Abstract
Past and present gold mining operations scattered throughout the Kharaa River basin, Mongolia, have been identified as a major source of heavy metal and metalloid contamination. However, the potential accumulation of toxic contaminates including Cr, Zn, As, Cd, Hg, Cu, Ni and Pb in the resident fish fauna and the subsequent human health risks associated with their consumption have previously not been quantified. In the current study, contaminates in water, sediment and five consumed fish species (Leuciscus baicalensis, Thymallus baicalensis, Brachymystax lenok, Lota lota and Silurus asotus) were examined. The results indicated that concentrations of As and Hg exceeded the national permissible limits for drinking water in the Gatsuurt tributary of 10 μg L-1 and 0.05 μg L-1 respectively, while Hg contents detected in the sediment of the Boroo tributary were highly elevated (0.78 μg g-1). Heavy metal and arsenic accumulation was evident in all five fish species sampled across the basin, with maximum muscle contents of Cr, As, Hg and Pb detected in several species caught in the middle and lower river reaches, while Zn was highly elevated in B. lenok collected in the upper tributaries. Elevated median contents of Cr, Cu, Hg and Pb increased with trophic level, with Hg accumulation posing the greatest threat to humans as 10.7 % of all fish sampled in the study exceeded the internationally recommended threshold for Hg in consumable fish tissue. Although recreational fishing is rapidly growing throughout Mongolia, the overall level of fish capture and consumption remains relatively low. However, increasing pollution and accumulation in resident fish species could lead to chronic heavy metal intoxication in people who consume them regularly from the most polluted regions of the basin, while additionally being exposed to other sources of contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, heavy metals and metalloids have been increasingly released into the environment as a result of a plethora of anthropogenic activities (von Tümpling et al. 1995; Durrieu et al. 2005; Dhanakumar et al. 2015; Pfeiffer et al. 2014). Upon release, these toxic pollutants are often transported to, and concentrated in, nearby rivers and lakes where they contaminate the water and sediment, and are ultimately incorporated into the aquatic biota (Dušek et al. 2005; Lü et al. 2011; Nyirenda et al. 2012). While trace amounts of chromium (Cr), copper (Cu), nickel (Ni) and zinc (Zn) are biologically essential for normal growth and functioning of organisms, they are damaging in high concentrations, whereas arsenic (As), cadmium (Cd), lead (Pb) and mercury (Hg) are all nonessential and highly toxic even at low levels (Shukla et al. 2007). Certain elements can also potentially accumulate and magnify along the food chain amassing hazardous concentrations in higher trophic level species such as fish. If these fish are then regularly consumed by people, potential serious health implications can occur including neurochemical and cardiovascular damage, cancers, restrictive lung disease, renal and gastrointestinal problems and prenatal abnormalities or death (Weil et al. 2005; Morea et al. 2007). The risk is amplified if people are simultaneously exposed to elevated levels of heavy metals in drinking water, other foods and/or their domestic or occupational environments (Järup 2003; Tchounwou et al. 2012).
In order to gain a complete overview of the heavy metal contamination within an ecosystem, including the potential threat to human health, it is necessary to not only identify the source and extent of the pollution through sampling water and sediment, but to also evaluate the magnitude of the bioaccumulation in the consumed fish species (Pérez-Cid et al. 2001). Numerous studies have identified elevated concentrations of one or more heavy metals in locally consumed freshwater fish, including multiply instances where national and international thresholds established for their safe consumption have been exceeded. These studies have also identified both biotic (e.g. fish length or age) and abiotic factors (e.g. water or sediment contents) which have influenced the heavy metal concentrations in resident fish species. For example, in the Puyango River basin of southern Ecuador and the Petit-Saut hydroelectric reservoir in French Guiana, Hg contamination from small-scale gold mining has made local piscivorous fish species a potential risk for human consumption (Tarras-Wahlberg et al. 2001; Durrieu et al. 2005). Tarras-Wahlberg et al. (2001) reported that bottom dwelling species were more likely to accumulate higher contents of Hg due to the ingestion of contaminated sediments from the river substrate compared to other species. While, in Lake Titicaca, pollution from intensive mining activities and urban sewage discharge had also elevated levels of Cu, Zn, Cd and Hg in four fish species, prompting recommendations by the authors to limit fish consumption in certain heavily polluted parts of the lake (Monroy et al. 2014). It was reported that metal bioaccumulation in fish was only weakly related to metal concentrations in the environment (water and sediment), although nonessential elements (Cd, Hg, Pb) were generally more consistent with environmental peaks than biologically essential ones, with the exception of Cu (Monroy et al. 2014). Additionally in France, 60 % of brown trout (Salmo trutta fario) sampled from an historical mining region in the Cévennes National Park exceeded the maximum allowed concentrations for human consumption of Pb and Cd in fish tissue (Monna et al. 2011). The heavy metals detected in trout from this study reflected the high content in the river sediment, although age-related effects were also identified as an influential factor determining contamination in fish (Monna et al. 2011). These results highlight the capacity of various heavy metals originating from mining operations to contaminate locally consumed freshwater fish stocks to the point where they potentially pose a health risk to people consuming them, even if the contamination occurred in the past.
In Mongolia, gold mining is currently a driving force in the national economy, with substantial operations located in the country’s northern regions including the Boroo and Gatsuurt tributaries of the Kharaa River Basin (KRB) (Sandmann 2012; Karthe et al. 2015a). However, recent reports indicate that these mining activities are often major sources of heavy metal and arsenic pollution in both river water and sediment (Hofmann et al. 2010; Enkhdul et al. 2010; Oyuntsetseg et al. 2012; Brumbaugh et al. 2013; Pfeiffer et al. 2014). Mercury is known to have been used extensively in the Boroo River subcatchment by the thousands of illegal small-scale, artisanal miners who have used and released Hg into the environment during the gold amalgamation process (Grayson et al. 2004; Steckling et al. 2011). This source of Hg has added to the existing pollution that has resulted from a factory accident in 1956 where a substantial amount of this highly toxic and persistent element escaped into the nearby Boroo River (Tumenbayar et al. 2000). Further upstream in the Gatsuurt tributary, the expanding open-cut gold mining operations have also been correlated with the release of As from the surrounding soil and rocks (Tsetsegmaa et al. 2009), elevating As concentrations in the tributary's water and sediments (Enkhdul et al. 2010; Pfeiffer et al. 2014). In addition, the Kharaa River upstream from Darkhan city at Khongor sum has also been the location of a second industrial accident that occurred at a mining ore postprocessing plant in 2007, which saw both Hg and cyanide escaping in large quantities (Hofmann 2008). As a result of these past and present gold mining operations and accidents, several locations across the KRB have been identified as pollution hotspots as they are known to have elevated concentrations of toxic heavy metals in the water and sediment (Hofmann et al. 2010; Batbayar et al. 2015; Kosheleva et al. 2015). This high level of pollution in certain regions is a cause for concern, as it is also a likely source of contamination for the resident fish species, including those that are increasingly being captured and consumed by the basin's small, but growing, recreational fishing community.
Traditionally, in Mongolia there has been little cultural connection to fishing or consuming fish, although in recent decades this has changed rapidly (Chandra et al. 2005; Jensen et al. 2009). Seventeen fish species inhabitat the KRB including the Siberian sturgeon (Acipenser baerii) and Siberian taimen (Hucho taimen) that are listed as critically endangered and endangered, respectively, in the Mongolian Red Book of Fishes. However, over recent years both have become extremely rare due to overfishing and are now likely locally extinct. Up to eight species of fish are regularly caught by the recreational fishers in the KRB, which fish year round but are mostly concentrated in the summer and autumn months between June and November. Fishers typically target two salmonid species including the sharp snout lenok (Brachymystax lenok) and the Baikal grayling (Thymallus baicalensis), but also consume incidentally caught fish including the Siberian dace (Leuciscus baicalensis), burbot (Lota lota) and the introduced Amur catfish (Silurus asotus), among others (Kaus pers. obs.). The catch is mostly consumed by the fisher, their family and friends, or sometimes sold to the public in roadside stands or in city markets. To date, the human health risks associated with the consumption of these potentially contaminated fish species remains unknown, but needs to be urgently determined. This study aimed to quantify the existing contents of four nonessential and highly toxic elements (As, Cd, Pb and Hg) and four biologically important, but potentially contaminating heavy metals (Cr, Zn, Ni and Cu) in the muscle and liver of five consumed fish species, surface water and river sediment from across the KRB. In addition, it was set out to identify in which species and regions has the heavy metal bioaccumulation in fish exceeded the internationally recommended thresholds for human consumption of fish tissue and thus evaluate, whether or not, health warnings are warranted considering the current level of fish capture.
