Abstract
The savanna formations of the Brazilian “Cerrado” present a high degree of resistance and resilience to the impacts of fire, although little is known of its forest formations. Given this, the present study evaluated the resistance and resilience of the “Cerradão” to fire impacts over a 7-year period. In March 2008, we established 50 permanent plots of 10 m × 10 m and measured all the woody individuals with a base diameter ≥5 cm. Six months later, all plots were burned by an accidental fire. In March 2012 and March 2015, we re-measured all surviving individuals, and measured the recruits. Species richness, the density of individuals, and the basal area were all significantly greater (P < 0.05) in 2008, prior to the fire, in comparison with 2012 and 2015, after the fire. Species richness and the density of individuals were also higher (P < 0.05) in 2015, about 7 years post-fire, in comparison with 2012. During the interval in which the fire occurred (2008–2012), the mortality and reposition time, and the stability were all higher (P < 0.05) than during the subsequent interval, post-fire (2012–2015). The recruitment rate was also lower between 2008 and 2012 (P < 0.05). Our results indicate that the “Cerradão” has reduced resistance and resilience to the disturbances caused by fire relative to savanna formations. Given this, frequent burn-off may cause drastic alterations to this phytophysiognomy, emphasizing the need for special care for the preservation of its biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fire plays a major role in the occurrence and distribution of tropical forest and savanna formations worldwide (Staver et al. 2011; Dantas et al. 2013). A number of recent studies have indicated that forest and savanna represent alternative states that are maintained primarily by the action of wildfires (Hoffmann et al. 2009; Staver et al. 2011). In this scenario, frequent fires favor the establishment of savanna species (Staver et al. 2011; Reis et al. 2015a), favoring the transformation of forest into savanna (Coutinho 1990; Bond et al. 2005). The suppression of fires in savanna formations may have the opposite effect, leading to the substitution of savanna species by forest ones, which results in a significant increase in the basal area, height, and density of individuals over time (Durigan and Ratter 2006; Cardoso et al. 2009).
Fire is a historical phenomenon (Bond et al. 2005), which is becoming increasingly frequent due to its use in the management of farmland (Medeiros and Fiedler 2011; Pivello 2011) and prolonged dry periods (Brando et al. 2014), ultimately interfering in the trophic equilibrium of the local plant communities (Smit et al. 2010; Reis et al. 2015a). The Brazilian “Cerrado” is formed by forest, savanna, and grassland formations (Ribeiro and Walter 2008), of which the forests are more sensitive to fire and have a reduced capacity for recuperation in comparison with the savanna (Moreira 2000; Hoffmann 2005). This difference in the sensitivity of woody species to fire is related to the morphological adaptations of the typical “Cerrado” species, such as the thickened bark, which provides thermal protection (Hoffmann 2005; Hoffmann et al. 2012) and the greater investment in root biomass, which increases the availability of carbohydrates for resprouting (Hoffmann 2005). Physiological adaptations, such as reduced nutritional requirements, also facilitate the re-establishment of the plant following a fire (Miranda et al. 2004). These adaptations result in the increased resistance (capacity to sustain structural integrity and growth) and resilience (recovery capacity) of the savanna formations to fire damage (Coutinho 1990; Miranda et al. 2004; Hoffmann et al. 2012).
A number of studies have evaluated the effects of fire on the woody vegetation of forest and savanna formations, and have shown that this type of impact generally leads to the exclusion of the rarest (Libano and Felfili 2006; Mews et al. 2013) and/or most sensitive species (Moreira 2000; Reis et al. 2015a), resulting in a reduction in species richness (Coutinho 1990; Ribeiro et al. 2012). The structure and dynamics of the vegetation are also affected, in particular in terms of the density of individuals (Lima et al. 2009; Gomes et al. 2014) and recruitment rates (Medeiros and Miranda 2005; Gomes et al. 2014), resulting from the increase in mortality. This is more pronounced in the smaller plants, which have yet to develop fire-resistant adaptations, such as the cork-like bark (Coutinho 1990; Hoffmann et al. 2009, 2012). These effects may result in the simplification or homogenization of species composition and community structure over time.
The present study provided an opportunity to study in situ the effects of fire on the species richness, structure, and dynamics of the woody vegetation of the “Cerradão”, the forest form of “Cerrado”, also known as savanna woodland (Ratter et al. 1973), formation typical of the transition between the “Cerrado” and Amazon biomes (Ratter et al. 1973; Reis et al. 2015a). Most of the agricultural burn-off in this ecotonal region (Araújo et al. 2012) occurs in the area known as the “arc of deforestation” of the southern Amazon basin (Nogueira et al. 2008).
In this context, studies that evaluate the effects of fire on plant communities are essential for the conservation of a region’s biodiversity, given the potential for the identification and understanding of the different processes (e.g., resistance and resilience) that determine the community’s species composition and diversity, and its structure (Melo and Durigan 2010). The findings of these studies can also contribute to the creation of effective fire management strategies, whether for the reduction of fire risk, which would contribute to the preservation of the forest formations, or the controlled management of fires, which would favor the maintenance of “Cerrado” savanna formations.
