Abstract
In Brazil’s state of Acre, in southwestern Amazonia, wildfires mediated by extreme droughts in 2005 and 2010 affected more than 500,000 ha of forest, causing changes in their structure, species diversity, and aboveground biomass (AGB), and the expansion of bamboo. Our objective was to analyze these changes in an open bamboo forest in Acre after forest fires occurred either in one of the extreme drought years (2005 or 2010) or in both years (2005 + 2010). We sampled 9.75 ha (in 2016 and 2017), distributed in 18 0.5 ha (100 m × 50 m) plots and three 0.25-ha (50 m × 50 m) plots. We identified a strong fire effect on the number of tree individuals per hectare, which declined by 50% if the forest was burned in only one year (2005 or 2010) and by 74% if burned in both years. This was inversely related to the expansion of bamboo stems, which increased in number by 7 to 9 times. Changes in forest structure and species composition after the fire were characterized by a high importance value for pioneer tree species; reductions in the number of trees with logging potential, in the basal area of trees, and in the number of lianas; and an increase in the density of bamboo stems. AGB in the burned forests was 51–73% that of the unburned forest. With the expansion of bamboo, its contribution to AGB increased from 1% in the unburned forest to 27% in the twice-burned forest. These forms of degradation represent serious threats to Amazon forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest fire is a major concern in the Amazon due to the loss of biomass (Anderson et al. 2015), drastic reduction of the biodiversity of flora and fauna (Barlow et al. 2016), and negative impact on the formation of rainfall (Andreae et al. 2004). Fire has been an important cause of degradation over the last 30 years, disturbing millions of hectares of forests (Anderson et al. 2015; Morton et al. 2013; Silva et al. 2018).
In addition to forest fires and other ongoing disturbances, such as deforestation and fragmentation (Numata and Cochrane 2012), Amazon forests have been severely damaged by the increasing frequency and intensity of extreme droughts (Brando et al. 2020; Davidson et al. 2012). These droughts have increased the occurrence of forest fires (Alencar et al. 2015; Barni et al. 2015; Morton et al. 2013) and tree mortality. Previous studies in the southern and southwestern Amazon found that forest fires in years of extreme drought represented 79 to 95% of the total area of forest fires mapped by Morton et al. (2013) and Silva et al. (2018). In drought years, tree mortality increases and a large amount of litter accumulates on the ground (Balch et al. 2011; Brando et al. 2014). These characteristics create conditions that trigger uncontrolled fires that burn large areas of rain forest. The fires severely alter forest structure and reduce both the number of trees and the biomass (Barlow et al. 2003; Brando et al. 2014; Xaud et al. 2013), increasing the proportion of dead trees (Barlow et al. 2003) and favoring the proliferation of pioneer tree species (Barlow and Peres 2008) and invasion of grasses (Balch et al. 2011).
Due to the increasing frequency of extreme-drought events and the large extent of forest-fire impacts, it is necessary to understand the drought-induced changes in forest structure and species composition at regional and pantropical scales (Brando et al. 2020; Davidson et al. 2012). In a controlled-fire experiment in Brazil’s state of Mato Grosso, the combination of extreme drought and fire resulted in an abrupt increase in tree mortality by approximately 200%, when compared to the fire experiment in years with normal dry seasons (Brando et al. 2014). Impacts on the forest can last for a long time period, affecting forest recovery. Barlow and Peres (2008) found little or no recovery of forest structure and floristic composition: species common in unburned forest were rare or totally absent even 9 years after a fire in Brazil’s Pará State. This effect is further aggravated by recurrent forest fires. For example, forest areas that burned multiple times within a decade had up to 94% less aboveground carbon than intact forests (Longo et al. 2016). These processes increase the emission of greenhouse gases (Fearnside 2012; Vasconcelos et al. 2013).
Acre was the epicenter of the 2005 and 2010 extreme droughts, two of the most intense drought events ever recorded in the Brazilian Amazon (Lewis et al. 2011). More than 3500 km2 of forest fires occurred in 2005 and more than 1200 km2 in 2010, mainly in the eastern portion of the state (Silva et al. 2018). Eastern Acre houses the largest native bamboo forest in the Amazon, covering 15.5 to 16.15 million ha (Carvalho et al. 2013; Dalagnol et al. 2018). However, few floristic studies, as well as studies of aboveground biomass, have been done in these forests. These forests are characterized by two predominant semi-scandent (climbing) woody bamboo species: Guadua weberbaueri Pilger and Guadua sarcocarpa Londoño & Peterson. These species have rapid growth rates, with height increasing by 3.4 m month−1 on average (Silveira 2001). Due to their semi-scandent characteristics, these bamboos cause physical damage to the canopies of 40 to 70% of trees with diameter at breast height (DBH) up to 29 cm (Griscom and Ashton 2006). With the impact of fire, the bamboo population can occupy adjacent bamboo-free forests by increasing the density of stems per hectare (note that for bamboo, “stems” are not the same as “individuals”). Once established in an area, bamboo dominates the vegetation structure over historical timescales (Dalagnol et al. 2018; McMichael et al. 2013).
Studies of the impacts of fire on species diversity show 17 to 27% higher densities of pioneer tree species and 4 to 21% higher mortality in lianas in burned forests as compared to unburned forests (Araujo et al. 2013; Numata et al. 2017). With an increasing frequency of drought events, Amazon forests are more subject to fires mediated by recurrent droughts (Barlow and Peres 2008; Berenguer et al. 2014). Both the degree of damage and the recovery of forest from fire vary as functions of fire frequency and intensity (Balch et al. 2011). However, our knowledge of the effects of fire recurrence on forests with bamboo in the southwestern Amazon is still limited.
The present study evaluates changes in the structure, floristic composition, and aboveground biomass of open bamboo forests that were affected by forest fires in 2005 and 2010 in southwestern Amazonia. We conducted forest inventories in the eastern portion of Acre to study the impacts of fires that occurred in two different drought years (2005 and 2010), considering impacts on forest structure, species composition, and aboveground biomass. These sampled areas were used to address different recovery processes and the impact of fire frequency after the drought events.
