Abstract
The environmental impacts of Boroo gold mine project in Mongolia was evaluated by chemical characterization of trace element concentrations in water, soils and tailing dam sediment samples. The results showed that concentrations of B, Cd, Ni and Se in the water samples were within the accepted levels of the Mongolia water quality standard (MNS4586: 1998). However, the concentrations of Al, As, Cu, Mn, Fe, Pb, U and Zn were higher than the maximum allowable concentration especially in the monitoring and heap leach wells. The average concentrations of As, Cd, Cu, Ni, Pb and Zn in the tailing dam sediment were 4419, 58.5, 56.0, 4.8, 20.6 and 25.7 mg/kg, respectively. Generally, arsenic and heavy metals in the soil samples were within the acceptable concentrations of the soil standard of Mongolia (MNS 5850: 2008). The chemical characterization of As solid phase in tailing dam sediment showed that the majority of As were found in the residual fraction comprising about 74% of total As. Assessing the potential risk to humans, simple bioavailability extraction test was used to estimate bioavailability of arsenic and heavy metals, and the concentrations extracted from tailing dam sediment were; 288.2 mg/kg As, 7.2 mg/kg Cd, 41.1 mg/kg Cu, 13.5 mg/kg Pb, 4.7 mg/kg Ni and 23.5 mg/kg Zn, respectively. From these results, the Boroo gold mine project has presently not significantly impacted the environment, but there is a high probability that it may act as a source of future contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
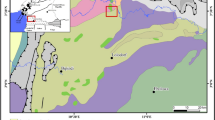
The Boroo Gold project is a gold mining and processing operation located in the Selenge province of Mongolia. It is situated approximately 140 km northwest of the capital city Ulaanbaatar at latitude 48°45′ N and longitude 106°10′ E. The project facilities including process plant, equipment workshop and tailing facility were constructed in 2002–2003. Boroo began commercial production in 2004 and Centerra Cold Inc. Canada has a 95% equity interest (Dalai and Dorjbaatar 2008).
The Boroo Gold Tailings facility was designed by Golder Associates Ltd. in order to provide the required capacity to contain all tailings generated over the anticipated life of the project. In addition, the facility has been designed as a zero discharge facility; that is, no supernatant water from the dam will be discharged to the environment. The tailing dam storage (TSD) is located in a wide valley approximately 6 km east of the open pits and the process plant. The valley is defined by low hills to the north, west and south. The geology at the site has been described as recent and quaternary deposits of eluvium, diluvium and proluvium. Bedrock at the site is generally of granite origin (Dalai and Dorjbaatar 2008).
Boroo gold deposits shows that the contact between the intrusive and the sedimentary rocks is highly irregular, with sedimentary xenoliths floating in the intrusive rocks in the border zone. A significantly younger igneous event of probably late Paleozoic age is restricted to narrow dikes and fissures of granitic to dioritic composition. Gold-sulphide zones host the largest proportion of gold mineralization at Boroo. This type manifests itself as an earlier, gold-pyrite-arsenopyrite-quartz phase that occurs in thin, irregular veinlets, less often in breccia zones, and disseminated within a pervasive zone of quartz-sericite alteration (“beresite” in the Soviet nomenclature). This earlier type is overprinted, and locally completely replaced, by a carbonate-bearing phase that is also quartz-sericite dominated and contains disseminated sulphides. It appears that the gold in this mineralization is relatively fine-grained. The overall intensity of the “beresite” alteration changes within the individual mineralized zones (Thalenhorst and Farquharson 2004).
The second major gold bearing phases are massive, white quartz-sulphide veins in which gold is commonly coarse-grained. From a volume point of view, this type is subordinate, but can carry very high gold values of up to several hundred grams per tonne. The sulphide content in both types is relatively low, typically a few percent. Geochemical assay results from the Boroo Gold Company (BGC) due diligence drilling indicates that, in keeping with the sulphide species observed, arsenic is highly anomalous (up to 21,500 ppm), but a positive correlation with gold is restricted to gold values up to about 2 g/t. Sulphur shows the same pattern, being noticeably lower in the higher gold grade ranges. This would appear to separate the two main types of mineralization/alteration described above. Silver values are generally low and are not obviously correlated with gold; with most samples below the detection limit of 2 g/t. Silver values can be higher in the quartz veins (Thalenhorst and Farquharson 2004).