Materials and methods
Study site
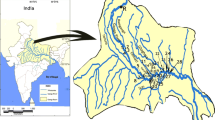
The Kharaa River Basin (14,534 km2) is located in northern Mongolia within the Selenga River catchment. The main river channel is 362 km long from its source in the Khan Khentii Mountains (2668 m a.s.l.) to its confluence with the Orkhon River (654 m a.s.l.). Annual air temperatures fluctuate between −40 °C in winter and +40 °C in summer, while the average annual rainfall varies between 250 and 350 mm (Karthe et al. 2015a). The population in the basin is approximately 147,000 with over half residing in the city of Darkhan. Gold mining remains an important industry and key source of income for thousands of people in the basin. Hofmann et al. (2010) has identified nine gold mines in operation, four gold mines out of use, six main centres of small gold mining activities and nine potentially contaminated regions including areas in the Boroo, Gatsuurt and Zagdalin tributaries and both upstream and downstream from Darkhan city (Fig. 1).
Field sampling
Surface water, river sediment and fish were collected from 11 sites across the KRB in June 2011 (see Fig. 1). In the upstream region, which is largely undisturbed and mountainous (Hofmann et al. 2015a) two tributaries were sampled, Sugnugr and Olgin, which were selected as the study’s reference sites (UP). In the mid-upper region (MID-UP), three sites were sampled including the Gatsuurt tributary, a small stream flowing directly through the Gatsuurt mining area; Kharaa 8.4, the most upstream main channel site, located approximately 1 km downstream from the Gatsuurt tributary and the Kharaa River confluence; and Kharaa 8, another main channel sampling location, which is a popular fishing spot in a lower impacted area of the basin. The middle region (MID) has two sampling sites including the Boroo River, a heavily polluted tributary draining a subcatchment with a high concentration of illegal small-scale mining activities as well as a large open-cut mine; and Kharaa 5.5, a main channel site located several kilometres downstream from the Boroo and Kharaa River confluence. The mid-down region (MID-DOWN) also consists of two sites including the Zagdalin River, another tributary with mining and agriculture dominating the subcatchment; and Kharaa 4, a main channel site located 2.6 kilometres downstream from the Zagdalin and Kharaa River confluence. Finally, in the downstream region (DOWN), sites Kharaa 3 and Kharaa 1 were sampled. Both are main channel sites located either side of Darkhan city and are characteristically slow flowing, meandering river reaches with little riparian vegetation and high levels of bank erosion (Hofmann et al. 2015a).
Kharaa River basin in northern Mongolia including the 11 sample sites marked with a red circle. UP stream reference sites: Sugnugr and Olgin. MID-UP sites: Gatsuurt, Kharaa 8.4 and Kharaa 8. MID sites: Boroo and Kharaa 5.5. MID-DOWN sites: Zagdalin and Kharaa 4, and DOWN stream sites: Kharaa 3 and Kharaa 1 (colour figure online)
Surface water samples were each collected with a new plastic syringe that had been triple-rinsed and strained through a single-use 0.45-µm membrane filter. One sample was collected at each site for the Hg analysis in an acid-washed glass bottle and acidified with hydrochloric acid, while a second sample was taken for analysis of the other considered heavy metals (Cr, Zn, As, Cd, Ni, Cu and Pb) in new polyethylene vials and acidified with redistilled nitric acid. Water samples were kept chilled during transportation and storage until final analysis at the Helmholtz Center for Environmental Research (UFZ) analytical laboratory in Magdeburg, Germany. Fine surface sediment samples were collected from the river's edge and small backwaters where sediment had accumulated as a result of the natural flow regime. A single sediment sample of approximately 0.3–0.5 kg was taken with a teflon scoop and kept in plastic containers for transportation back to the UFZ laboratory as well. Fish were captured using two backpack electrofishing machines (Hans Grassl GmbH, Germany; Type ELT 60) or obtained via angling and sampling catches of local fishers. Total lengths (cm) and weights (grams) were measured, and otoliths were taken for ageing purposes. A sample of muscle from the left dorsal fillet and the liver was removed from each fish for analysis. In order to avoid cross-contamination, all dissections were conducted using ceramic scalpels and pincers on a teflon cutting board that was cleaned thoroughly between each fish.
Laboratory analysis
Heavy metal concentrations were obtained from the filtered and acidified water samples prepared in the field without alteration. Concentrations of Cd, Cr, As, Zn, Cu, Ni and Pb were analysed with the ICP-MS, while total Hg concentrations were determined using the Mercury Analyser (Jena, Germany). Sediment samples were freeze-dried at −51 °C for 48 h (Christ Alpha 1–2 lyophiliser, B. Braun Biotech International, Melsungen, Germany) (Margetínová et al. 2008) and sieved to obtain a <63 µm homogenised fine sediment sample. From this sample, a 0.5 g subsample was extracted and digested overnight in acid-washed teflon containers in a combined 10 ml mixture of HCL and HNO3 (Aqua Regia) at room temperature. To ensure complete digestion of the sediment, the samples were then microwaved on a 40 min cycle and after diluted with Milli-Q water to obtain a standard volume of 20 ml for the final analysis.
Fish tissue samples were cleaned of all remaining skin, scales, bone and blood and a 1.00 g of muscle and 0.50 g of liver were subsampled and digested overnight in 2 ml of H2O2 and 8 ml of HNO3 (69 %) at room temperature. The acidified samples were further digested in a 40 min microwave cycle of heat and pressure before being transferred to new polyethylene vials where Milli-Q water was added to standardise the sample volume to 20 ml. Heavy metal analysis was conducted using the ICP-MS and the Mercury Analyser as per the water and sediment samples. Due to the small size of L. baicalensis, it was necessary to combine muscle and liver samples from two individuals captured at the same site and of similar sizes into a single sample to meet the required minimum analytical amount of 0.5 g. The tissues from both individuals were combined at a ratio of 50:50 where possible. Thus, as a result, 37 of the 46 L. baicalensis final samples contained two combined individuals. No aggregations of tissues were necessary for the other species. L. baicalensis individual ages were estimated using length and age data reported for Siberian populations by Lobón-Cerviá et al. (1996). For the remaining species, fish ages were obtained by counting annual growth rings of whole otoliths under a stereomicroscope with translucent light by two independent readers conducting two blind reads each.
Quality assurance
For every ten water, sediment or fish tissue samples, one blank and one standard sample was also analysed. The analysis of blanks was undertaken to determine potential contamination during the analysis, while the assessment of standard reference material was conducted to test the accuracy and precision of the analytical method and identify drifting errors. The standard reference materials used were from the National Institute of Standards and Technology (NIST) and the National Research Council of Canada (NRC). For sediment samples NIST 2704 was used, for fish muscle NRC Dorm-2 was used and for fish liver NBS DOLT was used.