In this context, the present study evaluated the resistance and resilience of the woody vegetation of the “Cerradão” to fire, by examining the following questions: (1) does fire change the species richness and composition, and the structure and dynamics of the woody vegetation of the “Cerradão”? (2) is the establishment of “Cerrado” savanna species in the “Cerradão” facilitated by fire? (3) are 7 years of fire suppression sufficient to guarantee the recovery of these aspects of the structure and composition of the “Cerradão” vegetation? Based on these questions, our operational hypotheses were as follows: (1) over the short term and the full extension of the study period, fire reduces species richness, the density of individuals, and their basal area, affecting principally the individuals of smaller size; (2) fire facilitates the establishment of savanna species in the “Cerradão”; (3) the resilience of the “Cerradão” takes longer than the 7 years of the study period.
Materials and methods
Study area
We conducted this study in a “Cerradão” in the Bacaba Municipal Park (14°41′S, 52°20′W) in the municipality of Nova Xavantina, Mato Grosso, Brazil, which is located in the southeastern portion of the Cerrado–Amazon transition zone. The soils are sandy loams of the yellow latosol type, acidic (pH < 5.0) and dystrophic (Ca2 + 0.4 cmolc kg−1), with high levels of exchangeable aluminum (Al3+~ 1.3 cmolc kg−1) (Marimon Junior and Haridasan 2005). The predominant vegetation is “Cerrado” sensu stricto, interspersed with areas of forest and tracts of “Cerradão” (Marimon Junior and Haridasan 2005). The region’s climate is of Köppen’s Aw type, that is, tropical with dry winters (Alvares et al. 2013). The mean monthly temperature is 25 °C, and the mean annual precipitation is 1500 mm (Marimon et al. 2010).
Vegetation survey
In March 2008, we established a linear transect 250 m long and 20 m wide, which was subdivided into contiguous plots of 10 m × 10 m, creating 50 permanent plots within a total area of 0.5 ha. In each plot, we identified and marked with numbered aluminum tags all live individuals with a ground-level diameter of ≥5 cm. In September 2008, an accidental fire affected all the study plots. In March 2012 and March 2015, we re-measured all the surviving individuals following the same procedure adopted in 2008, and measured all new recruits (individuals that had grown to the minimum size for inclusion in the sample).
We collected botanical samples for taxonomic identification and inclusion in the scientific collection of the NX herbarium of the Nova Xavantina campus of Mato Grosso State University. We adopted the arrangement suggested by the APG III (2009) for the classification of the plant families. We reviewed and updated the nomenclature of the taxa using the Species List of Brazilian Flora (http://floradobrasil.jbrj.gov.br/2015).
Data analysis
We tested for spatial autocorrelation using Moran’s I index (see Fig. S1 in Supplementary Material) and found no significant spatial dependence among the subplots for any of the variables evaluated (species richness, density of individuals, or basal area). We calculated the species richness for each plot and compared the values among years (2008, 2012, and 2015) using a repeated measures analysis of variance (ANOVA), followed by Tukey’s post hoc test (Zar 2010).
To evaluate the effects of fire on the establishment of “Cerrado” savanna species, we classified each species according to its habitat preference. Species were thus classified as savanna specialists (SA), found typically in savanna formations, e.g., dense, typical, sparse, or rocky “cerrado”; forest specialists (FO), found typically in forest formations, e.g., gallery forest, riparian forest, dry forest; and generalists (SA/FO), which can be found in both types of formation. This classification was based on the available literature (Silva Júnior et al. 2001; Felfili et al. 2001; Pereira 2002; Mendonça et al. 2008; Silva Júnior and Pereira 2009; Silva Júnior 2012). We used Friedman non-parametric test to compare the percentage of individuals and species per plot of each habitat preference (SA, FO, and SA/FO) between years (2008, 2012, and 2015), followed by Wilcoxon post hoc test (Zar 2010).
We used a repeated measures analysis of variance (ANOVA) to compare mean density and basal area per plot among years (2008, 2012, and 2015), followed by Tukey’s post hoc test (Zar 2010). We plotted histograms to represent the distribution of the individuals in the different diameter classes (5-cm intervals). We also used ANOVA to compare the distribution of individuals of different size classes among surveys.
Based on the number of individuals recorded during each of the three surveys, we calculated, for the intervals 2008–2012 and 2012–2015, the mean annual rates of mortality (M = {1 − [(N 0 − N d) N 0 −1]1/t} × 100), recruitment (R = [1 − (1 − N r N f −1)1/t] × 100) (Sheil et al. 1995, 2000), stability time (S = (t 1/2 − t 2), and reposition time (turnover) (Rep = (t 1/2 + t 2) 2−1) (Swaine and Lieberman 1987; Korning and Balslev 1994), where t is the time (years) between survey, N 0 the initial number of individuals, N d the number of dead individuals (dead stem), N r the number of recruits, N f the final number of individuals, t 1/2 the time of half-life, and t 2 is the time of duplication. The stability values close to zero represent a stable community. Reposition time indicates how dynamic a community is: the lower this value, the more dynamic the community. We consider all individuals which showed dead stems as dead.