Gold mining activities are important sources of arsenic and heavy metals in the environment, which may result in considerable water or soil contamination. Recently, heavy metals contamination of surface and ground water, agricultural soils and crops in mining areas have been identified as one of the most serious environmental problems in several countries of the world including Korea, Mexico, India, China, France and Spain (Kim et al. 1998, 2002; Lee et al. 2006; Huang et al. 2007; Ruiz-Chancho et al. 2007; Douay et al. 2008; Espinosa et al. 2009; Yellishetty et al. 2009). Ground water is the source of drinking water in the study area because drinking water is directly related to human health, and it is necessary to keep monitoring the quality of water to ensure it meets national and international standards. In the Boroo gold mine site, a number of water wells were drilled and are included in the Boroo Gold Company monitoring project. However, due to limitations in research capability, this joint study was undertaken between the Central Research Laboratory of Environment and Metrology (CLEM), National Agency for Meteorology and Environmental Monitoring, Mongolia and International Environmental Research Centre (IERC), Gwangju Institute of Science and Technology, Korea.
The major purpose of this work is to contribute to a better understanding of the geochemical distribution of As and heavy metals in the vicinity of the Boroo Gold Mine. The potential risks of environmental and human As contamination in the Boroo Gold mine are discussed through the evaluation of the leachability, bioaccessibility and speciation of As in the tailing dam sediment (or slurry).
Materials and methods
Water sampling and analysis
Ground water samples were collected twice in the months of February (winter) and September (summer) 2009 from the 14 ground water wells that are included in the Boroo Gold Company (BGC) water monitoring programme (Fig. 1). The wells include 7 monitoring wells (MW7–MW14) located east of the tailing storage dam; 4 borehole wells (BH1–BH5) located along the flow path of River Boroo and 3 ground water wells placed up and down gradient in the heap leach (HL) area of the mine. The up-gradient well (HL4 and HL5) gives information on the background water quality while the down-gradient well (HL6) would be indicative of any contributions from the heap leach. Control sample was collected from the drinking water well (DW) located near the factory workers living area. In total, 64 ground water samples were collected. Surface water samples were collected from River Boroo during the two sampling campaigns as well as waste water (TSD) discharging into the tailing storage dam. A total of 10 surface water samples and 4 waste water samples were collected.
All samples were collected in 1-l polypropylene bottles. The bottles were cleaned using a modified procedure of Laxen and Harrison (1981). On site pH was measured using a portable device TOA pH meter: HM-10P, Japan; similarly, electrical conductivity was measured using portable device TOA Conductivity meter: CM-11P, Japan. After collection, the bottles were stored in an ice box, transported to the Central Laboratory for Environment and Metrology (CLEM), Mongolia for further processing. In CLEM, the samples were split into 125-ml containers, acidified with analar grade HNO3 and stored in the refrigerator at 4°C. After two days when the samples had been completely frozen, they were shipped in ice boxes with dry ice packs to International Environmental Research Centre (IERC) laboratory, Korea. The remaining part of the samples were used for nutrients (NO3, PO4, SO4) measurement using UV–VIS spectrophotometer: Shimadzu UV-1201, Japan; SS by weighing method, F determination using photocolorimetric method and titration methods were used for the determination of Ca and Cl (Bulgan 2008). At IERC laboratory, the samples were digested using the method of Jarvas et al. (1992) and analysed for total dissolved arsenic and 10 trace elements using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES: Perkin elmer Optical 5300 DV, USA).
Tailing storage dam sediment and soil samples
Sediment and soil samples were collected during the February and September 2009, sampling period. The sediment sample was collected with a plastic shovel in the top 5 cm in banks of the dam avoiding input of ground materials, which was a composite formed by ten subsamples taken randomly from different areas of the dam. Soil sample was collected within a depth of 15 cm from surface and was a composite of 10 subsamples taken across the areas of ground and surface water sampling locations within the mine site. The samples were transported to CLEM laboratory where they were air-dried, disaggregated, sieved on a 10 mesh (<2 mm) screen, then quartered, pulverized and passed through an 80 mesh (<180 μm) sieve. The samples were then posted to IERC laboratory for further analysis.