Data analysis
Dissolved water (µg L−1) and fine sediment (µg g−1) results are presented as unaltered analytical values for each KRB sample site. Only water concentrations for Ni were taken from a similar 2007 sampling campaign (Ibisch unpublished). Heavy metal content data from fish tissue samples were tested for normality and homogeneity of variance using both Shapiro–Wilk and Levene’s tests. Significant differences within and between species muscle and liver heavy metal contents were calculated using the non-parametric Mann–Whitney U test due to the non-normal distribution of the data. In order to investigate the relative influence of different biotic and abiotic variables on the heavy metal accumulation patterns detected in KRB fish species, a generalised linear model (GLM) with a Gaussian distribution was fitted to log-transformed heavy metal data. Included as explanatory variables for all elements were fish tissue, fish length, fish age and site sediment contents from the location where the fish were caught. Site water concentrations from the fish sampling locations were added for As and Hg only, as all other heavy metals considered were below detection limits. Site sediment content and water (for As and Hg) concentrations were used to indicate the contamination level of each sampling locations and determine whether fish heavy metal content reflected site contamination. Fish length was also included over the highly correlated (R 2 = 0.98) fish weight measurements to reflect fish size. Multiple GLMs were completed per species and heavy metal, and the best model was selected by first using the automatic ‘step’ function in R which removes nonsignificant elements from full models containing full fixed factors and interactions, then using the manual backwards stepwise deletion method of remaining non-significant variables. All figures and statistics were produced using R (R Development Core Team 2010, version 3.1.3) or STASTICA 12 (STATSOFT 2013).
Results
Water concentrations (filtered to <0.45 µm)
Dissolved water concentrations (µg L−1) of Cr (<0.5), Ni (<0.5), Zn (<5), Cd (<0.2), Cu (<0.5) and Pb (<0.5) in the water samples were all below the analytical detection limits (see Table 1). Arsenic was detected at every site in the basin, with an overall mean concentration of 3.4 ± 4.29 µg L−1 (mean ± SD). The highest concentrations were recorded in the Gatsuurt (15.3 µg L−1) and Boroo (6.9 µg L−1) tributaries, while the lowest concentrations were detected in the Sugnugr (0.6 µg L−1) and Olgin (1.0 µg L−1) tributaries. Total dissolved Hg was at or below the detection limit of 0.008 µg L−1 in the surface water at Sugnugr, Zagdalin and Kharaa 1, with the highest concentrations in the basin detected at Gatsuurt (0.066 µg L−1), followed by Kharaa 3 (0.031 µg L−1) and Kharaa 8.4 (0.03 µg L−1). Mercury concentrations in the Boroo tributary were 0.025 µg L−1. The mean total dissolved Hg concentration for the Kharaa River basin surface water was 0.022 ± 0.02 µg L−1 (mean ± SD).
River sediment contents (sieved to <63 µm)
All heavy metals and As were detected in the fine river sediment across the basin (see Table 1). Only Hg was at or below the detection limit (<0.05 µg g−1) in eight of the 11 sites. The maximum total Hg content was in the Boroo River sediment (0.78 µg g−1), followed by Kharaa 4 (0.08 µg g−1) and Kharaa 5.5 (0.06 µg g−1). The Boroo River sediment also contained the highest content of Pb (17.6 µg g−1), Ni (23.6 µg g−1), Cu (21.7 µg g−1) and Cr (44.5 µg g−1), with the Gatsuurt tributary recording the next highest measurements for these four metals. The maximum total As content was detected in the Gatsuurt tributary sediment (30.8 µg g−1), followed by the Boroo tributary sediment (14.5 µg g−1). Sugnugr had the highest content of both Zn (112 µg g−1) and Cd (0.55 µg g−1), with Boroo (c Zn = 102 µg g−1) and Kharaa 4 (c Cd = 0.46 µg g−1), recording the next highest contents in the basin for these heavy metals, respectively.
Heavy metal contents (Cr, Zn, As, Cd, Ni, Cu, Hg and Pb) in liver (grey boxes) and muscle (white boxes) of five consumed fish species in the Kharaa River basin (L. baicalensis n = 14 liver samples/46 muscle samples, T. baicalensis n = 23/24, B. lenok n = 34/35, L. lota n = 10/11 and S. asotus n = 3/3) collected in June 2011. Letters (a, b, c, d) above each plot indicate significant differences between species groups—grey letters relate to liver, and black letters relate to muscle. Boxes indicate the median and 10/90 percentile, whiskers 2 × percentile range, open dots are outliers; b.d.l. indicates values are below detection limits. Dotted lines indicate international recommended thresholds for human consumption of fish tissue where applicable
Median heavy metal contents with standard error in the muscle of five fish species (L. baicalensis, LB; T. baicalensis, TB; B. lenok, BL; L. lota, LL and S. asotus, SA) grouped into five regions across the KRB; UP stream (Sugnugr and Olgin), MID-UP (Gatsuurt, Kharaa 8.4 and Kharaa 8), MID (Boroo and Kharaa 5.5), MID-DOWN (Zagdalin and Kharaa 4) and DOWN stream (Kharaa 1 and Kharaa 3). The dotted lines indicate the international recommended thresholds for human consumption of fish tissue. The line of numeric values above the bars represents the number of samples included in each region (n)
Fish muscle and liver contents
A total of 119 muscle samples and 74 liver samples were analysed for their heavy metal and metalloid contents from the five fish species captured across the KRB (Table 2). The basin-wide accumulation of heavy metals in fish tissue indicated Cr, Hg and Pb were typically present in higher amounts in fish muscle, while As, Zn, Cd and Cu were associated with fish liver (Fig. 2). Significant differences (p < 0.05) between heavy metal muscle and liver contents were identified for Cr, Zn, As, Cd, Ni, Cu and Hg in L. baicalensis, for Cr, As, Zn, Cd and Cu in T. baicalensis, for Cr, As, Hg, Zn, Cu and Pb in B. lenok and for all elements in L. lota, but no elements in S. asotus, most likely as a result of the small sample size. Median muscle contents of Cr, Hg and Pb generally increased from the lower trophic level species to the higher trophic level species (L. baicalensis < T. baicalensis < B. lenok < L. lota and S. asotus), while liver contents of these heavy metals remained lower than the levels in the muscle. Extremely elevated (p < 0.05) median liver contents were observed for As in L. lota, Cd in S. asotus and Zn in B. lenok, while liver contents of individual B. lenok had the maximum amounts of Ni (0.82 µg g−1) and Cu (33.8 µg g−1) detected in the study. In fish muscle, several individuals exceeded the maximal permissible limits for human consumption for Zn, Hg and Pb. Four B. lenok (53.2–109 µg g−1) had Zn contents in their muscle above the 40-µg g−1 threshold. For Hg, six L. baicalensis (0.52–1.85 µg g−1), one B. lenok (0.58 µg g−1), five L. lota (0.50–0.72 µg g−1) and two S. asotus (0.65–0.88 µg g−1) had contents in their muscle above the 0.5-µg g−1 threshold, and for Pb two B. lenok (0.59 and 0.73 µg g−1) and two L. lota (0.32 and 0.34 µg g−1) had contents in their muscle above the 0.3-µg g−1 threshold.
Generalised linear model (GLM)
The GLM determined the influence of five variables in explaining the observed heavy metal patterns detected in the sampled fish species (Table 3 Supplementary Material). The most important explanatory variables across species and heavy metals were fish age and length/tissue interactions, followed closely by fish tissue, fish length and site sediment contents. Site water concentrations for As and Hg were significant (p ≤ 0.05) for all species except S. asotus for Hg. For L. baicalensis, length/tissue interactions were the most important explanatory variable being significant for all heavy metals, while for T. baicalensis and B. lenok fish age was the only variable significant for each heavy metal and arsenic. Fish length, fish tissue and length/age interactions were all significant for L. lota and S. asotus, while fish tissue and fish age were both significant for all but one heavy metal (Pb and Cr), respectively.