For the basal area, we calculated the mean annual rates of loss (L = {1 − [(BA0 − BAd − BAl) BA0 −1]1/t} × 100), gain (G = {1 − [1 − (BAr + BAg) BAf −1]1/t} × 100) (Guimarães et al. 2008), stability time (S BA = (t 1/2BA − t 2BA), and reposition time (RepBA = (t 1/2BA + t 2BA) 2−1) of basal area (Oliveira Filho et al. 1997), where BA0 represents the initial basal area, BAd the dead basal area, BAr the basal area of the recruits, BAf the final basal area, BAl the loss in basal area, and BAg represents the gain in basal area. Based on the diameter of each individual, we calculated the periodic annual increase (PAI) (Encinas et al. 2005). We also calculated the mean annual rates of mortality, recruitment, stability, reposition, loss, gain, and PAI per plot for the intervals 2008–2012 and 2012–2015. We compared these parameters between the two intervals using the t test for dependent samples (Zar 2010). We adopted a 5% significance level in all analyses.
Results
Species composition
The species richness of woody plants in 2008 prior to the fire was 93 species, a significantly higher (F = 152.4; P < 0.01) number than that observed in 2012 (n = 72), 3 years and 7 months after the fire, and in 2015 (n = 81 species; F = 152.4; P < 0.01). The species richness recorded in 2015, approximately 7 years after the fire, was also significantly greater than in 2012 (F = 137.5; P = 0.04).
Considering the 93 species present in the community in 2008, 14 (15.1%) were found at the same density in 2012; 57 (61.3%) were reduced in density; and 21 (22.6%) had been excluded from the plots. However, one species, Mimosa laticifera Rizzini & A. Mattos, a habitat generalist, increased in density by 66.7% between 2008 and 2015. Of the 21 species excluded following the fire, only seven had been recruited back into the community by 2015. It is important to point out that some of these excluded species were still present at the site as resprouting plants, but were no longer present as stems ≥5 cm.
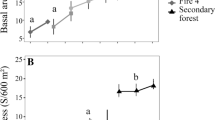
Of the typical savanna species, 21.9% (51.4% of individuals) were excluded between 2008 and 2012 (e.g., Myrcia lanuginosa O. Berg, n = 3 in 2008; Rourea induta Planch., n = 4), but the percentage of these species was not different between 2008 and 2012 (Fig. 1). By 2015, 57.1% of the savanna species excluded from the previous inventory had rejoined the community (e.g., Anacardium occidentale L., n = 1; Caryocar brasiliense Cambess., n = 1), but the percentage of these species was reduced significantly between 2008 and 2015 (Fig. 1). The percentage of individuals of the savanna species increased significantly between 2008 and 2012, but was the same between 2008 and 2015 (Fig. 1).
Percentage of individuals (A) and species (B) of savanna, generalist, and forest formations in the woody vegetation of a “Cerradão” in the Bacaba Municipal Park, Nova Xavantina, Mato Grosso (Brazil). Different letters indicate significant differences (P ≤ 0.05) between years (2008: white; 2012: dark gray; and 2015: light gray) in each habitat preference, based on Wilcoxon post hoc test
Of the habitat generalists, 18.5% (61.5% of individuals) were excluded between 2008 and 2012 (e.g., Matayba guianensis Aubl., n = 10; Cardiopetalum calophyllum Schltdl., n = 4; Leptolobium dasycarpum Vogel., n = 4). Among the generalists, 30% returned in 2015 (e.g., Coccoloba mollis Casar., n = 8; Tapirira guianensis Aubl., n = 8). The percentage of these species was the same between 2008 and 2012, but increased significantly in 2015 (Fig. 1). The percentage of individuals was the same between the years (Fig. 1).
Of the forest species, 50.0% (92.3% of individuals) were excluded between 2008 and 2012, including Erythroxylum engleri O.E. Schulz (n = 2) and Alchornea discolor Poepp. (n = 3). Half (50.0%) of the forest species (Alchornea discolor n = 1) also returned in 2015. The percentage of these species and individuals was the same between 2008, 2012, and 2015 (Fig. 1). In addition to these changes, three species not recorded prior to the fire were found in the plots in 2015, including two typical savanna species (Eugenia gemmiflora O. Berg and Stryphnodendron polyphyllum Mart.) and a generalist, Schefflera morototoni (Aubl.) Maguire, Steyerm. & Frodin.
Structural parameters
The density of individuals prior to the fire in 2008 (1505 individuals) was reduced significantly (F = 332.4; P < 0.01) by 2012 (648 individuals) and increased only slightly by 2015 (748 individuals). The density of individuals was also significantly higher in 2015 in comparison with 2012 (F = 332.4; P = 0.01). The basal area was also significantly greater (F = 4.117; P = 0.02) in 2008 (11.45 m2) in comparison with both 2012 (7.53 m2) and 2015 (7.66 m2), although the two latter years were similar to one another (F = 4.117; P = 0.99). The species that most accumulated basal area after the fire was Tachigali vulgaris L.G. Silva & H.C. Lima (30.9%). All other species together contributed the remaining 69.1%.