Total arsenic concentrations were determined after the digestion of samples with aqua regia. Triplicate samples of 0.4 g (modified to enhanced good recovery and accuracy while still maintaining solid to liquid ratio of 1:10) were digested at room temperature with 36% HCl and 62% HNO3 (ratio 3:1) mixture for at least 8 h (overnight) (Fuentes et al. 2004; Oliveira et al. 2007). After this, the solutions were digested at 70°C for 1 h under reflux condition and allowed to cool (Kim et al. 2002, 2005). The extract was separated by centrifugation for 10 min, collected in polyethylene bottles and made up to 50 ml with 2% HNO3 for analysis (Ure 1995). Arsenic, Cd, Cu, Pb, Ni and Zn were measured by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES: Perkin Elmer Optical 5300 DV, USA).
Arsenic sequential extraction
A sequential extraction procedure was employed to examine the chemical forms of As solid phases in the tailing dam sediment. The method proposed by Wenzel et al. (2001) for the sequential extraction of As in soils was simple to execute and targets the most abundant environmentally important forms of As. Fraction 1, employed as single extractant, has been shown to correlate well with As in field-collected soil solution and hence can be used for predicting solute As (Johnston and Barnard 1979). Such information is useful in risk assessment of As leaching to the ground water and the readily bioavailable fraction. Fractions 2–4 may provide information on potential lability of As from different solid phases as a result of soil remediation or alteration in soil (e.g. redox, pH) and environmental factors. High contributions of residual fractions (Fraction 5) in tailing sample should undoubtedly be considered as beneficial from the standpoint of environmental risk (Krysiak and Karczewska 2007). The sequential steps are described in Table 1, and the chemical forms of As were separated into non-specifically sorbed, specifically sorbed, amorphous and poorly crystalline hydrous oxides of Fe and Al, well-crystallized hydrous oxides of Fe and Al and residual fraction.
All analytical data were assessed for accuracy and precision using a quality control system during the analytical procedure (Ramsey et al. 1987). To check As recovery in aqua regia and sequential extraction, standard reference material (SRM) 2711 from National Institute of Standards and Technology, NSIT, USA was used. The recovery for As was 97 ± 8% for aqua regia extraction while a recovery of 110 ± 9% was obtained for the As sequential extraction experiment using SRM 2711.
Simple bioavailability extraction test (SBET)
The SBET was used to determine the bioavailability or bioaccessibility of arsenic and heavy metals in the tailing dam sediments. SBET is a simplified in vitro form, which has been used to determine the human bioavailability quotients for arsenic and toxic heavy metals from soils. The method involves the leaching of solid samples such as mine waste or contaminated soil in a simulated gastric fluid matrix, and the test was performed according to the method described by Kim et al. (2002). Synthetic stomach fluid was prepared by dissolving 60.6 g of glycine in 2 l of de-ionized water and adjusting to pH 1.5 with HCl (12.1 M). One hundred millilitres of this artificial stomach fled was placed in a wide-mouth HDPE bottle containing 1.00 g of sediment sample. The HDPE bottles had air-tight screw-cap seals, and care was taken the bottles did not leak during the extraction procedure. Each sample bottle was wholly immersed and placed into the extractor in a water bath at 37°C. The extractor was rotated end over end at 30 rpm for 1 h. When extraction was completed, a 10-ml aliquot was directly taken from each reaction bottle and passed through a 0.45-μm filter. The filtration time and the pH of the fluid remaining in the extraction bottle were recorded. If the fluid pH was not within ±0.5 pH units of the starting pH, the test was discarded. All sample aliquots were analysed for As, Cd, Cu, Pb and Zn by ICP-MS
Leaching experiment
Tailing dam sediments (100 g) were shaken in de-ionized water (1 l) at room temperature for 24 h. Ionic strength and pH were not regulated to keep the conditions close to the naturally occurring process (Ahn et al. 2005). At the end of the equilibration period, solution pH was determined for the suspension using a combination pH electrode (Thermo Orion). The filtered leachate samples were analysed for total dissolved arsenic concentrations by ICP-MS.