Regional patterns of heavy metal bioaccumulation in fish muscle
The regional differences between the median heavy metal muscle contents of sampled fish species were considerable (Fig. 3). The median Cr contents were generally low and well below the internationally recommended threshold of 1 µg g-1 for this heavy metal in consumed fish muscle. For Zn, only B. lenok in the UP region had elevated contents close to the 40 µg g-1 threshold, while for all other species and regions Zn accumulation remained low (<10 µg g-1). The highest As median muscle contents were detected in L. lota sampled from the DOWN region, although As was also elevated in S. asotus (DOWN) and B. lenok (UP). The median As contents in the muscle of all other fish species and regions was below 1 µg g-1, however, no threshold has been given for As in consumed fish, as the principle form in fish tissue is organic arsenic (arsenobetaine) and thus non-toxic to humans (FAO/WHO 2011). Cadmium (Cd) was below the analytical detection limits in all species and regions except for B. lenok in the upper tributaries (UP), although median muscle contents were still well below the recommended threshold of 0.05 µg g-1 for fish tissue. Median muscle contents of Ni and Cu were negligible in all fish species and regions sampled in the KRB. Mercury detected in fish muscle exceeded the international recommended threshold of 0.5 µg g-1 in three different species and two regions: L. baicalensis in the MID region and L. lota and S. asotus in the MID-DOWN region. S. asotus in the DOWN region also recorded an elevated median muscle Hg content of 0.46 µg g-1. Lead (Pb) was very low or below the detection limit for L. baicalensis, T. baicalensis and B. lenok in the UP, MID-UP and MID regions. Elevated median Pb contents were detected in four fish species (TB, BL, LL, SA) in the MID-DOWN region, although the 0.3 µg g-1 threshold was not exceeded.
Discussion
Several studies of surface water, groundwater and river sediment in the KRB have highlighted the localised heavy metal and arsenic pollution associated with gold mining activities (Hofmann et al. 2010, 2015b; Inam et al. 2011; Pfeiffer et al. 2014; Batbayar et al. 2015). In the current study, maximum surface water concentrations for both c As = 15.3 µg L−1 and c Hg = 0.066 µg L−1 were detected in the Gatsuurt tributary downstream from the mine site, where these elements were more than double any other concentration detected in the KRB during the study, but less than half that of previously reported maximum concentrations for As (30.1 µg L−1) and Hg (2.0 µg L−1) (Hofmann et al. 2010; Pfeiffer et al. 2014). However, the 2011 concentrations still exceeded the Mongolian drinking water thresholds of 10 µg L−1 for As and 0.05 µg L−1 for Hg, indicating a significant health risk for the local residence, their livestock and wildlife who rely on this tributary for their drinking water. The As content detected in the Gatsuurt tributary sediment was also the highest in the basin at 30.8 µg g−1, although well below the previously reported level of c As = 136 µg g−1 sampled from closer to the mine site (Enkhdul et al. 2010), and still below the recommended threshold for As (c As ≤ 40 µg g−1) in river sediment recommended for German Rivers (Schneider et al. 2003). In contrast to the elevated Hg in river water, Hg in Gatsuurt sediment was beneath the analytical detection limit, suggesting a more recent source of Hg pollution is contaminating the river water, but had not yet accumulated in the sediment. In any case, the Gatsuurt tributary is one of the most polluted in the KRB and considering the future expansion of the mining operations, the potential increased pollution could have significant impacts on the usability of the river water for downstream residents as well. Mitigating measures should be urgently implemented to minimise As leaching from the overburdened soil and rocks during the open-cut mining operations, while attempts should be made to identify the source of the high Hg concentration in the tributary water. Current residents must be made fully aware of the hazards of drinking the water from the Gatsuurt tributary, as prolonged exposure to even low concentrations of these toxic elements can induce serious health problems (Pfeiffer et al. 2014; Steckling et al. 2011).
A second polluted site identified in the KRB was the Boroo tributary. While water concentrations for As and Hg were elevated above reference levels, they did not surpass Mongolian drinking water thresholds even with the very high content of c Hg = 0.78 µg g−1 in the sediment. The Boroo River Hg sediment contamination can be characterised as a moderately polluted ecosystem (c Hg ≤ 0.8 µg g−1), according to the German Chemical Classifications (LAWA 1997a), and recorded the same maximum Hg content as Lake Titicaca sediment (c Hg = 0.78 µg g−1; Monroy et al. 2014), but a lower maximum content compared to the Puyango River in Ecuador (c Hg = 0.99 µg g−1; Tarras-Wahlberg et al. 2001), both regions that reported hazardous Hg contamination in resident fish tissue. Elevated contents of Cr, Zn, Pb, Cu and Ni were also detected in the Boroo tributary sediment; however, all heavy metals there and elsewhere in the KRB were below the German Chemical Classifications for river sediment Class I thresholds as related to an aquatic ecosystem without any anthropogenic interference (c Cr ≤ 80 µg g−1, c Ni ≤ 30 µg g−1 and c Pb ≤ 25 µg g−1), or below the Class I–II thresholds indicating levels of very low pollution (c Zn ≤ 150 µg g−1, c Cu ≤ 20 µg g−1 and c Cd ≤ 0.6 µg g−1; LAWA 1997a). Thus, the Boroo tributary was confirmed as a key source of Hg pollution in the KRB owing to the contamination related to artisanal, small-scale mining as well as the 1956 Hg industrial accident (Hofmann et al. 2010; Tumenbayar et al. 2000). Although the use of liquid Hg to amalgamate gold in Mongolia’s small-scale mining operations was prohibited in 2008, strict enforcement is lacking and so its use likely continues, potentially adding to the already high Hg levels in this subcatchment.
During high rainfall and snow melt events, contaminates are either washed or leached from mining sites into nearby streams and tributaries or are infiltrated into groundwater bodies (Chalov et al. 2015; Hofmann et al. 2015b), where they are influenced by processes including dispersion, sorption, dissolution—precipitation and different chemical reactions that ultimately bind them to sediments and suspended loads (Thorslund et al. 2012; Chalov and Romanchenko 2015). As a result, heavy metal concentrations in surface waters fluctuate greatly, making a single water sample capable of only providing an approximation of the local contamination at a specific point in time, whereas the analysis of the heavy metal sediment content can offer a more robust assessment of the long-term pollution status of a local area. Therefore, future water and sediment monitoring programmes in the KRB should have an increased temporal sampling regime in order to accurately follow the contamination levels stemming from the expanding open-cut and illegal, placer mining operations.
Palaearctic riverine fish communities are naturally separated into upstream salmonid-dominated and downstream cyprinid-dominated communities due to the species’ ecological and biological preferences for specific habitat conditions. Mature individuals of many fish, including the sampled species, typically have extended home ranges which include spawning, feeding and winter refuge pools in river reaches separated by up to 10s of km. Particularly, in the harsh conditions of the prolonged Mongolian winter, individuals are forced to exit the smaller tributaries before they freeze solid forcing fish to return to the main river channel and accumulate in the deeper pools under the river ice, only re-entering those tributaries in spring to access more optimal spawning and feeding sites. It is expected that for at least B. lenok and T. baicalensis, individuals have likely travelled between multiple sampling sites and potentially across adjacent regions during extended seasonal migrations (>45 km) as has been indicated by acoustic telemetry tagging and population genetics studies of these species in Mongolia (Kaus unpublished). Thus, individuals have potentially been exposed to different levels of heavy metal contamination, and therefore, in order to gain a complete understanding of the accumulation pattern of KRB fish, it was necessary and reasonable to evaluate fish both at a basin-wide scale by grouping individuals by species, as well as at a regional scale by dividing fish into sampling regions.