Prior to the fire, the smallest diameter class (5–10 cm) contained 70.6% of the live individuals. After the fire, however, there was a significant reduction in this class both in 2012 (67.6%) and 2015 (56.8%). The next largest size class also underwent a significant but less extreme reduction. The density of individuals in the first size class was also significantly greater in 2015 than in 2012 (Fig. 2).
Distribution of stem diameter classes in the woody vegetation of a “Cerradão” in the Bacaba Municipal Park, Nova Xavantina, Mato Grosso (Brazil). Different letters indicate significant differences (P ≤ 0.05) between years (2008: white; 2012: dark gray; and 2015: light gray), based on Tukey’s post hoc test
Vegetation dynamics
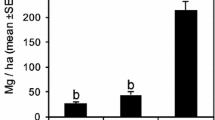
The density and basal area of the woody individuals that died (dead stem) during the first interval (2008–2012) were greater than those recorded in the second interval (2012–2015). The mean annual rates of mortality (based on the number of dead stem) and loss of basal area were higher during the former interval, reflecting the greater stability and reposition time. By contrast, the density and basal area of the recruits, the recruitment rate, and the gain in basal area were significantly greater in the second interval (Table 1).
Discussion
Resistance
The exclusion of species and the reduction in density and basal area following the fire indicate that the “Cerradão” has low resistance to this type of impact. Reis et al. (2015a) obtained similar finding in a second area of “Cerradão” impacted by fire. The low resistance of the “Cerradão” may be related to the presence of species typical of forest formations, which do not have adaptations to fire, increasing the vulnerability of this formation to wildfires and accidental burn-off (Moreira 2000; Hoffmann 2005; Walter and Ribeiro 2010). Resistance to fire is much greater in the region’s savanna formations (Gomes et al. 2014).
Other factors that contributed to the accentuated reduction in species richness were the predominance of smaller individuals (<10 cm in diameter) and the low density (rarity) of some species in the first survey, given that smaller individuals are more susceptible to the effects of fire (Hoffmann et al. 2009) and less abundant species are more susceptible to fluctuations at the density in consequence of probabilistic sampling (Felfili et al. 2000). Some of the more abundant species excluded following the fire in the present study (Matayba guianensis, Leptolobium dasycarpum, and Rourea induta) were also excluded or greatly reduced in density (58–90.9%) in the fire-affected “Cerrado” savanna formations (Mews et al. 2013; Gomes et al. 2014).
These findings indicate that even species typical of savanna formations may present reduced resistance to the effects of fire, especially because they are shrubs and thus more susceptible to the flames. However, Mimosa laticifera Rizzini & A.Mattos, which Gomes et al. (2014) considered to be fire-resistant, was the only species that increased in density after the fire in the present study. We observed that this species re-sprouted from the base after fire, favoring establishment in the community. In other words, while the fire altered the species composition of the savanna formations, a more pronounced effect was found in the “Cerrado” forest formations, as observed by Moreira (2000).
A significant reduction in the density of individuals (stem) after fire, as observed in the present study, has been recorded in a number of different savanna (Libano and Felfili 2006; Mews et al. 2013; Gomes et al. 2014) and forest formations (Reis et al. 2015a) in the Brazilian “Cerrado”, as well as in North American (Peterson and Reich 2001); African (Roques et al. 2001; Smit et al. 2010), and Australian (Scott et al. 2012; Beringer et al. 2015) savannas. However, the “Cerradão” presented a higher percentage of dead (dead stem) individuals (59.6%) in comparison with savanna formations (20.5% in Lima et al. (2009); 8.8 and 10.3% in Ribeiro et al. (2012); 43.6% in Gomes et al. (2014)), indicating that the “Cerradão” is more sensitive to fire. In this case, the recurrence of fires may lead to local extinctions.
Fire had the greatest impact (67.6%) on the smallest diameter class (5–10 cm), as observed in other studies of the “Cerradão” (Moreira 2000; Elias et al. 2013; Reis et al. 2015a). A similar pattern has also been recorded in “Cerrado” savanna formations (Lima et al. 2009; Gomes et al. 2014). However, the percentage of dead individuals (dead stem) in the smaller diameter classes is much lower in the savanna formations (37% in the 3–6 cm class in Lima et al. (2009); ≃9% in the 4.77–9 cm class in Ribeiro et al. (2012)), indicating that even the smaller individuals in savanna formations are more resistant to fire than those in forest formations.
The higher mortality rate and greater loss of basal area recorded in the first study interval (2008–2012) in comparison with the second (2012–2015) are directly related to the negative and proximate effects of the fire on the smaller individuals in the community. The reduced rates of recruitment and gain in basal area in the post-fire interval were similar to the results of studies of “Cerrado” savanna formations (2.08% year−1 in Ribeiro et al. (2012); 2.2% year−1 in Gomes et al. (2014)). This, together with the reduction in the density of individuals, results in a more open canopy, which favors the establishment and makes the vegetation more susceptible to new fires (Balch et al. 2011; Mews et al. 2013; Baudena et al. 2015).
