Abstract
A 70-year-old woman was referred to our hospital because of early gastric cancer (lesser curvature of the antrum, 0-IIc, tub1, 15 mm) and underwent endoscopic submucosal dissection. Microscopically, the lesion was found to be confined to the mucosa, and predominantly composed of well-differentiated tubular adenocarcinoma with a micropapillary component. On immunohistochemical examination, the characteristic “inside-out pattern” of the micropapillary component was observed; thus, we diagnosed the lesion as gastric cancer with a micropapillary component. Invasive micropapillary carcinoma is a rare subtype of gastric carcinoma, and, to our knowledge, this is the first case of invasive micropapillary carcinoma of the stomach confined to the mucosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive micropapillary carcinoma (IMPC) is a rare subtype of adenocarcinoma, and is defined as a carcinoma composed of small clusters of tumor cells located within clear spaces, which simulate lymphovascular channels [1]. This subtype has been previously described in various organs and tissues, including the breasts [1, 2], lungs [3], urinary bladder [4], major salivary glands [5], colon [6], bile duct [7], ampulla of Vater, and pancreas [8]. IMPC is known to have a high incidence of lymph node metastasis, and is generally associated with a poor prognosis. Although there are several previous reports of IMPC of the stomach [9–20], to our knowledge, IMPC of the stomach confined to the mucosa has never been reported. In the present report, we describe a case of IMPC of the stomach confined to the mucosa, which we believe is the first case. In addition, we assessed the pathological findings according to the Japanese classification of gastric carcinoma (3rd English edition) [21].
Case report
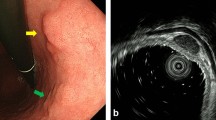
A 70-year-old woman was referred to our hospital with early gastric cancer. She had undergone endoscopic submucosal dissection (ESD) for early gastric cancer (M, tub1, 0-IIc, pM, 18 mm) two years previously. On admission, there were no remarkable physical or laboratory findings. Upper gastrointestinal endoscopy showed a red depressed lesion at the lesser curvature of the gastric antrum (Fig. 1a, b). Histological examination of specimens obtained by forceps biopsy indicated the presence of well-differentiated tubular adenocarcinoma. Enhanced computed tomography (CT) showed no lymph node or distant metastasis. Based on the preoperative examination, the lesion was diagnosed as early gastric cancer (tub1, 0-IIc, tumor depth M), and ESD was performed. Macroscopically, a depressed lesion in the center of the resected specimen was noted, the tumor was found to be predominantly composed of well-differentiated tubular carcinoma cells. In addition, we observed a low papillary structure and a micropapillary component in approximately 15 % of the mass (Fig. 2a–c). There was no lymphatic or venous invasion, and no cancer cells were observed at the edges of the resected specimen. Non-cancerous gastric mucosa was mainly composed of pyloric metaplasia, and intestinal metaplasia was found around the tumor. Although Helicobacter pylori (HP) infection was not detected, these findings may be associated with HP infection.
Histological findings: a, b Microscopically, it was composed mostly well-differentiated tubular carcinoma, and the micropapillary component was observed in this lesion (HE staining, ×40, ×100); c The mapping of the ESD red line well-differentiated tubular adenocarcinoma. Blue line invasive micropapillary carcinoma
Opon immunohistochemistrical examination, the IMPC component was found to be positive for mucin 5AC(MUC5AC), MUC6, MUC2, MUC1, cytokeratin7(CK7), epithelial membrane antigen (EMA), caudal type homeobox 2, and tumor suppressive protein p53, and negative for CK20, CD10, and podoplanin(D2-40). In addition, the tumor cell membranes were positive for E-cadherin and β-catenin. The positive mucin expression indicated that the lesion was a gastric intestinal type. The MIB-1 proliferation index was more than 90 %. Furthermore, the immunohistochemical stainging patterns of EMA and MUC1 showed an “inside-out pattern,” which is characteristic of the IMPC component (Fig. 3a–c). There was no difference between the well-differentiated tubular adenocarcinoma and the IMPC component as per immunohistochemical analysis mentioned above. Based on these findings, we made a final diagnosis of early gastric cancer with a micropapillary component (L, Less-Ant, 9 × 15 × 1 mm, Type 0-IIc, tub1 > IMPC, pT1(M), ly0, v0, pUl(-), pHM0, pVM0). This lesion was confined to the mucosa but contained a micropapillary component. Hence, careful follow-up of this patient was performed.