The heavy metal accumulation observed in KRB fish species was highly variable depending on the contaminate and the main depository tissue. S. asotus, like other catfish, are opportunistic predators that feed on most other fish species in their habitat (Stolyarov 1985). Thus, they are at the top of the aquatic food chain, where they tend to accumulate elevated contents of heavy metals such as Hg due to biomagnification processes (Paterson et al. 2009). This has evidently happened with regard to the high S. asotus Hg content in the KRB, and has likewise occured in the lower Selenga River basin near Lake Baikal, where S. asotus accumulated the highest Hg muscle content of the 13 fish species investigated there. S. asotus recorded a mean Hg muscle content of ~0.216 µg g−1 wet weight (ww) (1.08 µg g−1 dry weight, dw; Komov et al. 2014; Haines et al. 1992), which was 2.4 times lower than KRB S. asotus (mean = 0.52 ± 0.43 µg g−1 ww, median = 0.65 µg g−1 ww, n = 3). In addition, a related catfish species (Silurus glanis) in the Danube River, Serbia, also accumulated the highest Hg content of the four fish species investigated in that study, recording a mean Hg muscle content of ~0.326 µg g−1 ww (Subotić et al. 2013), although this was also less than the Hg detected in KRB S. asotus. The generalised linear model (GLM) results indicated fish length, fish tissue, fish age and site sediment all significantly contributed to the Hg accumulation in S. asotus, suggesting that older, larger fish accumulate more Hg in their muscle tissue and that this species close affinity to the river substrate has also played a substantial role. The elevated Cd observed in the liver of KRB S. asotus, compared to muscle, was related to biotic factors only (e.g. fish length and age), as site sediment was not significant. Danube catfish also showed higher mean contents of Cd in their liver (~0.004 µg g−1 ww) compared to muscle (~0.002 µg g−1 ww) (Subotić et al. 2013), although at far lower levels in relation to KRB S. asotus liver contents (mean = 0.655 ± 0.58 µg g−1 ww, median = 0.426 µg g−1 ww, n = 3). Therefore, in comparison with the lower Selenga basin and Danube River catfish, the Kharaa River S. asotus had accumulated considerably higher heavy metal contents in the muscle for Hg, Pb, As, Cr and Cd but lower contents of Zn and Cu thus highlighting the hazardous heavy metal contamination of this consumed Kharaa River species.
Lota lota are also close to the top of the aquatic food chain as they are a predatory species that consumes mainly fish, but also invertebrates at smaller sizes (Pääkkönen and Marjomäki 2000). L. lota have accumulated a median Hg muscle content of 0.178 µg g−1 ww (mean = 0.306 ± 0.28 µg g−1 ww, n = 11), with four individuals exceeding 0.5 µg g−1 ww. However, this was in contrast to L. lota sampled from the Taimyr Peninsula in northern Russia, which recorded a mean Hg muscle content of ~0.494 µg g−1 ww (Allen-Gil et al. 2003), or considerably above KRB L. lota levels. Although, in the Lena and Mezen rivers, also in Russia, mean Hg muscle contents of L. lota were lower with ~0.05 and ~0.15 µg g−1 ww, respectively (Castello et al. 2014), or well below KRB L. lota contents. The GLM results indicated a complex process of Hg accumulation in L. lota, with fish age appearing to play the most significant role. For Cr, L. lota accumulated the highest median muscle content of all KRB species with a content of 0.193 µg g−1 ww (mean = 0.171 ± 0.1 µg g−1 ww, n = 11), which was elevated compared to Danube River L. lota with ~0.008 µg g−1 ww (Subotić et al. 2013). Fish length, fish tissue and site sediment along with the interactions of these variables have influenced Cr contents in L. lota muscle as determined by the GLM. Arsenic was found to be extremely elevated in the liver of L. lota compared to the other species investigated, which was also the case for L. lota in the Danube River, Serbia (Subotić et al. 2013), and from the Taimyr Peninsula in Russia (Allen-Gil et al. 2003). KRB L. lota As liver contents (median = 1.2 µg g−1 ww, mean = 0.99 ± 0.56 µg g−1 ww, n = 11) were 4.6 and 3.5 times higher than the mean As liver contents from this species in the Danube River (~0.212 µg g−1 ww) and the Taimyr Peninsula (~0.28 µg g−1 ww), respectively, once dry weights were adjusted to wet weights (see Komov et al. 2014; Haines et al. 1992). According to the modelling results, As accumulation in L. lota was complex, with all variables and interactions significant, except for tissue/age. Previous studies have suggested that elevated As contents in fish can be explained by the geomorphological substratum (Rowland et al. 2011), but this does not appear to be the case in the KRB with low As sediment contents and water concentrations compared to references sites detected in the lower basin (Kharaa 1 and Kharaa 4) where L. lota with the highest As contents were captured.
B. lenok and T. baicalensis are both benthopelagic species that prefer clearer, faster flowing and well-oxygenated waters of the middle and upper KRB basin. The diet of these salmonids including zoobenthos, macro invertebrates, fish and terrestrial rodents (Chandra et al. 2005), which has along with their upstream habitat preferences, likely contributed to their low heavy metal contents detected in most of the KRB individuals. These species typically recorded lower median heavy metal contents in comparison with L. lota and P. asotus for all elements, except Zn in B. lenok sampled from the UP region. The reason behind the high Zn content in B. lenok in the upper reference tributaries was undetermined, although elevated levels were generally found in individuals sampled from the Olgin tributary site, which although itself did not have elevated Zn in the water or sediment (77.7 µg g−1), it is located in the same upstream region as the Sugnugr tributary where the highest Zn sediment contents were detected in the KRB (112 µg g−1). This contamination supports the idea that individuals of this species undertake extended seasonal movements between the main channel and various spawning and feeding tributaries, which was also observed in acoustic telemetry tagging studies (Kaus unpublished). In other regions that have examined heavy metal accumulation in B. lenok, including the Genhe and Ussuri rivers in north eastern China, heavy metal contents in the muscle were also very low, being below KRB levels for Cr, Cd, Zn, Ni and Pb in both rivers, and for Cu in the Ussuri River (Wang and Mou 2011). While for T. baicalensis sampled in Lake Baikal at the mouth of the Selenga River (0.076 µg g−1 ww–0.38 µg g−1 dw; Komov et al. 2014), mean Hg muscle contents were comparably low with the KRB individuals (mean = 0.084 ± 0.03 µg g−1 ww, median = 0.086 µg g−1 ww, n = 24), while further downstream in the Yenisei River near Krasnoyarsk City, Russia, the heavy metal burden was investigated in Thymallus arcticus and was again found to have a similar low muscle content of Pb, Zn, Ni and Cd, but elevated Cr contents compared to KRB grayling (Anishchenko et al. 2009).
Leuciscus baicalensis is a small bodied cyprinid that consumes periphyton, zoobenthos and terrestrial insects (Chandra et al. 2005) and thus occupies the bottom tier of the KRB food web. However, the elevated contents detected in this species are likely due to its benthic feeding behaviour, where it incidentally ingests contaminated fine sediments and suspended organic matter, which can increase its uptake of heavy metals, than what would generally be the case for a lower trophic level species. This has been previously described in other regions and species, where sediment contaminated with heavy metals has posed a direct risk to benthic feeding fish (Köse et al. 2015; Monroy et al. 2014). Although the GLM results determined site sediment as a significant factor influencing the accumulation of most heavy metals in L. baicalensis, it also identified water concentrations for As and Hg, fish length, fish age, fish tissue and multiply interactions of these variables as also being significant, thus suggesting that heavy metal accumulation in L. baicalensis in the KRB is more complex than just considering heavy metal contamination in the river sediment. In similar studies, L. baicalensis sampled in the Russian section of the lower Selenga River basin and nearby lakes were reported as having mean Hg muscle contents between ~0.136 (0.68 µg g−1 dw) and ~0.054 µg g−1 ww (0.27 µg g−1 dw) (Komov et al. 2014), which is substantially lower than in the KRB where the mean muscle Hg content was 0.362 µg g−1 ww (median = 0.254 ± 0.32 µg g−1 ww, n = 45). While, in the Pechora River, northern Russia, Leuciscus idus also had lower muscle contents for Cd and Pb, but higher contents for Cu and Zn (Allen-Gil and Martynov 1995), further illustrating the increased bioavailability of contaminating heavy metals in the KRB compared to other similar rivers and species.