The imbalance between mortality and recruitment rates in the first interval (2008–2012) resulted in an increase in individual reposition time and the stability of the community, a pattern also observed in basal area. These findings re-emphasize the reduced resistance and resilience of the “Cerradão” to fire.
Resilience
Even 7 years after the fire, the species richness, individual density, and basal area were all lower than the values recorded in 2008, prior to the fire, indicating that the “Cerradão” also has low resilience, in addition to its reduced resistance. This contrasts with the findings for savanna formations. For example, Gomes et al. (2014) evaluated the effects of fire on rocky “Cerrado”, a typical savanna formation, and concluded that 4 years was a sufficient period for the recovery of the species composition to its pre-fire level. Even after 7 years, however, the species richness and composition of the “Cerradão” monitored in the present study had not returned to the levels observed prior to the fire.
In addition, the greater percentage of individuals of species typical of savanna formations, after fire, reflects the intense impact of the fire on the species composition of the “Cerradão” and indicates a potential for the savannization of this environment, under continuing fire impact. This may be related to the loss of canopy cover resulting from the high mortality, even of large individuals, such as those of Tachigali vulgaris. Furthermore, species typical of savanna formations, e.g., Mimosa laticifera, re-sprouted from the base post-fire. This would favor the establishment of species typical of “Cerrado” savanna formations, to the detriment of forest species (Staver et al. 2011). Thus, fires can favor the establishment of individuals of species typical of savanna formations (observed in 2012) and the suppression of fire-favoring species typical of forest formations or generalists (observed in 2015). Similar results were observed by Durigan and Ratter (2006) and Morandi et al. (2015). These results indicate that savanna and forest formations are alternative states (Staver et al. 2011).
While the density of individuals appeared to be recovering, given that it was higher in 2015 in comparison with 2012, this was not the case for basal area. This may be related to the establishment of young individuals in the community, which increases density, but has a limited contribution to the total basal area of the community.
Tachigali vulgaris was the species that most contributed to the gain in basal area after the fire, as observed by Reis et al. (2015a) in individuals of ≥10 cm in diameter in an area of “Cerradão”. This is a fast-growing pioneer species (Reis et al. 2015a). A similar pattern has been observed in seasonal semi-deciduous rainforest in southeastern Brazil (Melo and Durigan 2010). These observations confirm the importance of the role of this species in the recovery of basal area and thus of the forest carbon lost during the fire. They also indicate that Tachigali vulgaris is a key species in the process of recovery of this type of vegetation (Reis et al. 2015a), given that it contributes directly to the formation of the canopy.
The pattern of lower mortality rate recorded in the second interval (2012–2015) was similar to that observed in a preserved area of “Cerradão” (4.36% year−1) studied by Reis et al. (2015b), indicating that fire-related mortality in the “Cerradão” does not exceed 4 years. The same situation has been observed in forests in the central Amazon basin (Barlow et al. 2003) and in transition forests in the southeastern Amazon (Balch et al. 2011).
In addition, during the second interval (2012–2015), which was free of fire, the recruitment rate (8.19% year−1) (some of the recruits in 2012 and 2015 were re-sprouted of individuals topkilled (stem dead) by fire in 2008) was higher than that recorded (2.67% year−1) by Reis et al. (2015b) in a well-preserved area of “Cerradão”. In this case, the thinning of the canopy and the reduced competition may favor the more rapid establishment of new individuals (Melo and Durigan 2010), benefitting the recruitment process. Subsequently, as the canopy closes, the recruitment rate decreases gradually (Oliveira and Felfili 2005). Even so, the recruitment rate recorded here in the “Cerradão” was considerably lower than that recorded in a savanna formation 2 years after a fire (16.4% year−1) by Gomes et al. (2014), further reinforcing the conclusion that “Cerrado” savanna habitats are more resilient than that of the forest formations.
The recruitment rate in the second interval (2012–2015) was higher than the mortality rate, resulting in lower reposition and reduced stability time in comparison with the values recorded (28.4% year−1 and 17.89 years, respectively) in a preserved “Cerradão” (Reis et al. 2015b). In contrast with the pattern observed in the present study, Gomes et al. (2014) observed that most parameters of the dynamics of the savanna vegetation had returned to their pre-fire levels approximately 3 years after the fire event. These results emphasize the greater resilience of the savanna formations to the effects of fire.
All three of our hypotheses were upheld, given that all the parameters tested were affected significantly by fire. The sum of the evidence permits us to conclude that the “Cerradão” is a formation characterized by low resistance and resilience to fire impacts, given that (1) there were a reduction in species richness, changes in the structure and dynamics of the vegetation, with high mortality, principally in the smallest individuals, and low rates of recruitment; (2) the establishment of individuals of species typical of savanna formations was favored; (3) 7 years was not a sufficient period for the recovery of species richness, individual density, or basal area to anywhere near the levels observed prior to the fire.
