP component 21:50:28.5%, pT-matched controls 14:37:49%), or in the macroscopic type of the lesion (IMP component, type 0-IIc:type 1:type 2 or 3 = 21.4:7.1:71.4%; pT-matched controls, type 0-IIc:type 1:type 2 or 3 = 24:7:69%).
Histopathological manifestations of gastric cancers with an IMP component
The percentages of well-differentiated adenocarcinoma with a papillary pattern are shown in Fig. 2. Well-differentiated adenocarcinoma with a papillary pattern was found in 29% of cases with gastric cancer with an IMP component, whereas 6% of the pT-matched controls showed well-differentiated adenocarcinoma with a papillary pattern (p < 0.01). There were no differences in the distribution of moderately differentiated adenocarcinoma orpoorly differentiated adenocarcinoma between the groups (p = 0.83) (Table 2).
Histopathological types of the primary lesions in patients with an invasive micropapillary component (IMP). In the patients with an invasive micropapillary component, 29% showed well-differentiated (W/D) adenocarcinoma with a papillary pattern as the co-existent histological component, whereas only 6% of the pT-matched controls showed well-differentiated adenocarcinoma with a papillary pattern (p < 0.01)
The histopathological manifestations in patients with an IMP component compared with those in the pT-matched controls are shown in Table 2. Between patients with gastric cancer with an IMP component and pT-matched controls, no significant differences were found in the size of the primary lesion (median size 61.5 vs 56.2 mm: p = 0.23), in the depth of invasion of the tumor (mucosa-muscular layer 42.8%, subserosal-serosal layer 57.1% vs mucosa-muscular layer 43.0%, subserosal-serosal layer 57.0%: p = 0.96), in the dominant histological grade of the lesion (percentage of well-differentiated adenocarcinoma 42.8 vs 28.0%, moderately differentiated adenocarcinoma 50.0 vs 56.0%, poorly differentiated adenocarcinoma 7.1 vs 14.0%, other histological type 0 vs 2.0%: p = 0.83), in the perineural invasion of the tumor (percentage of ne2, 3; cases 28.5 vs controls; 25.0%: p = 0.96). On the other hand, statistically significant differences were observed in the degree of lymphatic infiltration (ly2 or 3; 78.5 vs 32.0%: p < 0.01), in the degree of venous invasion (incidence of v2 or 3; 62.4 vs 30.0%: p = 0.02), in the median number of metastatic lymph nodes (number/case; 16.3 vs 3.2: p < 0.01), and in the frequency of lymph node metastasis (percentage of cases; 100 vs 57.0%: p < 0.01). In particular, all patients with the IMP component showed marked lymph node metastasis.
Outcome for patients with an IMP component
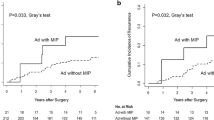
The results of the present study indicate that patients with an IMP component frequently showed marked lymphatic infiltration and lymph node metastasis, which are clinicopathological characteristics similar to those of IMP carcinoma of the breast. Thus, in order to assess the outcome for patients with gastric cancer associated with an IMP component, we evaluated the survival rate in these patients and compared it with the survival rate in 100 stage-matched controls randomly assigned over the same period. The patients’ demographic data and characteristics are shown in Table 3. Patients with an IMP component showed a significantly higher incidence of aggressive lymphatic infiltration (p < 0.01), venous invasion (p < 0.01), and lymph node metastasis (p < 0.01). As shown in Fig. 3, the 3-year disease-free survival rate of patients with an IMP component was 40.5%, whereas that of the stage-matched controls was 72.6% (p = 0.02). Further, as shown in Fig. 4, the 3-year overall survival rate of patients with an IMP component was 59.3%, whereas that of the stage-matched controls was 80.6% (p = 0.07).
Overall survival (OS) rate of the patients with gastric cancer with a regional invasive micropapillary structure. The 3-year survival rate of the patients with an invasive micropapillary structure was lower than that of the stage-matched controls (80.6 vs 59.3%, p = 0.07), although the difference was not significant
Univariate analysis and multivariate analysis
To explore factors with potential prognostic significance, various pathological parameters were investigated in the 14 patients with the IMP component and in the 100 stage-matched controls (total 114 cases). The results of univariate analysis revealed that the following factors were significant indicators of survival in patients after the operation: IMP component (p = 0.02), depth of tumor invasion (p < 0.01), lymphatic infiltration (p = 0.01), venous invasion (p = 0.04), perineural invasion (p = 0.03), and lymph node metastasis (p < 0.01). To further evaluate the significance of these 6 factors, multivariate analysis was carried out. Results of the multivariate analysis with the Cox proportional hazard model showed that depth of tumor invasion [hazard ratio (HR) 4.28, 95% confidence interval (CI) 1.493–12.320, p < 0.01] and lymph node metastasis (HR 6.29, 95% CI 1.749–22.686, p < 0.01) were independent prognostic factors for disease-free survival, and the IMP component was not an independent prognostic factor for disease-free survival (Table 4).