Upper gastrointestinal endoscopy 29 months after the second ESD showed a red depressed lesion at the site of the previous ESD scar. The lesion was diagnosed as a well-differentiated tubular adenocarcinoma by forceps biopsy, and considered a metachronous lesion. Accordingly, distal gastrectomy was performed, and we subsequently made a final diagnosis of early gastric cancer (L, Less, Type 0-IIc[ul− IIs], 10 × 12 × 1 mm, tub1 > por2, pT1[M], ly0, v0, pN0[0/23], pPM0, pDM0). The patient has been disease-free for 15 months after the distal gastrectomy.
Discussion
IMPC is a rare subtype of adenocarcinoma found in various organs and tissues, and is associated with a high tumor grade and a poor prognosis [1–8]. Histologically, it is characterized by the presence of small clusters of tumor cells located within clear spaces that simulate vascular channels. In addition, immunohistochemical staining for EMA, MUC1, and D2-40 in such cases shows a characteristic “inside-out pattern” [1]. There are currently only 11 reports on IMPC of the stomach. In previous reports, IMPC of the stomach accounted for between 0.07 and 13.4 % of all gastric cancers [9–20], and, in the majority of the studies, IMPC of the stomach was described as an aggressive variant associated with a high incidence of nodal metastases and a poor prognosis. However, according to one of the reports, there were no differences between gastric carcinomas with and without IMPC histology in terms of overall and disease-free survival among patients with Stages III–IV of the disease. Therefore, the clinical and histopathological features of IMPC are somewhat controversial.
In four of the previously reported cases, IMPC components were observed in the mucosa, with each lesion invading the submucosa or deeper [9, 17]. One of these cases consisted mainly of a mucosal lesion, with a submucosal invasion of only 200 μm. However, despite of the presence of only minimal invasion into the submucosa, the lesion also presented with both lymphatic and venous invasion. Similarly, in one of the other cases, the IMPC component was reported to be found in front of the deep invasive part of an early differentiated gastric cancer, while the submucosal invasion was again only 200 μm [12]; in this case, both lymphatic invasion and regional lymph node metastasis were observed. IMPC of the stomach may not fulfill the conditions necessary for ESD on the basis of the Japanese gastric cancer treatment guidelines [22], and therefore, the curability of the lesions should be assessed carefully.
In the present case, the lesion was confined to the mucosa, and no lymphatic or venous invasion was observed. However, we followed-up our patient carefully due to the potential for lymph node metastases.
As suspected, a metachronous lesion was found 29 months after the second ESD, which involved a gastric mucosal tumor at the site of the post-ESD scar. Histologically, the low papillary structure and IMPC component were not found in the recurrent lesion. In addition, no cancer cells were found at the edge of the second ESD specimen, and we therefore diagnosed this recurrent lesion, not as a local recurrence, but rather as a metachronous occurrence. However, the prevalence of metachronous occurrences of gastric cancer with an IMPC component is currently unknown, as there are only three previous reports of this phenomenon [10, 12, 20]. This may indicate that the properties of background gastric mucosa facilitate the development of metachronous gastric lesions. Various factors have been implicated as a cause of the metachronous occurrence of gastric cancer, including environmental and lifestyle factors, and most importantly, persistent HP infection [23, 24]. Further studies are necessary to clarify the triggers of metachronous occurrences of gastric cancer with an IMPC component.
Currently, we believe that this subtype of gastric cancer should be treated while considering that it may not be indicated for ESD and may be associated with the risk of a metachronous occurrence. Moreover, in the present case, the IMPC component had a sharp margin and we could not observe the lesion by magnifying endoscopy with narrow band imaging. Therefore, further studies are required on the usefulness of this method for identifying the IMPC component of gastric cancer.
Conclusion
In the present report, we described a case of gastric mucosal cancer with an IMPC component, which we believe is the first such case in the literature. However, additional studies with a greater number of cases are required to clarify whether ESD can be indicated for these tumors, and also assess their malignant potential.