Regional patterns of heavy metal bioaccumulation in the KRB fish fauna was evident for Cr, Zn, As, Hg and Pb, while Cd, Ni and Cu accumulation showed no obvious regional differences in fish muscle content. In the unimpacted reference sites (UP region), B. lenok had still accumulated elevated Zn and As due to the increased background levels in the sediments. In the upper basin, B. lenok is the largest species and also likely moves substantial distances from deeper overwintering pools into smaller tributaries to spawn and feed in spring. Thus, the bigger B. lenok sampled in this region has likely been exposed over multiple years to these elevated heavy metals during their extensive seasonal movements and through the ingestion of their contaminated prey items. In both the MID-UP and MID regions, where only L. baicalensis, T. baicalensis and B. lenok were collected, contamination levels were comparable to the upstream reference region, except for Zn and As in B. lenok and the elevated Hg levels detected in L. baicalensis in the MID region. As L. baicalensis was the only species collected in the heavily polluted Boroo tributary, it was not unexpected that the Hg contamination from the sediment was reflected in these individuals. There was also no apparent contamination in the MID-UP region fish fauna from the expanding mining operations in Gatsuurt, even with the hazardous As and Hg concentrations detected in the tributary’s water. Heavy metal and metalloid contamination was most apparent in the MID-DOWN and DOWN regions of the KRB, particularly as the larger trophic species were collected there. Both L. lota and S. asotus recorded Hg contents above international thresholds (0.5 µg g-1) in the MID-DOWN region, although the number of individuals sampled was very low. As Hg in the sediment was below the detection limits in the Zagdalin tributary, it is expected that the Hg recorded in the main channel site (Kharaa 4) had been transported downstream from its source in the Boroo tributary and thus the persistent and accumulative capability of Hg in the environment has likely contaminated these fish species in the neighbouring MID-DOWN region as well. Lead (Pb), and to a small degree Cr, contamination was limited somewhat to the MID-DOWN region even though the maximum contents in the river sediment was also detected in the Boroo tributary. It is likely that both of these heavy metals (Pb and Cr) have also been transported downstream, increasing sediment contents in the adjacent MID-DOWN region main channel site and further exposing the higher trophic level species to this contamination, which has subsequently accumulated in the individuals that were capture there. However, why T. baicalensis and B. lenok, which were sampled in both regions showed increased Pb and Cr accumulation in the MID-DOWN compared to the more polluted MID region is unclear. In the DOWN region, elevated As in L. lota and S. asotus was also unexpected as there was low As in both water and sediment sampled in this region. Individuals with the higher As contents were also generally smaller (<20 cm TL; n = 6), as was the case concerning L. lota, potentially indicating that a specific nursery area, that was not sampled, contains a higher As content than the main river sites sampled in the DOWN region. Only one S. asotus sampled in the DOWN region had a high Hg muscle content of 0.88 µg g-1, while a second individual recorded 0.04 µg g-1. Both fish were also similar lengths of 49 and 48.6 cm TL respectively. The reason for this elevated Hg content in a single individual in the DOWN region is unclear, but it is likely related to either the fish’s own movements or movements of its prey items potentially having been exposed to Hg contamination elsewhere. Considering the sediment contents were similar for Hg both above and below Darkhan (Kharaa 3 and Kharaa 1), it appears that neither Darkhan city nor the 2007 Hg spill has had a major influence on fish contamination in the region, although the Hg concentration in the water closest to the spill location remained elevated above references levels (0.031 µg L−1).
The Kharaa River basin fish fauna has been exposed to and accumulated elevated contents of several toxic heavy metals in their edible muscle tissue, with Hg posing the greatest threat to human health if fish are consumed frequently. Even though, in 2011 only 10.7 % of the total fish sampled from the five species had accumulated Hg above the internationally recommended threshold of 0.5 µg g−1 ww, this low level of contamination should still be considered carefully in relation to the amount, frequency and sensitivity of the people consuming fish from the KRB. Therefore, preliminary information was obtained from a recreational fishing survey conducted during the summer of 2012 (Kaus unpublished), and indicated that while fish consumption was generally low for most fishers, it varied considerably from once or twice a year to almost every day in the summer and autumn months for unemployed and retired residents. The most common species and mean size caught was B. lenok of 34 cm (25–40 cm), which had a median Hg muscle content of 0.14–0.16 µg g−1 ww. So considering the average consumption rate of KRB recreational fishers was approximately one B. lenok per week during the fishing period, then the Provisional Tolerable Weekly Intake (PTWI), as related to the safe human consumption of methyl mercury (~80 % of total Hg) in fish muscle, these fishers are ingesting between 21–24 % of the recommended weekly intake by the World Health Organisation (WHO) (MeHg 1.6 μg kg-1 body weight-1 week-1). With this weekly threshold potentially being exceeded at the maximum consumption rate reported in the survey (5–7 fish/week) or if a highly contaminated L. lota or S. asotus are consumed. These potential health risks also need to be seen in the context of other sources of heavy metal exposure, of which several are relevant in Mongolia. If the human body burden for Hg or other heavy metals is already elevated due to occupational exposure (e.g. artisanal gold miners) (Steckling et al. 2011), ingestion of contaminated water (e.g. Gatsuurt tributary), consumption of other contaminated foods (plant products grown on soils enriched in heavy metals; Kasimov et al. 2011), or exposure to significant air pollution (locally elevated levels of several heavy metals; Sorokina et al. 2013), then frequently eating contaminated fish species will serve to intensify the chronic absorption levels in a person. This is critical considering the most sensitive community members, including pregnant women and young children, may already be facing potential serious health implications from other sources of heavy metal contamination as well (Steckling et al. 2011).
Conclusions
Although the demand for direct intervention (e.g. restrictions of fish consumption) is not immediately warranted, it is advisable to implement an investigative monitoring programme in order to quantify pollution levels and determine trends of heavy metal contamination within the KRB fish fauna. A meaningful set-up with regard to spatial sampling strategies, analytical methods and potential biological indicator species may be derived from the results of the current study. In a broader context, the data presented here has filled an important knowledge gap for integrative water resource management planning, which in the case of the KRB has typically been impeded by poor data availability (Karthe et al. 2015b). For the first time in the KRB or anywhere in Mongolia, it has been shown that heavy metal emissions related to gold mining activities are not only theoretical risks, but that accumulation from the environment into consumed fish species is evident and has reached concerning levels in the worst affected regions e.g. in the middle and lower river reaches. The described findings from the KRB, as a model study area, are also important to better understand mining-related risks across the vast Selenga-Baikal basin, which is characterised by a similar natural environment and comparable anthropogenic pressures (Karthe et al. 2015c).