Results and discussion
Physico-chemical parameters in water samples
Average concentrations of chemical parameters measured in water samples from Boroo Gold mine site are presented in Table 2. The results were compared with the water quality standard MNS 4586: 1998 used for the evaluation of ground and surface water in Mongolia and the World Health Organization guidelines for drinking water quality (WHO 2006).
The pH varied in the range of 7.4–9.1 with the summer (S) values being higher than winter (W) values in most stations. The Mongolia standard recommends pH value of 6.5–8.5, which was complied with by some of the stations except summer readings of the following stations River Boroo, BH 5, HL6, MW 7, 8, 9, 10, 11 and 12. Similar higher pH values during summer have been reported by Borba et al. (2003) in ground water studies from a gold-producing area of Brazil. Conductivity values were between 49 and 3,040 μS/cm; the monitoring wells had the highest values of conductivities. Monitoring well 7 (MW 7) exhibited the highest values of 2,940 μS/cm in winter and 3,040 μS/cm in summer, respectively. A concentration range of 4.1–1,670 mg/l was obtained for sulphate with MW 7 having the highest values of sulphate (1,233 mg/l in winter and 1,670 mg/l in summer). Generally, all the monitoring wells and heap leach wells (except HL6) exceeded the 100 mg/l maximum allowable concentration (MAC) for sulphate by Mongolia water quality standard (MNS 4586: 1998). Similarly, highest values of Ca (199 mg/l in winter and 189 mg/l in summer) and Mg (132 mg/l in winter and 157 mg/l in summer) were found in MW7. The monitoring wells are located around the tailing storage dam, with MW 7 located about 10 m from the dam. During the detoxification of the dam, copper sulphate and sodium meta-bisulphite are added to the weak acidic environment in the dam to dissociate cyanide to less than 1 mg/l. Hydrated lime is also added to maintain the pH; therefore, it is likely that there is underground seepage from the dam which is affecting the water quality of MW 7 and surrounding monitoring wells. Nitrates and phosphates were all within the accepted ranges of Mongolia water quality standard (9.0 and 0.1 mg/l, respectively) in all the samples for the two seasons.
The concentrations of B, Cd, Ni and Se in all the samples analysed were within the acceptable levels of both the Mongolia water quality standard (MNS 4586: 1998) and the WHO guideline (WHO 2006) for water quality. However, the concentrations of Al, As, Cu, Mn, Fe, Pb, U and Zn were higher than the maximum concentrations allowable for Mongolia (MNS 4586: 1998) and the WHO guideline especially in the monitoring wells and heap leach wells. In general, the control samples, River Boroo samples and the Borehole (BH) samples contained the concentrations of most parameters (except U) within acceptable levels. Aluminium ranged from 16 to 9,275 μg/l with the highest value of 9,275 μg/l in MW 10 winter sampling. MW 7, 9, 10, 11, 12, and HL 4 and 5 had Al concentrations greater than the 500 μg/l WHO guideline (WHO 2006) especially in the winter. A similar trend was obtained for Fe, a concentration range of 10–7,744 μg/l, with MW 10 having the highest Fe value of 7,744 μg/l. MW 7, 11, 12, 14 and HL 4, 5, 6 all having values above the 500 μg/l guideline recommended by WHO. Similarly, MW 7, 9, 10, 11, 12, 14, HL 4, 5 and 6 have values above the 300 μg/l guideline of Mongolian standard (MNS 4586: 1998). The Cu concentrations of 21.8, 34.6 and 11.6 μg/l for winter measurements in MW7, MW9 and HL6 were higher than the 10 μg/l MAC in the Mongolia water quality standard. Although the control sample from the drinking water well (DW) had more than 10 μg/l Cu, this concentration is permissible based on the drinking water safety standard for Mongolia (MNS 900: 2005). Manganese was above the MAC 100 μg/l of Mongolia water quality standard (MNS 4586: 1998) in MW 7, MW10 and HL 4 with a range of 0.4–1077 μg/l. Arsenic concentration ranged from 0.1 to 46.1 μg/l, with winter measurements in MW7, MW10, MW11 and HL4 being 46.1, 20.3, 14.9 and 30.3 μg/l, respectively. These values are all above the 10 μg/l MAC of Mongolia water quality standard (MNS 4586: 1998) and WHO drinking water guideline (WHO 2006). Lead had concentration range of 0.1–54 μg/l, and MW 12 had the highest value of 54 μg/l. Other station with values above the 10 μg/l Mongolia and WHO guideline included MW 9, 10, 11, 14 and HL6. Uranium was generally above the 15 μg/l WHO guideline in all the stations except River Boroo. The concentration range was 3.1–150 μg/l, and there was no clear pattern between winter and summer samples among the stations; the highest value of 150 μg/l was obtained in summer at MW10. River Boroo and the borehole wells (BH1-5) did not exceed the 10 μg/l MAC level for Zn, other stations all had values greater than 10 μg/l especially in the winter measurements.