Discussion
The present study provides the first analysis of the clinicopathological features of IMP carcinoma of the stomach. Cancers with an IMP component have been reported not only in the breast but also in various other organs [3–7]. The accumulated evidence indicates that most breast cancers with an IMP component show marked tumor invasion of the lymphatic system, resulting in aggressive tumor behavior and a poor clinical course [3]. A similar IMP pattern has been reported in cancers originating from the gastrointestinal tract [8–10]. However, to the best of our knowledge, because the number of reports to date is limited, the clinical and histopathological features of this specific subtype of stomach cancer are largely unknown and are not being addressed in cases of IMP carcinoma of the stomach. Therefore, we first investigated the incidence of gastric cancer with an IMP component and found that there were no cases that exhibited the pure form of IMP carcinoma, which differs from the situation in breast cancer [13]. We found that, in the regional form of the invasive component, 1.5% (14/930) of cases showed an IMP pattern. Categorizing these cases as gastric cancer with a regional IMP component, we investigated the clinical and histopathological features of such cancers and found that all these cases shared common histopathological findings, such as a higher incidence of lymphatic infiltration and lymph node metastasis, which is consistent with the features of IMP carcinoma of the breast. In the present study, higher rate of papillary carcinoma was found in cancers with IMP component compared with those without IMP component. We have no definitive explanation to clarify the underlying mechanism why the high rate of papillary pattern is found in cancer of IMP component, and we cannot exclude a potential bias, because the number of gastric cancers with an IMP component was so small. In a recent report, however, differences in tumor grades were also demonstrated in colon cancer with an IMP component compared with colon cancer without an IMP component. Moreover, differences in the molecular background, such as differences in p53 or MMR gene expression and microsatellite instability status, were reported in gastrointestinal cancers with an IMP component compared with those without the IMP component. Thus, it is not unreasonable that these differences in molecular background could be involved in the patterns of tumor growth.
The prognosis for patients with IMP carcinoma has not been clarified. Due to the peculiar proclivity for lymphatic infiltration and the high incidence of lymph node metastasis, a poorer prognosis for IMP carcinoma was shown than that for usual invasive ductal carcinoma of the breast [14]. However, other reports have observed no poorer survival rates in patients with IMP carcinoma of the breast when data were adjusted for stage of disease, reasoning that a higher incidence of lymph node metastasis is usually categorized as advanced stage disease [15, 16]. In the present study, although the number of patients with an IMP component was small, we demonstrated a significant difference in disease-free survival rates in patients with IMP carcinoma of the stomach compared with stage-matched controls during the same period. Considering that lymph node metastasis is an important parameter for the determination of the stage of disease, the reasons for the poor prognosis in gastric cancer patients with the IMP component could also have been due to the aggressive tumor behavior in infiltration of the venous system.
The molecular background of IMP carcinoma has been examined in gastric cancer. Previous immunohistochemical analysis of this subtype have revealed the downregulation of E-cadherin compared with that in normal gastric epithelia [8]. Because E-cadherin is generally recognized as an invasion-suppressor gene [17, 18] and loss of E-cadherin expression has been demonstrated to be associated with tumor invasion in adenocarcinoma of the stomach [19], the aggressive tumor behavior of this subtype could be partly attributed to its molecular characteristics. Although there are conflicting data, several previous reports have demonstrated the molecular profile of IMP carcinoma in the breast. Estrogen receptor expression has been found in 25% [20] to 90% [21] of all pure IMP carcinoma cases, whereas HER2 protein overexpression has been observed in 36–100% [20, 22]. Other previous reports on breast cancer demonstrated that 72% of cases with IMP carcinoma expressed HER2 protein and 45% showed amplification of HER2 gene levels [23]. Considering the usual incidence of overexpression of HER2 protein and amplification of the HER2 gene, IMP carcinoma of the breast could have a higher incidence of overexpression of HER2 status. Also, taking into consideration a recent report from a large clinical trial in patients with gastric cancer (ToGA study) [24], it appears that information based on an investigation of HER2 status would provide interesting insights into IMP carcinoma. The ToGA study demonstrated a significantly better survival rate in patients treated with additional administration of trastuzumab (a monoclonal antibody against HER2) [24]. Therefore, it is potentially of great importance to evaluate the HER2 level in this subtype of gastric cancer when considering the future treatment strategy for patients with gastric cancer with a regional IMP component.
The present study has several limitations. Due to the low incidence of patients diagnosed with a regional IMP component, the number of patients was too small to perform more rigorous statistical evaluations, and the clinicopathological investigation was possibly biased. Furthermore, the present study covered a period of almost 4 years, during which preoperative diagnostic accuracy and postoperative follow-up regimens differed slightly. However, the performance of histopathological explorations was consistent, and this consistency may be considered a strong point of the study.
In conclusion, the results of the present study indicate the following: (1) there were no significant differences among patients with gastric cancer with an IMP component compared with controls in terms of age or gender or the location or macroscopic type of the tumor. (2) In patients with gastric cancer with an IMP component, significantly higher incidences of lymphatic infiltration, venous invasion, and lymph node metastasis were apparent compared with controls. (3) Survival rates of patients with an IMP component did tend to be lower than those of the stage-matched controls. Further analysis including the molecular background of the lesions and investigations of a large number of cases in a prospective setting should provide more detailed clues for understanding the prognosis and clinicopathological features of gastric cancer with an IMP component.