References
Allen-Gil SM, Martynov VG (1995) Heavy metal burdens in nine species of freshwater and anadromous fish from the Pechora River, northern Russia. Sci Total Environ 160/161:653–659. doi:10.1016/0048-9697(95)93634-T
Allen-Gil SM, Ford J, Lasorsa BK, Monetti M, Vlasova T, Landers DH (2003) Heavy metal contamination in the Taimyr Peninsula, Siberian Arctic. Sci Total Environ 301:119–138. doi:10.1016/S0048-9697(02)00295-4
Anishchenko OV, Gladyshev MI, Kravchuk ES, Sushchik NN, Gribovskaya IV (2009) Distribution and migration of metals in trophic chains of the Yenisei ecosystem near Krasnoyarsk city. Water Qual Prot Environ Asp 36(5):623–632. doi:10.1134/S0097807809050121
Batbayar G, Karthe D, Pfeiffer M, von Tümpling W, Kappas M (2015) Influence of urban settlement and mining activities on surface water quality in northern Mongolia. Chapter II. Environmental pollution impacts of anthropogentic activities. In: Karthe D, Chalov S, Kasimov N, Kappas M (eds) Water and environment in the Selenga-Baikal Basin: international research cooperation for an ecoregion of global relevance. Stuttgart, pp 73–86 (ISBN 978-3-8382-0853-4)
Brumbaugh WG, Tillitt DE, May TW, Javzan Ch, Komov VT (2013) Environmental survey in the Tuul and Orkhon River basins of north-central Mongolia: metals and other elements in streambed sediment and floodplain soil. Environ Monit Assess 185:8991–9008. doi:10.1007/s10661-013-3229-9
Castello L, Zhulidov AV, Gurtovaya TY, Robarts RD, Holmes RM, Zhulidov DA, Lysenko VS, Spencer RGM (2014) Low and declining mercury in Arctic Russian rivers. Environ Sci Tech 48:747–752. doi:10.1021/es403363v
Chalov S, Romanchenko A (2015) Linking catchments to rivers: flood-driven sediment and contaminant loads from catchment and in-channel sources in the Selenga River. Chapter II. Environmental pollution impacts of anthropogentic activities. In: Karthe D, Chalov S, Kasimov N, Kappas M (eds) Water and environment in the Selenga-Baikal basin: international research cooperation for an ecoregion of global relevance. Stuttgart, pp 101–118 (ISBN 978-3-8382-0853-4)
Chalov SR, Jarsjö J, Kasimov NS, Romanchenko AO, Pietroń J, Thorslund J, Promakhova E (2015) Spatio-temporal variation of sediment transport in the Selenga River Basin, Mongolia and Russia. Environ Earth Sci 73:663–680. doi:10.1007/s12665-01403106-z
Chandra S, Gilroy D, Purevdorj S, Erdenebat M (2005) The feeding behaviour of fish from the upper Lake Baikal watershed of the Eroo River in Mongolia. Mong J Biol Sci 3(1):39–45
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Saf 113:145–151. doi:10.1016/j.ecoenv.2014.11.032
Durrieu G, Maury-Brachet R, Boudou A (2005) Gold mining and mercury contamination of the piscivorous fish Hoplian aimara in French Guiana (Amazon basin). Ecotoxicol Environ Saf 60:315–323. doi:10.1016/j.ecoenv.2004.05.004
Dušek L, Svobodova Z, Janouškova D, Vykusova B, Jarkovsky J, Šmĺd R, Pavlis P (2005) Bioaccumulation of mercury in muscle tissue of fish in the Elbe River (Czech Republic): multispecies monitoring study 1991–1996. Ecotoxicol Environ Saf 61:256–267. doi:10.1016/j.ecoenv.2004.11.007
Enkhdul T, Darjaa T, Dorj D (2010) Arsenic elimination in the artificial lake of the Gatsuurt gold mining area Mongolia. In: Proceedings of the 2nd international symposium on health hazards of arsenic contamination of groundwater and its countermeasures. Miyazaki, pp 145–148
FAO/WHO (2011) Codex Alimentarius Commission: Joint FAO/WHO Food Standards Program Codex Committee on Contaminates in Food. Fifth Session, Arsenic Part 1. The Hague, The Netherlands. p 11. ftp://ftp.fao.org/codex/meetings/CCCF/cccf5/cf05_INF.pdf
Grayson R, Delgertsoo T, Murray W, Tumenbayar B, Batbayar M, Tuul U, Bayarbat D, Erdene-Baatar C (2004) The people’s gold rush in Mongolia—the rise of the Ninja Phenomenon. World Placer J 4:1–112
Haines TA, Komov VT, Jagoe CH (1992) Lake acidity and mercury content of fish in Darwin National Reserve, Russia. Environ Pollut 78:107–112. doi:10.1016/0269-7491(92)90017-5
Hofmann J (2008) Bericht über die Untersuchungen von Grundwasser ind Boden auf Schwermetalle und Cyanid in Khonger Sum (Investigations on heavy metals and cyanide in groundwater and soil of Khonger Sum 2008)—Report for the Mongolian Water authority. p 56. (with Mongolian Summary)
Hofmann J, Venohr M, Behrendt H, Opitz D (2010) Integrated water resources management in central Asia: nutrient and heavy metal emissions and their relevance for the Kharaa River Basin, Mongolia. Water Sci Technol 62:353–363. doi:10.2166/wst.2010.262
Hofmann J, Karthe D, Ibisch R, Schäffer M, Kaus A, Avlyush S, Heldt S (2015a) Initial characterization and water quality assessment of stream landscapes in northern Mongolia. Water 7(7):3166–3205. doi:10.3390/w70x000x
Hofmann J, Watson V, Scharaw B (2015b) Groundwater quality under stress: contaminants in the Kharaa River basin (Mongolia). Environ Earth Sci 73(2):629–648. doi:10.1007/s12665-014-3148-2
Inam E, Khantotong S, Kim KW, Tumendemberel B, Erdenetsetseg S, Puntsag T (2011) Geochemical distribution of trace element concentrations in the vicinity of the Boroo gold mine, Selenge Province, Mongolia. Environ Geochem Health 33:57–69. doi:10.1007/s10653-010-9347-1
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68(1):167–182. doi:10.1093/bmb/ldg032
Jensen O, Gilroy DJ, Hogan Z, Allen BC, Hrabik TR, Weidel BC, Chandra S, Vander Zanden MJ (2009) Evaluating recreational fisheries for an endangered species: a case study of taimen, Hucho taimen, in Mongolia. Can J Fish Aquat Sci 66:1707–1718. doi:10.1139/F09-109
Karthe D, Heldt S, Houdret A, Borchardt D (2015a) IWRM in a country under rapid transition: lessons learnt from the Kharaa River Basin, Mongolia. Environ Earth Sci 72(3):681–695. doi:10.1007/s12665-014-3435-y
Karthe D, Hofmann J, Ibisch R, Heldt S, Westphal K, Menzel L, Avlyush S, Malsy M (2015b) Science-based IWRM implementation in a data-scarce central Asian region: experiences from a research and development project in the Kharaa River basin, Mongolia. Water 7(7):3486–3514. doi:10.3390/w7073486
Karthe D, Chalov SR, Borchard D (2015c) Water resources and their management in central Asia in the early twenty first century: status, challenges and future prospects. Environ Earth Sci 73(2):487–499. doi:10.1007/s12665-014-3789-1
Kasimov NS, Kosheleva NE, Sorokina OI, Bazha SN, Gunin PD, Enkh-Amgalan S (2011) Ecological-geochemical state of soils in Ulaanbaatar (Mongolia). Eurasian Soil Sci 44(7):709–721. doi:10.1134/S106422931107009X
Komov VT, Pronin NM, Mendsaikhan B (2014) Mercury content in muscles of fish of the Selenga River and Lakes of its basin (Russia). Aquat Toxicol 7(2):178–184. doi:10.1134/S1995082914020059
Köse E, Cicek A, Tokatli C, Emiroğlu Ö, Arslan N (2015) Heavy metal accumulations in water, sediment and some cyprinid species in Porsuk Stream (Turkey). Water Environ Res 87(3):195–204. doi:10.2175/106143015X14212658612993
Kosheleva NE, Kasimov NS, Gunin PD, Bazha SN, Sandag EA, Sorokina O, Timofeev I, Alexeenko A, Kisselyeva T (2015) Hot spot pollution assessment: cities of the Selenga River Basin. Chapter II. Environmental pollution impacts of anthropogentic activities. In: Karthe D, Chalov S, Kasimov N, Kappas M (eds) Water and environment in the Selenga-Baikal Basin: international research cooperation for an ecoregion of global relevance. Stuttgart, pp 119–136 (ISBN 978-3-8382-0853-4)
LAWA-AK “ZV” (1997a) Erprobung der Zielvorgaben für Schwermetalle für die Schutzgüter “Aquatische Lebensgemeinschaften”, “Schwebstoffe und Sedimente”, “Trinkwasserversorgung”, “Fischerei” und “Bewässerung landwirtschaftlich genutzter Flächen”. Basis: Länderberichte 1996 (Stand: 10.01.1997)