These findings indicate that the “Cerradão” is not undergoing a process of savannization, but rather, one of recovery, probably due to the contribution of key species for the recovery of the canopy, such as Tachigali vulgaris. Even so, this process may be reverted where fires are frequent events, given the prolonged period of time needed for the recovery of the vegetation from impacts. Furthermore, a period longer than 7 years is necessary for the “Cerradão” to re-establish its original conditions, highlighting its vulnerability to the impacts of fire, emphasizing the need for special care for the preservation of its biodiversity.
References
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728
APG—Angiosperm Phylogeny Group (2009) An update of the angiosperm Phylogeny group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Araújo FM, Ferreira LG, Arantes AE (2012) Distribution patterns of burned areas in the Brazilian Biomes: an analysis based on satellite data for the 2002–2010 period. Remote Sens 4:1929–1946
Balch JK, Nepstad DC, Curran LM, Brando PM, Portela O, Guilherme P, Reuning-Scherer JD, Carvalho Jr O (2011) Size, species, and fire behavior predict tree and liana mortality from experimental burns in the Brazilian Amazon. For Ecol Manag 261:68–77
Barlow J, Peres CA, Logan BO, Haugaasen T (2003) Large tree mortality and the decline of forest biomass following Amazonian wildfires. Ecol Lett 6:6–8
Baudena M, Dekker SC, van Bodegom PM, Cuesta B, Higgins SI, Lehsten V, Reick CH, Rietkerk M, Scheiter S, Yin Z, Zavala MA, Brovkin V (2015) Forests, savannas and grasslands: bridging the knowledge gap between ecology and dynamic global vegetation models. Biogeosciences 12:1833–1848
Beringer J, Hutley LB, Abramson D, Arndt SK, Briggs P, Bristow M, Canadell JG, Cernusak LA, Eamus D, Edwards AC, Evans BJ, Fest B, Goergen K, Grover SP, Hacker J, Haverd V, Kanniah K, Livesley SJ, Lynch A, Maier S, Moore C, Raupach M, Russell-Smith J, Scheiter S, Tapper NJ, Uotila P (2015) Fire in Australian savannas: from leaf to landscape. Glob change biol 21:62–81
Bond WJ, Woodward FI, Midgley GF (2005) The global distribution of ecosystems in a world without fire. New Phytol 165:525–538
Brando PM, Balch JK, Nepstad DC, Morton DC, Putz FE, Coe MT, Silvério D, Macedo MN, Davidson EA, Nóbrega CC, Alencar A, Soares-Filho BS (2014) Abrupt increases in Amazonian tree mortality due to drought-fire interactions. PNAS 111:6347–6352
Cardoso E, Moreno MIC, Bruna EM, Vasconcelos HL (2009) Mudanças fitofisionômicas no cerrado: 18 anos de sucessão ecológica na estação ecológica do Panga, Uberlândia-MG. Caminhos Geogr 10:254–268
Coutinho LM (1990) Fire ecology of the Brazilian cerrado. In: Goldammer JG (ed) Fire in the tropical biota. Springer, Berlin, pp 82–105
Dantas VL, Batalha MA, Pausas JG (2013) Fire drives functional thresholds on the savanna–forest transition. Ecology 94:2254–2263
Durigan G, Ratter JA (2006) Successional changes in cerradão and cerrado/forest ecotonal vegetation in western São Paulo State, Brazil, 1962–2000. Edinb J Bot 63:119–130
Elias F, Marimon BS, Gomes L, Forsthofer M, Abreu MF, Reis SA, Lenza E, Frankzak D, Marimon Junior BH (2013) Resiliência de um cerradão submetido a perturbações intermediárias na transição Cerrado-Amazônia. Biotemas 26:49–61
Encinas JMI, Silva GF, Pinto JRR (2005) Idade e crescimento das árvores, 7v. Comunicações técnicas florestais, Brasília
Felfili JM, Rezende AV, Silva MC Jr, Silva MA (2000) Changes in the floristic composition of cerrado sensu stricto in Brazil over a nine-year period. J Trop Ecol 16:579–590
Felfili JM, Mendonça RD, Walter BMT, Silva Júnior MC, Fagg CW, Nóbrega MGG, Sevilha AC, Silva MA (2001) Flora fanerogâmica das matas de galeria e ciliares do Brasil Central. In: Ribeiro JF, Fonseca CEL, Souza-Silva JC (eds) Cerrado: caracterização e recuperação de Matas de Galeria. Embrapa, Planaltina, pp 195–263
Gomes L, Maracahipes L, Marimon BS, Reis SM, Elias F, Maracahipes Santos L, Marimon Junior BH, Lenza E (2014) Post-fire recovery of savanna vegetation from rocky outcrops. Flora 209:201–208
Guimarães JCC, Van Den Berg E, Castro GC, Machado ELM, Oliveira Filho AT (2008) Dinâmica do componente arbustivo-arbóreo de uma floresta de galeria aluvial no planalto de Poços de Caldas, MG, Brasil. Rev Bras Bot 31:621–632