References
Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6:660–2.
Tavassoli FA, Devilee P. WHO classification of tumours, pathology and genetics, tumour of the breast and female genital organs. Lyon: IARC Press; 2003. p. 35–6.
De La Cruz C, Moriya T, Endoh M, Watanabe M, Takeyama J, Yang M, et al. Invasive micropapillary carcinoma of the breast: clinicopathological and immunohistochemical study. Pathol Int. 2004;54:90–6.
Amin MB, Ro JY, El-Sharkawy T, Lee KM, Troncoso P, Silva EG, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol. 1994;18:1224–32.
Oh YL, Kim KR. Micropapillary variant of transitional cell carcinoma of the ureter. Pathol Int. 2000;50:52–6.
Amin MB, Tamboli P, Merchant SH, Ordöňez NG, Ro J, Ayala AG, et al. Micropapillary component in lung adenocarcinoma: distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–64.
Michal M, Skalova A, Mukensnabl P. Micropapillary carcinoma of the parotid gland arising in mucinous cystadenoma. Virchows Arch. 2000;437:465–8.
Shimoda M, Okada Y, Hayashi Y, Hatano S, Kawakubo H, Omori T, et al. Primary invasive micropapillary carcinoma of the stomach. Pathol Int. 2008;58:513–7.
Sakamoto K, Watanabe M, De La Cruz C, Honda H, Ise H, Mitsui K, et al. Primary invasive micropapillary carcinoma of the colon. Histopathology. 2005;47:79–84.
Trabelsi A, Ali AB, Yacoub-Abid LB, Stita W, Mokni M, Korbi S. Primary invasive micropapillary carcinoma of the colon: case report and review of the literature. Pathologica. 2008;100:428–30.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 2nd English edition. Gastric Cancer 1998;1:10–24.
Maehara Y, Orita H, Okuyama T, Moriguchi S, Tsujitani S, Korenaga D, et al. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245–7.
Peterse JL. Breast carcinoma with an unexpected inside out growth pattern. Rotation of polarization associated with angioinvasion. Pathol Res Pract. 1993;189:780. (abstract).
Pettinato G, Manivel CJ, Panico L, Sparano L, Petrella G. Invasive micropapillary carcinoma of the breast: clinicopathological study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Surg Pathol. 2004;121:857–66.
Chen L. Breast carcinoma with micropapillary features: clinicopathological study and long-term follow-up of 100 cases. Int J Surg Pathol. 2008;16:155–63.
Kuroda H, Sakamoto G, Ohnishi K, Itoyama S. Clinical and pathologic features of invasive micropapillary carcinoma. Breast Cancer. 2004;11:169–74.
Roger L, Jullien L, Gire V, Roux P. Gain of oncogenic function of p53 mutant regulates E-cadherin expression uncoupled from cell invasion in colon cancer cells. J Cell Sci. 2010;123:1295–305.
Yonemura Y, Ninomiya I, Kaji M, Sugiyama K, Fujimura T, Tsuchihara K, et al. Decreased E-cadherin expression correlates with poor survival in patients with gastric cancer. Anal Cell Pathol. 1995;8:177–90.
Chen HC, Chu RY, Hsu PN, Hsu PI, Lu JY, Lai KH, et al. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett. 2003;201:97–106.
Middleton LP, Tressera F, Sobol ME, Bryant BR, Alburquerque A, Grases P, et al. Infiltrating micropapillary carcinoma of the breast. Mod Pathol. 1999;12:499–504.
Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol. 2001;32:583–9.
Luna-More S, Casquero S, Perez-Mellado A, Rius F, Weill B, Gornemann I. Importance of estrogen receptors for the behavior of invasive micropapillary carcinoma of the breast. Review of 68 cases with follow-up of 54. Pathol Res Pract. 2000;196:35–9.
Varga Z, Zhao J, Ohlschlegel C, Odermatt B, Heitz PU. Preferential HER2/neu overexpression and/or amplification in aggressive histopathological subtypes of invasive breast cancer. Histopathology. 2004;44:332–8.
Okines AF, Cunningham D. Trastuzumab in gastric cancer. Eur J Cancer. 2010;46:1949–59.
Acknowledgments
We thank the members of the Division of Digestive Surgery for their critical discussion of this study. We greatly appreciate Dr. Satoshi Fujii for providing suggestions of the histopathological findings. We also appreciate the members of the Division of Gastrointestinal Medicine for providing us with information about the endoscopic examinations.
Conflict of interest
There are no financial supports or relationships that may pose a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujita, T., Gotohda, N., Kato, Y. et al. Clinicopathological features of stomach cancer with invasive micropapillary component. Gastric Cancer 15, 179–187 (2012). https://doi.org/10.1007/s10120-011-0094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-011-0094-5