References
Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6:660–2.
Pettinato G, Manivel CJ, Panico L, et al. Invasive micropapillary carcinoma of the breast: clinicopathologic study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Clin Pathol. 2004;121:857–66.
Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–64.
Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol. 1994;18:1224–32.
Nagao T, Gaffey TA, Visscher DW, et al. Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol. 2004;28:319–26.
Sakamoto K, Watanabe M, De La Crutz C, et al. Primary invasive micropapillary carinoma of the colon. Histopathology. 2005;47:479–84.
Kondo T. Bile duct adenocarcinoma with minor micropapillary component: a case report. Cases J. 2009;2:51.
Khayyata S, Basturk O, Adsay NV. Invasive micropapillary carcinomas of the ampullo-pancreatobiliary region and their association with tumor-infiltrating neutrophils. Mod Pathol. 2005;18:1504–11.
Shimoda M, Okada Y, Hayashi Y, et al. Primary invasive micropapillary carcinoma of the stomach. Pathol Int. 2008;58:513–7.
Kondo T, Kitazawa R, Kitazawa S. Gastric remnant adenocarcinoma with micropapillary component. Dig Dis Sci. 2008;53:2287–9.
Nakamura E, Hirota M, Kanzaki A, et al. Gastric carcinoma with invasive micropapillary pattern: a case report with immunohistochemical analysis. Jpn J Diagn Pathol. 2008;25:306–10.
Asaumi Y, Sakatoku M, Kaneko M, et al. A case of early gastric carcinoma with micropapillary carcinoma. Jpn J Gastroenterol Surg. 2009;42:1791–4.
Roh JH, Srivastava A, Leuwers GY, et al. Micropapillary carcinoma of stomach: a clinicopathologic and immnohistochemical study of 11 cases. Am J Surg Pathol. 2010;34:1139–46.
Lee JH, Kim JH, Choi JW, et al. The presence of a micropapillary component predicts aggressive behavior in early and advanced gastric adenocarcinomas. Pathology. 2010;42:560–3.
Okada A, Arai T, Saeki S, et al. A case of primary invasive micropapillary carcinoma of the stomach. Jpn J Gastroenterol Surg. 2010;43:1112–6.
Eom DW, Kang GH, Han SH, et al. Gastric micropapillary carcinoma: a distinct subtype with a significantly worse prognosis in TNM stages I and II. Am J Surg Pathol. 2011;35:84–91.
Ushiku T, Matsusaka K, Iwasaki Y, et al. Gastric carcinoma with invasive micropapillary pattern and its association with lymph node metastasis. Histopathology. 2011;59:1081–9.
Fujita T, Gotohda N, Kato Y, et al. Clinicopathological features of stomach cancer with invasive micropapillary component. Gastric Cancer. 2012;15:179–87.
Ninomiya S, Sonoda K, Shiroshita H, et al. Five-year survival after surgery for invasive micropapillary carcinoma of the stomach. Case Rep Surg. 2013;2013:560712.
Ohtsuki Y, Kuroda N, Yunoki S, et al. Immunohistochemical analysis of invasive micropapillary carcinoma pattern in four cases of gastric cancer. Med Mol Morphol. 2013;46:114–21.
Japanese gastric cancer association. Japanese classification of gastric carcinoma (3rd English edition). Gastric Cancer. 2011;14:101–12.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver.3). Gastric Cancer. 2011;14:113–23.
Ferrari F, Reis MA. Study of risk factors for gastric cancer by populational databases analysis. World J Gastroenterol. 2013;19:9383–91.
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9.
Disclosures
Conflict of interest
H. Tanaka, Y. Baba, T. Sase, Y. Isono, S. Matsusaki, T. Saito, H. Okano, K. Mukai, T. Murata, G. Watanabe declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, H., Baba, Y., Sase, T. et al. Gastric intramucosal adenocarcinoma with an invasive micropapillary carcinoma component. Clin J Gastroenterol 8, 14–17 (2015). https://doi.org/10.1007/s12328-014-0541-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-014-0541-z