Materials and methods
History of forest fire in Acre
Based on a 33-year series (1984–2016) of annual fire maps for Acre (Silva et al. 2018), we found that the years with the greatest areas of forest-fire scars were 2005 and 2010, with impacted areas of 351,285 ha and 120,459 ha, respectively. Forest-fire scars in the years of extreme drought (1987, 1998, 2005, 2010, and 2016) represented 98% of the sum of the areas of all forest fires that occurred in the 33-year series (Fig. 1; Table 1). Dense forest was the most-affected forest type, with 49% of its total area impacted by fire. This is explained by the fact that most of this forest type is located in eastern Acre, which also has the most deforestation and, consequently, the most burning.
The forest type with the greatest area burned was open forest with bamboo, with this forest type representing 64% of the total forest-fire area. This was followed by open forest with palms and dense forest (Table 1). Information on the years of the forest fires and the types of forest was the basis for choosing the locations for the forest inventories.
Study area
Based on the historical forest-fire maps, the study area was set in an open forest with bamboo affected by fire either once (in either 2005 or 2010) or twice (in both 2005 and 2010) (Fig. 1). Our study area encompasses the municipalities (counties) of Rio Branco and Bujari along the AC-90 road in eastern Acre (Fig. 1). The climate is of the Am type in the Köppen classification, with annual precipitation of 1900–2200 mm and average temperature of 24–26 °C (Alvares et al. 2013). The predominant forest type in the area is open forest with bamboo according to Acre’s Ecological Economic Zoning (Acre 2010). This type of forest has an average of 302 trees ha−1 (Salimon et al. 2011) and 1242 to 2884 bamboo stems ha−1 with the most abundant tree species being Bertholletia excelsa, Couratari sp., Tetragastris altissima, Aspidosperma sp., and Acacia polyphylla (Rockwell et al. 2014; Silveira 2001). Soil types are yellow-red Ultisols and Alfisols with expansive clays (Acre 2010).
The study area is in one of the portions of Acre where forests are most fragmented as a result of logging and deforestation. The main land use in the study area is cattle pasture (Almeida et al. 2016), which is the principal source of forest-fire ignition in Amazonia, including the state of Acre (Silva et al. 2018).
Forest inventories
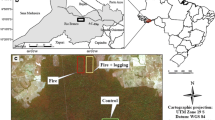
The forest inventories were carried out between August 2016 and July 2017 in the western portion of the municipality of Rio Branco (Fig. 2a, b). The plots were installed in four fragments of upland open forests with bamboo and palms. The selected fragments were at least 9 km apart in a straight line, and the plots were located at least 100 m from the nearest forest edge. Forest inventories for measurement of trees, palms, and vines with diameter at breast height (DBH) ≥ 10 cm were carried out in 21 plots totaling 9.75 ha in four areas with different fire histories: unburned forest (UF) (three plots of 100 m × 50 m and three plots of 50 m × 50 m), burned forest in 2005 (BF05) 11 years after the fire (six plots of 100 m × 50 m), burned forest in 2010 (BF10) 6 years after the fire (three plots of 100 m × 50 m), and burned forest in both 2005 and 2010 (BF05–10) 11 and 6 years after the respective fires (six plots of 100 m × 50 m) (Fig. 2b; Supplementary Material, Fig. S1). Three smaller plots (50 m × 50 m) from UF were adapted as they were placed in a small unburned area of 2 ha protected from a 10,000 ha forest fire. For counting and measuring the DBH of bamboo stems, 8 subplots of 5 m × 5 m were installed in three plots in each of the four areas (Fig. 2c).
Location of forest inventories carried out in 2016 and 2017. (a) Location of Acre and of the Amazon region; (b) location of forest inventory areas in the western portion of the municipality of Rio Branco; and (c) illustration of a 100-m × 50-m plot for trees with diameter at breast height (DBH) ≥ 10 cm and of the eight 5-m × 5-m subplots for bamboo
Botanical identification to either the species or genus level and the bamboo inventory were done in three of the plots in each treatment (UF, BF05, BF10, and BF05–10). Scientific names and families were confirmed based on online services that are compatible with the main plant taxonomic databases (Boyle et al. 2013). Thanks to the presence of flowers and fruits during the inventory (Supplementary Material, Fig. S2), the bamboo species was identified as Guadua weberbaueri based on its morphological characteristics (Olivier and Poncy 2009).
We recognize the limitation of our forest inventory due to its lack of chronological data (i.e., before and after fire events in the same plots). Nevertheless, our data show that the characteristics (or conditions) of forests with bamboo differ between areas with and without fire impacts.
Analyses
In order to evaluate the impacts of fires in the structure, floristic composition, and aboveground biomass of open bamboo forests, we conducted several statistical analyses. We used R software for all statistical analyses (R Core Team 2020). In these analyses, the areas with different fire impacts were considered to be treatments and the plots were considered to be replicates. For averaging tests between treatments, we analyzed the normality of the data by the Shapiro–Wilk test. Due to the lack of normality of the data, the non-parametric Kruskal–Wallis method and the Dunn post hoc test were used for test significant difference. To analyze the similarity of diameter distributions between the areas, the Kolmogorov–Smirnov test was applied. To analyze the correlation among variables studied, we applied the Spearman non-parametric correlation test.
For analysis of different impacts of fire on forest structure, we used the following parameters of the forest’s horizontal structure (Magurran 1989; Mueller-Dombois and Ellenberg 1974): (1) number of individual trees and species richness per area, (2) basal area of trees (m2 ha−1), (3) DBH distribution of trees among five diameter classes (10–19.9 cm, 20–29.9 cm, 30–39.9 cm, 40–49.9 cm, and ≥ 50 cm), (4) density of all trees (individuals ha−1), (5) density of all bamboo stems per hectare, 6) relative species density (DR = number of individuals of the species/number of individuals of all species × 100); 7) relative dominance (DoR = total basal area of the all species and pioneer species are excluded /total basal area of all species × 100) and frequency (FR = frequency of a species /sum of the frequencies of all species); and 8) the importance value per species (IV = DR + FR + DoR). The basal area is indicative of vegetation density and of the amount of commercially exploitable wood, so we analyzed the total basal area ha−1 of all species and basal area ha−1 excluding pioneer species.