The results obtained for the waste water from tailing processing plant was compared with the effluent standard of Mongolia (MNS 4943: 2000). Arsenic and Cu were the only elements having elevated concentrations which exceeds the requirement of 50 μg/l As and 300 μg/l Cu. The concentration of As was 1,746 μg/l during winter and 1,059 μg/l in summer while Cu concentrations were 2,687 and 2,811 μg/l for winter and summer seasons.
The monitoring wells (MW 7–14) may receive some input from the tailing storage dam as evident in elevated concentrations of Al, As, Fe, Pb and U in comparison with the control samples (DW). The surrounding geology of the location of the monitoring wells has been described as recent and Quaternary deposits of eluvium, diluvium and proluvium. Bedrock at the significant depth is generally of granitic origin with rock erosion products being loam, sandy loam, sand and gravel (Dalai and Dorjbaatar 2008). Therefore, in addition to anthropogenic sources, there could be a natural enrichment of these elements.
The highest elemental concentrations were found in samples collected in the winter with a few exceptions. It is assumed the dilution effect is less pronounced as the winter is associated with dry season. The same trend has been reported by Borba et al. (2003).
Arsenic and heavy metals concentrations in the tailing dam sediment and soils
The ranges and average concentrations of As and heavy metals in the tailing dam sediment and soils are listed on Table 3. The results were compared with the soil standard of Mongolia (MNS 5850: 2008) and the tolerable level established by Kabata-Pendias and Pendias (1984). The average concentrations of As, Cd, Cu, Ni, Pb and Zn in the tailing dam sediment were 4,419, 58.5, 56.0, 4.8, 20.6 and 25.7 mg/kg, respectively. Arsenic and Cd values were higher than natural soils (Bowen, 1979) and also higher than the soil standard of Mongolia (MNS 5850:2008) as well as the tolerable levels, which are considered as phytotoxically excessive; 20 mg/kg of As and 3 mg/kg of Cd (Kabata-Pendias and Pendias 1984). The average concentrations of Cu, Ni, Pb and Zn were, however, acceptable from this standpoint. Arsenic is derived from gold-pyrite-arsenopyrite-quartz minerals. During the tailing treatment process, arsenic is precipitated using ferric sulphate in the ratio 8:1 ferric sulphate/arsenic before the slurry, then flows by gravity from the tailings tank to the tailing storage dam. However, the focus of the treatment is to remove residual cyanide concentration going into the tailings dam to <1.0 mg/l as required in the environmental impact assessment (Dalai and Dorjbaatar 2008). Cadmium is associated with mine waste ore in the gold mining process.
The concentrations of As and heavy metals in soils were quite variable according to the sample collection area within the gold mine. The highest concentration of 264 mg/kg As was found in soil from the heap leach area. However, Cd levels were below the analytical detection limit of 0.03 mg/kg in all the soil samples including the control samples. Generally, As was above the acceptable concentrations of the soil standard of Mongolia (MNS 5850: 2008) in soils except the control area soil. The tailing dam is constructed to meet international standards to minimize the impacts to the environment, but As still finds its way into the environment.
Chemical fractionation of arsenic in tailing dam sediment
In order to provide information on the speciation of As in the tailing dam sediments for a future decommission plan, sequential extractions were performed. The results of sequential extractions of the tailing dam sediment are shown in Table 4.