Hoffmann WA (2005) Ecologia comparativa de espécies lenhosas de cerrado e mata. In: Scariot A, Sousa-Silva JC, Felfili JM (eds) Cerrado: ecologia, biodiversidade e conservação. Ministério do Meio Ambiente, Brasília, pp 155–165
Hoffmann WA, Adasme R, Haridasan MT, Carvalho MT, Geiger EL, Pereira MAB, Gotsch SG, Franco AC (2009) Tree topkill, not mortality, governs the dynamics of savanna-forest boundaries under frequent fire in central Brazil. Ecology 90:1326–1337
Hoffmann WA, Geiger EL, Gotsch SG, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, Haridasan M, Franco AC (2012) Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol Lett 15:759–768
Korning J, Balslev H (1994) Growth rates and mortality patterns of tropical lowland tree species and the relation to forest structure in Amazonian Ecuador. J Trop Ecol 10:151–166
Libano AM, Felfili JM (2006) Mudanças temporais na composição florística e na diversidade de um cerrado sensu stricto do Brasil Central em um período de 18 anos (1985–2003). Acta Bot Bras 20:927–936
Lima ES, Lima HS, Ratter JA (2009) Mudanças pós-fogo na estrutura e composição da vegetação lenhosa, em um cerrado mesotrófico, no período de cinco anos (1997–2002) em Nova Xavantina-MT. Cerne 15:468–480
Lista de Espécies da Flora do Brasil. 2015. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/2015. Accessed 17 Jul 2015
Marimon BS, Felfili JM, Lima ES, Duarte WMG, Marimon Junior BH (2010) Environmental determinants for natural regeneration of gallery forest at the Cerrado/Amazonia boundaries in Brazil. Acta Amazon 40:107–118
Marimon Junior BH, Haridasan M (2005) Comparação da vegetação arbórea e características edáficas de um cerradão e um cerrado sensu stricto em áreas adjacentes em solos distróficos no leste de Mato Grosso, Brasil. Acta Bot Bras 19:913–926
Medeiros MB, Fiedler NC (2011) Heterogeneidade de ecossistemas, modelos de desequilíbrio e distúrbios. Biodivers Bras 2:4–11
Medeiros MD, Miranda HS (2005) Mortalidade pós-fogo em espécies lenhosas de campo sujo submetido a três queimadas prescritas anuais. Acta Bot Bras 19:493–500
Melo ACG, Durigan G (2010) Impacto do fogo e dinâmica da regeneração da comunidade vegetal em borda de floresta estacional semidecidual (Gália, SP, Brasil). Rev Bras Bot 33:37–50
Mendonça RC, Felfili JM, Walter BMT, Silva Júnior MC, Rezende AV, Filgueiras TS, Nogueira PE (2008) Flora Vascular do Cerrado. In: Sano SM, Almeida SP (eds) Cerrado: ambiente e flora. Embrapa, Planaltina, pp 289–556
Mews HA, Silvério DV, Lenza E, Marimon BS (2013) Influência de agrupamentos de bambu na dinâmica pós-fogo da vegetação lenhosa de um cerrado típico, Mato Grosso, Brasil. Rodriguésia 64:211–221
Miranda HS, Sato MN, Andrade SMA, Haridasan M, Morais HC (2004) Queimadas de Cerrado: caracterização e impactos. In: Aguiar LMS, Camargo AJA (eds) Cerrado: ecologia e caracterização. Embrapa Cerrados, Planaltina, pp 69–123
Morandi PS, Marimon BS, Oliveira EA, Reis SM, Xavier Valadão MB, Forsthofer M, Passos FB, Marimon BS (2015) Vegetation succession in the Cerrado/Amazonian forest transition zone of Mato Grosso state, Brazil. Edinb J Bot 72:1–11
Moreira AG (2000) Effects of fire protection on savanna structure in Central Brazil. J Biogeogr 27:1021–1029
Nogueira EM, Fearnside PM, Nelson BW, Barbosa RI, Keizer EWH (2008) Estimates of forest biomass in the Brazilian Amazon: new allometric equations and adjustments to biomass from wood-volume inventories. For Ecol Manag 256:1853–1857
Oliveira ECL, Felfili JM (2005) Estrutura e dinâmica da regeneração natural de uma mata de galeria no Distrito Federal, Brasil. Acta Bot Bras 19:801–811
Oliveira Filho AT, Mello JM, Scolforo JR (1997) Effects of past disturbance and edges on tree community structure and dynamics within a fragment of tropical semideciduous forest in south-eastern Brazil over a five-year period (1987–1992). Plant Ecol 131:45–66
Pereira BAS (2002) Árvores do Brasil central: espécies da região geoeconômica de Brasília. IBGE/Diretoria de Geociências, Rio de Janeiro
Peterson DW, Reich PB (2001) Prescribed fire in oak savanna: fire frequency effects on stand structure and dynamics. Ecol Appl 11:914–927
Pivello VR (2011) The use of fire in the Cerrado and Amazonian rainforests of Brazil: past and present. Fire Ecol 7:24–39