To understand fire impacts on species composition, we chose number of individuals ha−1 of the pioneer tree species and species of commercial value as indicators of disturbances/recovery (Barlow and Peres 2008; Numata et al. 2017) and the economic importance (Brazil, SFB 2018; Supplementary Material, Table S2), respectively. For pioneer species, we used the parameters of relative density and selected the five species with the highest importance values (Supplementary Material, Table S1). Species of commercial value were identified according to the list of the Brazilian Forestry Service (Brazil, SFB 2018).
We use the term “bamboo expansion” to represent the expansion of the spatial occupation of the bamboo population. This is determined by analyzing the density of stems per hectare in areas impacted by fire.
For analysis of differences in means forest aboveground biomass (AGB) in burned and unburned areas, we estimated means AGB by available allometric equations from literatures. Allometric equations for AGB estimation (Mg ha−1) of living arboreal individuals, palms, vines (lianas), and bamboo are given below (Eqs. 1 to 4). A correction factor for logarithmic models (Sprugel 1983) was applied.
We assessed the impacts of these variables on mean AGB and on the forest structure (density of trees, density of pioneer trees, and density of commercially valuable trees) as response variables and fire and environmental variables as explanatory variables in the generalized linear model (GLM) using GLM. Environmental variables, including slope, distance to the nearest forest edge, and altitude, were quantified from remote sensing, including digital elevation data from the Shuttle Radar Topography Mission (SRTM) and optical satellite data from Landsat images. Variables related to fire included time after the first fire—TFF (0, 6, or 11 years), time after the last fire—TLF (0, 6, or 11 years), and number of occurrences of fire—NFF (0, 1, or 2 times). We assessed multicollinearity of explanatory variables by generalized variance inflation factors (VIF), excluding variables with VIF values > 10, represent a multicollinearity problem (O’Brien 2007). We observed that TFF had high collinearity with other variables, being excluded from the analysis. After checking the nature of response variables and residual dispersion, we assumed two types of distributions for response variables from models: gamma distribution for the AGB variable and negative binomial for forest structure variables. Gamma distribution is adequate for continuous and positive values (e.g., biomass) (Zuur et al. 2009), whereas binomial distribution characterizes well rates values (e.g., individual trees ha−1) belonging to the Poisson family. However, over-dispersion was found in the residual analysis of forest structure data; thus, the negative binomial error distribution was used (Crawley 2012). Selection of models was performed by the backward method. During this process, explanatory variables for the prediction of the response variable were removed sequentially from the models using likelihood ratio tests (LRT) assuming an approximate χ2 distribution, less until no variable could be removed without a significant loss of explanation (p < 0.05) (Ives 2015). We compared and ranked best-fitting models using Akaike’s information criterion corrected for small sample size (AICc) (Burnham et al. 2010).
Results
Differences in species diversity
We observed differences in the proportions of pioneer tree species between the areas analyzed (Kruskal–Wallis test, p < 0.01). In BF05–10, pioneer species accounted for 50 ± 14% of the tree species, while in the unburned forest they accounted for only 11 ± 4% (Fig. 3a). Pioneer species differed in terms of dominance between the study areas. Figure 3b shows the five most frequent species in each area. The genus in common between the areas was Cecropia, this genus being most abundant in BF10, followed by BF05, BF05–10, and UF (84 ± 43 ha−1, 17 ± 19 ha−1, 14 ± 21 ha−1, and 5 ± 5 ha−1, respectively; Kruskal–Wallis test, p = 0.05). The species Spondias mombin, Acacia polyphylla, and Inga thibaudiana only occurred in unburned forest (Fig. 3b).
We analyzed the five species with the highest importance values (IVs) for each area. In the unburned forest, only two of the five species were pioneers with low IVs of 2.3 (Table 2). In the areas affected by fire, the number of pioneer species was higher (3 to 5), with IVs ranging from 2 to 16 (Table 2). Pioneer species with the highest IVs, i.e., Ceiba samauma and Spondias mombin, only occurred in the UF area, whereas the most common genus in all burned-forest plots was Cecropia sp. The species Ochroma pyramidale occurred only in BF10 (the most recently burned forest). The species Urera baccifera was identified in UF, BF05, and BF10 (Table 2).
The relative density (number of individuals of each species ha−1) of the pioneer species differed significantly between UF (2% of the individuals) and BFs (11% in BF05, 34% in BF10, and 12% in BF05–10) (p < 0.05) (Supplementary Material, Fig. S3). The number of commercially valuable trees with potential for logging differed significantly among study sites (p < 0.01), being lower in burned forests (29% in BF05, with 37 ± 18 individuals ha−1; 54% in BF05–10, with 24 ± 10 individuals ha−1; and 72% in BF10, with 15 ± 6 individuals ha−1) than in UF (52 ± 15 individuals ha−1) (Fig. 6).
Differences in forest structure associated with forest fire
Total basal area (m2 ha−1) differed significantly among study sites (p < 0.01), indicating a decreasing trend in this parameter from UF to BF05, followed by BF10 and BF05–10 (Table 3). The largest difference as compared to UF was in the area affected by fire twice (BF05–10), both for density of trees (67% less) and total basal area (53% less), followed by BF10 with a density difference of 42% and a total basal area difference of 36%. Even 11 years after the fire, forest burned in 2005 showed strong reductions in absolute density and in total basal area as compared to unburned forest (45% and 12%, respectively).