Lobón-Cerviá J, Dgebuadza Y, Utrilla CG, Rincón PA, Granado-Lorencio C (1996) The reproductive tactics of dace in central Siberia: evidence for temperature regulation of the spatio-temporal variability of its life history. J Fish Biol 48:1074–1087. doi:10.1111/j.1095-8649.1996.tb01805.x
Lü C, He J, Fan Q, Xue H (2011) Accumulation of heavy metals in wild commercial fish from the Baotou Urban section of the Yellow River, China. Environ Earth Sci 62(4):679–696. doi:10.1007/s12665-010-0508-4
Margetínová J, Houserová-Pelcová P, Kubáň V (2008) Speciation analysis of mercury in sediments, zoobenthos and river water samples by high-performance liquid chromatography hyphenated to atomic fluorescence spectrometry following preconcentration by solid phase extraction. Anal Chim Acta 615:115–123. doi:10.1016/j.aca.2008.03.061
Monna F, Camizuli E, Revelli P, Biville C, Thomas C, Losno R, Scheifler R, Bruguier O, Baron S, Chateau C, Ploquin A, Alibert P (2011) Wild brown trout affected by historical mining in the Cévennes National Park, France. Environ Sci Technol 45:6823–6830. doi:10.1021/es200755n
Monroy M, Maceda-Veiga A, de Sostoa A (2014) Metal concentration in water, sediment and four fish species from Lake Titicaca reveals a large scale environmental concern. Sci Total Environ 487:233–244. doi:10.1016/j.scitotenv.2014.03.134
Morea MF, Surico-Bennett J, Vicario-Fisher M, Crane D, Gerads R, Gersberg RM, Hurlbert SH (2007) Contaminants in tilapia (Oreochromis mossambicus) from the Salton Sea, California, in relation to human health, piscivorous birds and fish meal production. Hydrobiologia 576:127–165. doi:10.1007/s10750-006-0299-5
Nyirenda M, Thekiso VV, Dzoma BM, Motsei LE, Ndou RV, Bakunzi FR (2012) Heavy metal poisoning as a possible cause of massive fish mortality and mongoose in the gold mining area around Khutsong, north west province, South Africa. Life Sci J 9(3):2533–2537 (ISSN: 1097-8135)
Oyuntsetseg B, Kawasaki K, Watanabe M, Ochirbat B (2012) Evaluation of the pollution by toxic elements around the small-scale mining area, Boroo, Mongolia. ISRN Anal Chem. doi:10.5402/2012/153081
Pääkkönen JJ, Marjomäki T (2000) Feeding of burbot, Lota lota, at different temperatures. Environ Biol Fishes 58(1):109–112. doi:10.1023/A:1007611606545
Paterson SA, Ralston NVC, Peck DV, Van Sickle J, Robertson JD, Spate VL, Morris JS (2009) How might selenium moderate the toxic effects of mercury in stream fish of the western US? Environ Sci Technol 43:3919–3925. doi:10.1021/es803203g
Pérez-Cid B, Boia C, Pombo L, Rebelo E (2001) Determination of trace metals in fish species of the Ria de Aveiro (Portugal) by electrothermal atomic absorption spectrometry. Food Chem 75:93–100. doi:10.1016/S0308-8146(01)00184-4
Pfeiffer M, Batbayar G, Hofmann J, Siegfried K, Karthe D, Hahn-Tomer S (2014) Investigating arsenic (As) occurrence and sources in ground, surface, waste and drinking water in northern Mongolia. Environ Earth Sci 73(2):649–662. doi:10.1007/s12665-013-3029-0
Rowland H, Omoregie E, Millot R, Jimenez C, Mertens J, Baciu C, Hug S, Berg M (2011) Geochemistry and arsenic behaviour in groundwater resources of the Pannonian Basin (Hungary and Romania). Appl Geochem 26:1–17. doi:10.1016/j.apgeochem.2010.10.006
Sandmann R (2012) Gier nach Bodenschätzen und Folgen für die Mongolei. Geogr Rundsch 64(12):26–33
Schneider P, Neitzel P, Schaffrath M, Schlumprecht H (2003) Leitbildorientierte physikalisch—chemische Gewässerbewertung—Referenzbedingungen und Qualitätsziele. Umweltforschungsplan des Bundesministeriums für Umwelt, Naturschutz und Reaktorsicherheit. ISSN 0722-186X
Shukla V, Dhankhar M, Prakash J, Sastry KV (2007) Bioaccumulation of Zn, Cu and Cd in Channa punctatus. J Enivron Biol 28(2):295–397
Sorokina OI, Kosheleva NE, Kasimov NS, Golovanov DL, Bazha SN, Dorzhgotov D, Enkh-Amgalan S (2013) Heavy metals in the air and snow cover of Ulan Bator. Geogr Nat Resour 34(3):291–301. doi:10.1134/S1875372813030153
Steckling N, Boese-O’Reilly S, Gradel C, Gutschmidt K, Shinee E, Altangerel E, Badrakh B, Bonduush I, Surenjav U, Ferstl P, Roider G, Sakmoto M, Sepai O, Drasch G, Lettmeier B, Morton J, Jones K, Siebert U, Hornberg C (2011) Mercury exposure in female artisanal small-scale gold miners (ASGM) in Mongolia: an analysis of human biomonitoring (HBM) data from 2008. Sci Total Enivron 409(5):994–1000. doi:10.1016/j.scitotenv.2010.11.029
Stolyarov IA (1985) Dietary features of catfish, Silurus glanis, and pike-perch, Stizostedion lucioperca, in Kizlyarsk Bay, northern Caspian Sea. J Ichthyol 25:140–145
Subotić S, Spasić S, Višnjić-Jeftić Ž, Hegediš A, Krpo-Ćetković J, Mićković B, Skorić S, Lenhardt M (2013) Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube River (Serbia). Ecotoxicol Environ Saf 98:196–202. doi:10.1016/j.ecoenv.2013.08.02
Tarras-Wahlberg NH, Flachier A, Lane SN, Sangfors O (2001) Environmental impacts and metal exposure of aquatic ecosystems in rivers contaminated by small scale gold mining: the Puyango River basin, southern Ecuador. Sci Total Enivron 278:239–261. doi:10.1016/S0048-9697(01)00655-6
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. In: Luch A (ed) Molecular, clinical and environmental toxicology: environmental toxicology. Springer, Basel, pp 133–164
Thorslund J, Jarsjö J, Chalov SR, Belozerova EV (2012) Gold mining impact on riverine heavy metal transport in a sparsely monitored region: the upper Lake Baikal Basin case. J Environ Monit 14:2780–2792. doi:10.1039/c2em30643c
Tsetsegmaa T, Darjaa T, Dorj D (2009) Use of nitrous oxide–acetylene flame for determination of arsenic by AAS in geological samples. Mong J Chem Sci 7(315):4–7
Tumenbayar B, Batbayar M, Grayson R (2000) Environmental hazard in Lake Baikal watershed posed by mercury placer in Mongolia. World Placer Journal 1:134–159
von Tümpling Jr W, Zeilhofer P, Ammer U, Einax JW, Wilken RD (1995) Estimation of mercury content in tailings of the gold mine area of Poconé, Mato Grosso, Brazil. Environ Sci Pollut Res Int 2(4):225–228. doi:10.1007/BF02986770/0375-6742(94)00040-I
Wang H, Mou Z (2011) Detection and comparison of 10 metals in Brachymystax lenok from the Genhe River and Ussuri River. Asian J Chem 23(10):4715–4716
Weil M, Bressler J, Parsons P, Bolla K, Glass T, Schwartz B (2005) Blood mercury levels and neuro-behavioral function. JAMA 293(15):1875–1882. doi:10.1001/jama.293.15.1875
Acknowledgments
This research was conducted as part of the IWRM in Central Asia: Model Region Mongolia (IWRM MoMo) project in its second phase (2010–2013). Funding was provided by the German Federal Ministry of Education and Research (BMBF; Grant No. 033L003) and the Helmholtz Interdisciplinary Graduate School for Environmental Research (HIGRADE). Thank you to S. Avlyush, B. Tsedendorj, N. Natsagnyam, M. Hartwig and P. Theuring for assistance in the field and to C. Hoffmeister, U. Block, J. Hagemann and R. Wild for assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 3
Generalised linear model (GLM) results table per species with heavy metal contents as the response variable and fish length, fish tissue, fish age, site sediment content and site water concentrations added as predictor variables, where available (DOCX 122 kb)
Rights and permissions
About this article
Cite this article
Kaus, A., Schäffer, M., Karthe, D. et al. Regional patterns of heavy metal exposure and contamination in the fish fauna of the Kharaa River basin (Mongolia). Reg Environ Change 17, 2023–2037 (2017). https://doi.org/10.1007/s10113-016-0969-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10113-016-0969-4