Ratter JA, Richards PW, Argent G, Gifford DR (1973) Observations on the vegetation of the northeastern Mato Grosso. I. The woody vegetation types of the Xavantina-Cachimbo expedition area. Phil Trans Roy Soc London B 266:449–492
Reis SM, Lenza E, Marimon BS, Gomes L, Forsthofer M, Morandi PS, Marimon Junior BH, Feldpausch TR, Elias F (2015a) Post-fire dynamics of the woody vegetation of a savanna forest (Cerradão) in the Cerrado-Amazon transition zone. Acta Bot Bras 29:408–416
Reis SM, Marimon BS, Marimon Junior BH, Gomes L, Morandi PM, Freire EG, Lenza E (2015b) Resilience of savanna forest after clear-cutting in the Cerrado-Amazon transition zone. Biosci J 31:1519–1529
Ribeiro JF, Walter BMT (2008) As principais fitofisionomias do Bioma Cerrado. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora. Embrapa, Planaltina, pp 151–212
Ribeiro MN, Sanchez M, Pedroni F, Peixoto KS (2012) Fogo e dinâmica da comunidade lenhosa em cerrado sentido restrito, Barra do Garças, Mato Grosso. Acta Bot Bras 26:203–217
Roques KG, O’connor TG, Watkinson AR (2001) Dynamics of shrub encroachment in an African savanna: relative influences of fire, herbivory, rainfall and density dependence. J Appl Ecol 38:268–280
Scott K, Setterfield S, Douglas M (2012) Does long-term fire exclusion in an Australian tropical savanna result in a biome shift? A test using the reintroduction of fire. Aust Ecol 37:693–711
Sheil D, Burslem DFRP, Alder D (1995) The interpretation and misinterpretation of mortality rate measures. J Ecol 83:331–333
Sheil D, Jennings S, Savill P (2000) Long-term permanent plot observations of vegetations dynamics in Budongo, a Ugandan rain forest. J Trop Ecol 16:765–800
Silva Júnior MC (2012) 100 árvores do cerrado sentido restrito: guia de campo. Rede de Sementes do Cerrado, Brasília
Silva Júnior MC, Pereira BS (2009) Mais 100 árvores do cerrado—Matas de Galeria: guia de campo. Rede de Sementes do Cerrado, Brasília
Silva Júnior MD, Felfili JM, Walter BMT, Nogueira PE, Rezende AV, Morais RO, Nóbrega MGG (2001) Análise da flora arbórea de Matas de Galeria no Distrito Federal: 21 levantamentos. In: Ribeiro JF, Fonseca CEL, Sousa-Silva JC (eds) Cerrado: caracterização e recuperação de Matas de Galeria. Embrapa Cerrados, Planaltina, pp 142–191
Smit IP, Asner GP, Govender N, Kennedy Bowdoin T, Knapp DE, Jacobson J (2010) Effects of fire on woody vegetation structure in African savanna. Ecol Appl 20:1865–1875
Staver AC, Archibald S, Levin SA (2011) The global extent and determinants of savanna and forest as alternative biome states. Science 334:230–232
Swaine MD, Lieberman D (1987) Note on the calculation of mortality rates. J Trop Ecol 3:ii–iii
Walter BMT, Ribeiro JF (2010) Diversidade fitofisionômica e o papel do fogo no bioma Cerrado. In: Miranda HS (ed) Efeitos do regime do fogo sobre a estrutura de comunidades de cerrado: resultados do projeto fogo. Ibama, Brasília, pp 59–76
Zar JH (2010) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgements
We are grateful to the research team of the Nova Xavantina Plant Ecology Laboratory for their assistance in the field, in particular Leandro Maracahipes, Karla Monique Carneiro, and Nayane Prestes. We also thank the Brazilian national Council for Scientific and Technological Development (CNPq) through project CNPq/PELD 558069/2009-6 (Cerrado–Amazon Forest Transition: ecological and socio-environmental strategies for conservation) and CNPq/PPBio 457602/2012-0 (Phytogeography of the Amazon–Cerrado Transition). We are also grateful to the Brazilian Coordination of Higher Education Training (CAPES) for providing graduate stipends to S.M. Reis, L. Gomes, P.S. Morandi, E.C. Neves, and F. Elias; the Foundation Support Research of the State of Mato Grosso (FAPEMAT), for the concession of scientific initiation stipends to E.A. Oliveira during the 2008 survey; and to CNPq for research productivity grants to B.S. Marimon, B.H. Marimon Junior, and E. Lenza.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reis, S.M., de Oliveira, E.A., Elias, F. et al. Resistance to fire and the resilience of the woody vegetation of the “Cerradão” in the “Cerrado”–Amazon transition zone. Braz. J. Bot 40, 193–201 (2017). https://doi.org/10.1007/s40415-016-0336-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0336-1