Liana density decreased significantly (p < 0.05) in burned forests. As compared to unburned forest (111 individuals ha−1), density was reduced by 79% (23 individuals ha−1) in BF-05, 89% (12 individuals ha−1) in BF10, and 95% (6 individuals ha−1) in BF05–10 (Fig. 4). By contrast, the density of bamboo stems was 7 to 9 times higher in the BFs than in UF (Fig. 4). The density of bamboo stems in the unburned forest was 667 stems ha−1, whereas much higher densities were found in the burned forest: 5200 stems ha−1 in BF05, 4500 stems ha−1 in BF10, and 5930 stems ha−1 in BF05–10 (p = 0.05). The densities of lianas and bamboo were negatively correlated due to the effect of forest fires (p < 0.01, r = 0.81) (Fig. 4).
Since basal area is an indicator of the quantity of wood that is commercially exploitable in the forest, we divided the data on basal area into two subsets: all trees including pioneer species (Fig. 5a) and trees excluding the pioneer species (Fig. 5b). Basal areas all trees differed significantly (p < 0.01) between the unburned forest (25 ± 3 m2 ha−1) and the burned forest twice in 2005 and 2010 (Fig. 5a—BF05, 17 ± 3 m2 ha−1; BF10, 12 ± 4 m2 ha−1; BF05–10, 9 ± 2 m2 ha−1), with a reduction in basal areas by 33 to 65% between unburned and burned areas. In both analyses, UF differed significantly from the burned forests (p < 0.01). There was a reduction of basal area without the pioneer species by 3 to 16% between UF and BFs, with basal areas for the trees being 21 ± 5, 14 ± 5, 8 ± 4, and 7 ± 3 m2 ha−1 for UF, BF05, BF10, and BF05–10, respectively.
Basal area in the inventoried areas considering all trees (a) and excluding pioneer species (b). Different letters indicate significantly different means (Kruskal–Wallis test, p < 0.01). UF = unburned forest; BF05 = burned forest in 2005; BF10 = burned forest in 2010; BF05–10 = burned forest in 2005 and 2010
Differences in aboveground biomass
Large differences were observed in the number of trees (p < 0.01) and in the aboveground biomass (p < 0.05) between the UF and the BFs. Compared to the mean tree density (individuals ha−1 ± SE) of the UF (548 ± 91), densities were lower by 51, 53, and 73% in BF10, BF05, and BF05–10 (254 ± 44, 267 ± 47, and 149 ± 23 individuals ha−1), respectively (Fig. 7a). AGB was lower by 26 to 66% in BF05, BF10, and BF05–10 (202, 146, and 92 Mg ha−1, respectively). Aboveground biomass and tree density were significantly different (p < 0.05) between UF and BF in 2005 and 2010, with the greatest effect of fire being on individuals in the 10–20-cm-diameter class (Fig. 7b).
Densities of bamboo stems in BFs increased 7 to 9 times the density in UF. Increase in the density of bamboo stems after forest fires increases the contribution of bamboo to forest aboveground biomass, representing 3, 26, 23, and 26 Mg ha−1 in UF, BF05, BF10, and BF05–10, respectively (p = 0.05; Fig. 8a). The contribution of bamboo to the total aboveground biomass of the forest increased from 1% in UF to 13% in BF05, 15% in BF10, and 27% in BF05–10 (Fig. 8b).
Drivers of change in the forest after the fires
The numbers of occurrences of forest fire, time after the first fire, and time after the last fire were the variables that best explained our results on the forest aboveground biomass, the number of commercially valuable trees, and the densities of all trees and of pioneer species, either in isolation or in combination (Table 4). Among the environmental variables, slope and distance from the forest edge had the strongest effects. Altitude did not show any relation with the structure of the forest. Secondary effects were not found in the models with combinations of independent variables.
Discussion
We found that fire effects occurred with different timings under different frequencies of fire events in open forest with bamboo in southwestern Amazonia.
Changes in species composition
The changes in tree density and in floristic composition that we found (which were especially great in BF05–10) corroborate results found in other parts of Amazonia, such as in Pará (Barlow and Peres 2008; Barlow et al. 2016; Berenguer et al. 2014) and Roraima (Xaud et al. 2013).
Analyzing the change in species composition between areas of burned and unburned forest, we found a significantly larger number of pioneer species in the forest areas after the fire than in unburned forest. This phenomenon is analogous to the “secondarization” of primary forest reported by Barlow and Peres (2008), who coined the expression “secondarization” to characterize the effect of fire-induced mortality in leading to a rapid collapse in the abundance of old trees and an increased abundance of pioneer species.
As observed in other studies (Barlow et al. 2003; Barlow and Peres 2008; Xaud et al. 2013), the recurrence of fire causes a collapse in the abundance of trees and leads to a secondarization of the forest, with an increase in the number of pioneer species and in the abundance of individuals of these species. Nobre and Borma (2009) suggested that secondarization can occur from interactions between longer dry seasons, forest fires, and forest fragmentation, inducing substitution by fire-tolerant savanna plant species such as grasses. Our results show strong secondarization, with high density and relative dominance of pioneer species after forest fires, especially after repeated fire. These factors change the structure of the forest with respect to basal area and biomass, which could pass a tipping point leading to bamboo-dominated forests.
The impact of the fire is of economic concern due to its effect on the forest’s potential for logging. Using the list of species with commercial potential compiled by the Brazilian Forest Service, there is a reduction by 29 to 72% in the number of individuals with potential for exploitation in burned forest compared to unburned forest. This scenario could modify the perception of landowners regarding the value and use of these forests, and could be a decisive factor in the choice between forest management and deforestation. Silva et al. (2018) showed that 26–27% of the forest that burned either once or twice in Acre was cleared in later years.
Effect of forest fire on bamboo abundance
Bamboo expansion was the most dramatic change identified in our study. We observed that there was an increase of 7 to 9 times in the number of bamboo stems and a decline in the number of woody lianas in the burned forest (BF05, BF10, and BF05–10). Our result for the density of bamboo stems per hectare in unburned forest was similar to results reported by Griscom and Ashton (2006) for forest with bamboo without fire disturbance in the recent past in Peru’s department of Madre de Dios, which borders on Acre. Evidence of bamboo expansion in the forests of southwestern Amazonia has important implications, since approximately 58% of the forests in Acre do not have bamboo in their floristic composition or, when present, bamboo is not dominant (Acre 2010; Salimon et al. 2011). Our study showed that the impact of fire caused a reduction by 33 to 65% in the basal area of trees in open bamboo forests and that it can be maintained in the future due to the heavy occupation of bamboo, a condition that can suppress tree-seedling recruitment. Griscom and Ashton (2003) showed that the basal area of trees in bamboo-dominated forest can be up to 8 times smaller than the basal area of trees in adjacent forest without bamboo.
We observed an inversion of the relationship between liana and bamboo densities after the fire. Because bamboos in the genus Guadua are semi-scaling (climbing) species, they use trees to reach the canopy and damage the crowns of trees with DBH ≤ 30 cm (Griscom and Ashton 2006). Reduction in density of woody lianas and increase in the density of bamboo are the combined result of the increased mortality of lianas due to fire, decrease in the number of anchor trees, and the beneficial effect of fire on bamboo growth and recruitment. Our data corroborate the results of Gerwing (2002), who reported mortality of large-diameter woody lianas and an increase of up to 8 times in the abundance of vines with diameter < 1 cm after burning and logging in forests in Pará. The rapid increase in height of Guadua weberbaueri in Acre (3.4 m month−1; Silveira 2001) may explain the high density of bamboo after forest fire.
Once bamboo is established in an area, it can dominate the vegetation structure over historical timescales; for example, McMichael et al. (2013) attribute current bamboo-dominated forests in southwestern Amazonia to fires that left charcoal in the soil 1200 years ago. This has important implications for carbon storage.
Declines in aboveground biomass
Our results show declines of 51 to 73% of the aboveground biomass in burned forest as compared to unburned forest, even 11 years after a fire event, demonstrating the slow recovery of the forest. At a site near our study area, Ziccardi et al. (2019) found 45% lower aboveground biomass in forests disturbed by fire and logging 11 years after the fire event and nine after logging. A study in Acre by Numata et al. (2017) found aboveground biomass 34% lower than in undisturbed forest 4 years after the 2010 fire, and 23% lower than in undisturbed forest 9 years after the 2005 fire. Longo et al. (2016) found a 55% reduction in aboveground biomass as compared to unburned forest at a site burned twice (2005 and 2010) 9 years after the last fire event. Also in Acre, Sato et al. (2016) found a reduction of 6 to 23% in aboveground biomass 4 to 10 years after the fire as compared to unburned forest. In another study in Acre, Vasconcelos et al. (2013) compared measurements in permanent plots monitored before and after the 2005 fires and showed a 14.4% loss of aboveground biomass 4 years after the fire. Barlow et al. (2012a) compared burned and unburned forests 3 years after the 2005 fires in the Chico Mendes Extractive Reserve in Acre and were unable to detect a significant biomass change, although they found a significant reduction in the number of trees.
Among these studies in Acre, the highest biomass decline (the 55% reduction found by Longo et al. 2016) was at Fazenda Talismã, where the forest type is the same as at our study sites: open forest with bamboo. Even without the impact of recent fire, forests with bamboo can have 30 to 50% less carbon than forests without bamboo (Silveira 2001). This could be an indication of the long-term impact of fire in southwestern Amazonia.
Forest inventories in Amazonia have only considered trees and palms and not included bamboo in estimates of aboveground biomass after the impact of forest fires (e.g., Berenguer et al. 2014; Longo et al. 2016; Numata et al. 2017; Vasconcelos et al. 2013). In southwestern Amazonia (including portions of Peru and Bolivia, in addition to Brazil), there are 15.5 to 16.15 million ha of native forest with bamboo (Carvalho et al. 2013; Dalagnol et al. 2018), but the contribution of bamboo to forest biomass has been neglected. Our study provides the information on the contribution of bamboo to forest biomass after the effect of fire.
We show that the contribution of bamboo to AGB is significant in forests in Acre. In unburned open forest with bamboo, bamboo stems represent about 3% of the aboveground forest biomass. With the impact of fire, our data show that bamboo’s contribution to aboveground forest biomass can reach 27%. Fire not only reduces forest biomass, it also facilitates the growth of tree species that had been less abundant before the fire. This, together with the effect of fire in favoring bamboo, can decisively change the structure of the forest. Ziccardi et al. (2019) studied a forest in Acre where bamboo’s contribution to unburned forest biomass was around 7% (greater than in our study), with the contribution of bamboo increasing to 13% after the impact of fire and fire + logging. Our study and that of Ziccardi et al. (2019) show the importance of including bamboo in biomass accounting.
One of the unanswered questions concerns how bamboo expansion in burned forests would impact forest recovery (Ferreira et al. 2020). Future climate projections for Amazonia suggest less rain and higher temperature in the dry period (Faria et al. 2017; Fu et al. 2013), which would increase the probability of forest fires. Brando et al. (2020) modeled fire scenarios showing that forest fires could double, affecting up to 16% of Amazonian forests by 2050. These predictions would imply further bamboo expansion in southwestern Amazonia, altering forest conditions and recovery processes in burned forests in the future.
The decrease by 51 to 73% in the aboveground biomass after fire events, even 11 years after the forest fire, raises concern about the ecosystem services of the forest. This is relevant to public policies to reduce emissions from deforestation and forest degradation (REDD+) (Aragão and Shimabukuro 2010; Barlow et al. 2012b; Fearnside 2012). This concern has been raised by several studies, showing that, under future climate scenarios with more extreme drought events, Amazonian forests will be increasingly degraded and will have less carbon stock and less resistance to fire events (Aragão et al. 2014; Barlow et al. 2012b; Brando et al. 2020; Fearnside 2018).
Conclusion
Fires cause a substantial degradation of the Amazon forest. The forests we studied in Brazil’s state of Acre differ in structure and floristic composition as functions of both the time elapsed after the fire events and of the recurrence of these events. Burned forests had higher densities of pioneer tree species and bamboo, lower basal area of trees, lower aboveground biomass, and lower numbers of lianas and of individuals of tree species with potential for commercial timber. Our forest areas affected by repeated fires (burned both in 2005 and 2010) showed the largest declines in forest biomass (reduction by up to 73%) and the largest increases in the contribution of bamboo to aboveground biomass (by 27% in the burned forest twice).
Given the trend to increasing occurrence of extreme drought events and associated forest fires, much larger forest areas are under this threat. Our results represent a harbinger of changing forest conditions and potential forest transformation in Amazonia due to repeated forest fires. The changes in forest condition observed in this study are important indicators of forest quality. For example, the combination of a reduction of the number of trees with logging potential and the expansion of bamboo may be decisive for rural property owners in choosing whether to keep the forest standing or to fell the forest for agriculture and ranching. Widespread fire impact can also jeopardize forest conservation and biodiversity. This level of degradation represents a serious threat to the forest and to its potential for sustainable management for timber.
References
Acre (2010) Zoneamento Ecológico-Econômico do Estado do Acre: Fase II (Escala 1:250.000), 2nd edn. SEMA, Rio Branco
Alencar A, Brando PM, Asner GP, Putz FE (2015) Landscape fragmentation, severe drought, and the new Amazon forest fire regime. Ecol Appl 25:1493–1505. https://doi.org/10.1890/14-1528.1
Almeida CA, Coutinho AC, Esquerdo JCDM, Adami M, Venturieri A, Diniz CG, Dessay N, Durieux L, Gomes AR (2016) High spatial resolution land use and land cover mapping of the Brazilian Legal Amazon in 2008 using Landsat-5/TM and MODIS data. Acta Amazonica 46:291–302. https://doi.org/10.1590/1809-4392201505504
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Anderson LO, Aragão LEOC, Gloor M, Arai E, Adami M, Saatchi SS, Malhi Y, Shimabukuro YE, Barlow J, Berenguer E, Duarte V (2015) Disentangling the contribution of multiple land covers to fire-mediated carbon emissions in Amazonia during the 2010 drought. Glob. Biogeochem. Cycles 29:1739–1753. https://doi.org/10.1002/2014GB005008
Andreae MO, Rosenfeld D, Artaxo P, Costa AA, Frank GP, Longo KM, Silva-Dias MAF (2004) Smoking rain clouds over the Amazon. Science 303:1337–1342. https://doi.org/10.1126/science.1092779
Aragão LEOC, Shimabukuro YE (2010) The incidence of fire in Amazonian forests with implications for REDD. Science 328:1275–1278. https://doi.org/10.1126/science.1186925
Aragão LEOC, Poulter B, Barlow JB, Anderson LO, Malhi Y et al (2014) Environmental change and the carbon balance of Amazonian forests. Biol Rev 1–19. https://doi.org/10.1111/brv.12088
Araujo HJB, Oliveira LC, Vasconcelos SS, Correia MF (2013) Danos provocados pelo fogo sobre a vegetação natural em uma floresta primária no Estado do acre, Amazônia Brasileira. Ciênc Florest 23:297–308. https://doi.org/10.5902/198050989276
Balch JK, Nepstad DC, Curran LM, Brando PM, Portela O, Guilherme P, Reuning-Scherer JD, de Carvalho Jr. O (2011) Size, species, and fire behavior predict tree and liana mortality from experimental burns in the Brazilian Amazon. For Ecol Manag 261:68–77. https://doi.org/10.1016/j.foreco.2010.09.029
Barlow JB, Peres CA (2008) Fire-mediated dieback and compositional cascade in an Amazonian forest. Philos Trans R Soc B Biol Sci 363:1787–1794. https://doi.org/10.1098/rstb.2007.0013
Barlow JB, Lagan BO, Peres CA (2003) Morphological correlates of fire-induced tree mortality in a central Amazonian forest. J Trop Ecol 19:291–299. https://doi.org/10.1017/S0266467403003328
Barlow JB, Parry L, Gardner TA, Ferreira J, Aragão LEOC, Carmenta R, Berenguer E, Vieira ICG, Souza C, Cochrane MA (2012a) The critical importance of considering fire in REDD+ programs. Biol Conserv 154:1–8. https://doi.org/10.1016/j.biocon.2012.03.034
Barlow JB, Silveira JM, Mestre LAM, Andrade RB, D’Andrea GC, Louzada J, Vaz-de-Mello FZ, Numata I, Lacau S, Cochrane MA (2012b) Wildfires in bamboo-dominated Amazonian forest: impacts on above-ground biomass and biodiversity. PLoS ONE 7:1–11. https://doi.org/10.1371/journal.pone.0033373
Barlow J, Lennox GD, Ferreira J, Berenguer E, Lees AC, Nally RM, Thomson JR, Ferraz SF de B, Louzada J, Oliveira VHF, Parry L, Solar RRC, Vieira ICG, Aragão LEOC, Begotti RA, Braga RF, Cardoso TM, Oliveira Jr RC, Souza Jr CM, Moura NG, Nunes SS, Siqueira JV, Pardini R, Silveira JM, Vaz-de-Mello FZ, Veiga RCS, Venturieri A, Gardner TA (2016) Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535:144–147. https://doi.org/10.1038/nature18326
Barni PE, Pereira VB, Manzi AO, Barbosa RI (2015) Deforestation and forest fires in Roraima and their relationship with phytoclimatic regions in the northern Brazilian Amazon. Environ Manag 55:1124–1138. https://doi.org/10.1007/s00267-015-0447-7
Berenguer E, Ferreira J, Gardner TA, Aragão LEOC, de Camargo PB, Cerri CE, Durigan M, Oliveira RCD, Vieira ICG, Barlow J (2014) A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Change Biol 20:3713–3726. https://doi.org/10.1111/gcb.12627
Boyle B, Hopkins N, Lu Z, Garay, JAR, Mozzherin, D, Rees T, Matasci N, Narro ML, Piel WH, McKay SJ, Lowry S, Freeland C, Peet RK, Enquist BJ (2013) The taxonomic name resolution service: An online tool for automated standardization of plant names. BMC Bioinformatics 14:16. https://doi.org/10.1186/1471-2105-14-16
Brando PM, Balch JK, Nepstad DC, Morton DC, Putz FE, Coe MT, Silvério D, Macedo MN, Davidson EA, Nóbrega CC, Alencar A, Soares-Filho BS (2014) Abrupt increases in Amazonian tree mortality due to drought–fire interactions. Proc Natl Acad Sci 111:6347–6352. https://doi.org/10.1073/pnas.1305499111
Brando PM, Soares-Filho B, Rodrigues L, Assunção A, Morton D, Tuchschneider D, Fernandes ECM, Macedo MN, Oliveira U, Coe MT (2020) The gathering firestorm in southern Amazonia. Sci Adv 6, eaay1632. https://doi.org/10.1126/sciadv.aay1632
Brazil, SFB (Serviço Florestal Brasileiro) (2018) Espécies madeireiras de interesse comercial. http://www.florestal.gov.br/snif/recursos-florestais/especies-florestais?modal=1&tipo=tableau. Accessed 30 Jan 2020
Burnham KP, Anderson DR, Huyvaert KP (2010) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. https://doi.org/10.1007/s00265-010-1029-6
Carvalho AL, Nelson BW, Bianchini MC, Plagnol D, Kuplich TM, Daly DC (2013) Bamboo-dominated forests of the southwest Amazon: Detection, spatial extent, life cycle length and flowering waves. PLOS ONE 8:1–13. https://doi.org/10.1371/journal.pone.0054852
Crawley MJ (2012) Generalized linear models. In: The R book. John Wiley & Sons, Ltd, pp 557–578. https://doi.org/10.1002/9781118448908.ch13
Dalagnol R, Wagner FH, Galvão LS, Nelson BW, Aragão LEOC (2018) Life cycle of bamboo in southwestern Amazon and its relation to fire events. Biogeosci Discuss:1–28. https://doi.org/10.5194/bg-2018-207
Davidson EA, de Araújo AC, Artaxo P, Balch JK, Brown IF, Bustamante MMC, Coe MT, DeFries RS, Keller M, Longo M, Munger JW, Schroeder W, Soares-Filho BS, Souza Jr CM, Wofsy SC (2012) The Amazon basin in transition. Nature 481:321–328. https://doi.org/10.1038/nature10717
Faria BL, Brando PM, Macedo M, Panday P, Soares-Filho B, Coe M (2017) Current and future patterns of fire-induced forest degradation in Amazonia. Environ Res Lett 119601. https://doi.org/10.1088/1748-9326/aa69ce
Fearnside PM (2012) Brazil’s Amazon forest in mitigating global warming: unresolved controversies. Clim Policy 12:70–81. https://doi.org/10.1080/14693062.2011.581571
Fearnside PM (2018) Brazil’s Amazonian forest carbon: the key to southern Amazonia’s significance for global climate. Reg Environ Chang 18:47–61. https://doi.org/10.1007/s10113-016-1007-2
Ferreira E, Kalliola R, Ruokolainen K (2020) Bamboo, climate change and forest use: a critical combination for southwestern Amazonian forests? Ambio 49:1353–1363. https://doi.org/10.1007/s13280-019-01299-3
Fu R, Yin L, Li W, Arias PA, Dickinson RE, Huang L, Chakraborty S, Fernandes K, Liebmann B, Fisher R, Myneni RB (2013) Increased dry-season length over southern Amazonia in recent decades and its implication for future climate projection. Proc Natl Acad Sci U. S. A. 110:18110–18115. https://doi.org/10.1073/pnas.1302584110
Gerwing JJ (2002) Degradation of forests through logging and fire in the eastern Brazilian Amazon. For Ecol Manag 157:131–141. https://doi.org/10.1016/S0378-1127(00)00644-7
Goodman RC, Phillips OL, Torres DC, Freitas L, Cortese ST, Monteagudo A, Baker TR (2013) Amazon palm biomass and allometry. For Ecol Manag 310:994–1004. https://doi.org/10.1016/j.foreco.2013.09.045
Griscom BW, Ashton PMS (2003) Bamboo control of forest succession: Guadua sarcocarpa in southeastern Peru. For Ecol Manag 175:445–454. https://doi.org/10.1016/S0378-1127(02)00214-1
Griscom BW, Ashton PMS (2006) A self-perpetuating bamboo disturbance cycle in a neotropical forest. J Trop Ecol 22:587–597. https://doi.org/10.1017/S0266467406003361
Ives AR (2015) For testing the significance of regression coefficients, go ahead and log-transform count data. Methods Ecol Evol 6:828–835. https://doi.org/10.1111/2041-210X.12386
Lewis SL, Brando PM, Phillips OL, Heijden GMF, Nepstad DC (2011) The 2010 Amazon drought. Science 331:554–554. https://doi.org/10.1126/science.1200807
Longo M, Keller M, Santos MN, Leitold V, Pinagé ER, Baccini A, Saatchi S, Nogueira EM, Batistella M, Morton DC (2016) Aboveground biomass variability across intact and degraded forests in the Brazilian Amazon. Glob Biogeochem Cycles 30:2016GB005465. https://doi.org/10.1002/2016GB005465
Magurran AE (1989) Diversidad Ecológica y su Medición. Vedrà, Barcelona
McMichael CH, Bush MB, Silman MR, Piperno DR, Raczka M, Lobato LC, Zimmerman M, Hagen S, Palace M (2013) Historical fire and bamboo dynamics in western Amazonia. J Biogeogr 40:299–309. https://doi.org/10.1111/jbi.12002
Melo AWF (2017) Alometria de árvores e estoque de carbono Florestal na Amazônia Sul-Ocidental. (Doctoral thesis in tropical forest sciences) Instituto Nacional de Pesquisas da Amazônia, Manaus, Amazonas, Brazil
Morton DC, Page YL, DeFries RS, Collatz GJ, Hurtt GC (2013) Understorey fire frequency and the fate of burned forests in southern Amazonia. Philos Trans R Soc B Biol Sci:368. https://doi.org/10.1098/rstb.2012.0163
Mueller-Dombois D, Ellenberg H (1974) Aims and methods vegetation ecology. John Wiley & Sons, New York
Nobre CA, Borma LDS (2009) ‘Tipping points’ for the Amazon forest. Curr Opin Environ Sustain 1:28–36. https://doi.org/10.1016/j.cosust.2009.07.003
Nogueira EM, Fearnside PM, Nelson BW, Barbosa RI, Keizer EWH (2008) Estimates of forest biomass in the Brazilian Amazon: new allometric equations and adjustments to biomass from wood-volume inventories. For Ecol Manag 256:1853–1867. https://doi.org/10.1016/j.foreco.2008.07.022
Numata I, Cochrane MA (2012) Forest fragmentation and its potential implications in the Brazilian Amazon between 2001 and 2010. Open J For 2:265–271. https://doi.org/10.4236/ojf.2012.24033
Numata I, Silva SS, Cochrane MA, d’Oliveira MV (2017) Fire and edge effects in a fragmented tropical forest landscape in the southwestern Amazon. For Ecol Manag 401:135–146. https://doi.org/10.1016/j.foreco.2017.07.010
O’Brien RM (2007) A caution regarding rules of thumb for variance inflation factors. Qual Quant 41:673–690. https://doi.org/10.1007/s11135-006-9018-6
Olivier J, Poncy O (2009) A taxonomical revision of Guadua weberbaueri Pilg. and Guadua sarcocarpa Londoño & P. M. Peterson (Poaceae). Candollea 64:171–178. https://dialnet.unirioja.es/servlet/articulo?codigo=4213778
R Core Team (2020) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rockwell CA, Kainer KA, d’Oliveira MVN, Staudhammer CL, Baraloto C (2014) Logging in bamboo-dominated forests in southwestern Amazonia: caveats and opportunities for smallholder forest management. For Ecol Manag 315:202–210. https://doi.org/10.1016/j.foreco.2013.12.022
Salimon CI, Putz FE, Menezes-Filho L, Anderson A, Silveira M, Brown IF, Oliveira LC (2011) Estimating state-wide biomass carbon stocks for a REDD plan in Acre, Brazil. For Ecol Manag 262:555–560. https://doi.org/10.1016/j.foreco.2011.04.025
Sato LY, Gomes VCF, Shimabukuro YE, Keller M, Arai E, dos-Santos MN, Brown IF, Aragão LEOC (2016) Post-fire changes in forest biomass retrieved by airborne LiDAR in Amazonia. Remote Sens 8:839. https://doi.org/10.3390/rs8100839
Schnitzer SA, DeWalt SJ, Chave J (2006) Censusing and measuring lianas: a quantitative comparison of the common methods. Biotropica 38:581–591. https://doi.org/10.1111/j.1744-7429.2006.00187.x
Silva SS, Fearnside PM, Graça PMLA, Brown IF, Alencar A, Melo AWF (2018) Dynamics of forest fires in the southwestern Amazon. For Ecol Manag 424:312–322. https://doi.org/10.1016/j.foreco.2018.04.041
Silveira M (2001) A floresta aberta com bambu no sudoeste da Amazônia: padrões e processos em múltiplas escalas. (Doctoral thesis in ecology) Universidade de Brasília, Brasilía, DF, Brazil
Sprugel DG (1983) Correcting for bias in log-transformed allometric equations. Ecology 64:209–210. https://doi.org/10.2307/1937343
Vasconcelos SS, Fearnside PM, Graça PMLA, Nogueira EM, Oliveira LC, Figueiredo EO (2013) Forest fires in southwestern Brazilian Amazonia: Estimates of area and potential carbon emissions. For Ecol Manag 291:199–208. https://doi.org/10.1016/j.foreco.2012.11.044
Xaud HAM, Martins FSRV, Santos JR (2013) Tropical forest degradation by mega-fires in the northern Brazilian Amazon. For Ecol Manag 294:97–106. https://doi.org/10.1016/j.foreco.2012.11.036
Ziccardi LG, Graça PMLA, Figueiredo EO, Fearnside PM (2019) Decline of large-diameter trees in a bamboo-dominated forest following anthropogenic disturbances in southwestern Amazonia. Ann For Sci 76:1–13. https://doi.org/10.1007/s13595-019-0901-4
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We thank Antonio Barreto (Tunico) for the botanical identification. We are grateful for funding from the State of Acre Research Support Foundation (FAPAC 03/2013), the NASA Terrestrial Ecology Program (NNX14AD56G), National Council for Scientific and Technological Development (CNPq) (708565/2009, 429795/2016-5, 610042/2009-2, 575853/2008-5, 311103/2015-4), and the Coordination for Improvement of Higher Education Personnel (CAPES) INPA/UFAC Interinstitutional Doctoral Program (459/2013), Foundation for the Support of Research of the State of Amazonas (FAPEAM) (708565), and National Institute for Research in Amazonia (INPA) (PRJ13.03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Anne Bousquet-Melou
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1837 kb)
Rights and permissions
About this article
Cite this article
da Silva, S.S., Numata, I., Fearnside, P.M. et al. Impact of fires on an open bamboo forest in years of extreme drought in southwestern Amazonia. Reg Environ Change 20, 127 (2020). https://doi.org/10.1007/s10113-020-01707-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10113-020-01707-5