The amounts of readily labile As extracted by (NH4)2 SO4 was small (<1%), but represent the most important fraction related to environmental risk. This fraction represents the soluble As minerals and exchangeable forms of As. The fraction of As extracted by (NH4) H2PO4 represented about 3% of total As and may be useful in providing a measure of specifically sorbed As in being potentially mobilized due to changes in pH. The NH4-oxalate extractable phase accounted for 12–18% of total As, and the oxalate reagent targets the dissolution of the amorphous Fe oxyhydroxides (Keon et al. 2001). A minor amount of As in the tailing dam sediments were associated with the crystalline Fe oxides. The majority of As in tailing dam sediment were found in the residual fraction comprising about 78–82% of the total As. The results showed a predominance of fractions where As is more strongly retained by sediment components and such high contributions of the residual fractions should undoubtedly be considered beneficial from the standpoint of environmental risk. However, it must be noted that the chemical reagents employed in the sequential extraction targeted As in exchangeable forms and As sorbed by, and co-precipitated with, iron oxides excluded other As minerals that may be present in tailings (Ahn et al. 2005). The sediment samples used in this study represents the processed tailings for which ferric sulphate has already been applied to precipitate As. There were differences in the fractionation of As in the summer and winter samples with summer values being higher than the winter values except in fraction 3. The As concentration of the summer sample associated with Fraction 4 was almost 4 times higher than those of winter sample.
The sequential extraction procedure is aimed at the simulation of conditions whereby trace elements associated with certain components of the solids can be released (Devesa-Rey et al. 2008). Although As associated with Fe (and Mn) oxides presents a low mobility in non-acidic oxidizing environment, there is a risk of releasing upon changes in redox potential or pH. Under reducing conditions, Fe (and Mn) oxides will partly transform into Fe(II) and Mn(II) forms and dissolve, leading to release of arsenates and their reduction to arsenites (As III) (Krysiak and Karczewska 2007). This may happen in sediments rich in organic matter undergoing decomposition after sediments burial. On the other hand, strongly acidic environments leading to the dissolution of Fe oxides and a consequent increase in As solubility are not common in fluvial environments (Bose and Sharma 2002).
Bioavailability of As and heavy metal bioavailability in tailing dam sediment
In order to assess the potential risk to humans, SBET method was used to estimate the bioavailability of arsenic and heavy metals in tailing dam sediment. The simple bioavailability extraction test or SBET is a simplified in vitro form, which has been used to determine the human bioavailability quotients for arsenic and toxic heavy metals from soils. The method involves the leaching of solid samples such as mine waste or contaminated soil in a simulated gastric fluid matrix (Kim et al. 2002).
The results of the SBET for percentage bioavailability of toxic heavy metals in tailing dam sediments are shown in Table 5. The concentration of elements extracted within the simulated human stomach for 1 h from tailing dam sediment were 288.2 mg/kg As, 7.2 mg/kg Cd, 41.1 mg/kg Cu, 13.5 mg/kg Pb, 4.7 mg/kg Ni and 23.5 mg/kg Zn, respectively. In particular, the extracted concentrations of As and Cd exceeded the tolerable levels of 20 mg/kg As and 3 mg/kg Cd. Besides, the As bioavailability of 6.5% is lower compared to that of the other heavy metals followed by Cd 12.3%. This trend was also observed by Kim et al. (2002), where % bioavailability of As was lower than Cd, Cu, Pb and Zn from paddy soils, using SBET method. They explained that stomach pH is less important in controlling As bioavailability than it is for other heavy metals. This result indicates that the mine workers may potentially take up these toxic elements through normal work activities.
Estimates of the bioavailability of toxic elements are relevant to improve risk analysis especially during decommissioning phase of the mining plant. The results of the SBET indicates that there is a potential health threat through long-term exposure if tailing dam is not properly remediated after decommissioning.
When the tailing dam sediment samples were equilibrated with de-ionized water, the solution phase pH ranged from 9.4 to 9.5 (Table 6). The dissolution of soluble salts in tailings directly affects the pH (Ahn et al. 2005). The concentrations of As in the solution was 1.3 mg/l, which is higher than the WHO water guideline (WHO 2006) of 0.01 mg/l. The release of As under alkaline conditions can be explained by its anionic character, and the equilibrium between sorption and desorption process (Bowell 1994; Liu et al. 2001; O’Neil 1995). Cadmium and Pb exhibited similar trend. The results of de-ionized water/sediment equilibration experiments suggested that As and heavy metals in the tailings, sediment may dissolve and become mobile with periodic wetting by rain water to contaminate water bodies.
Conclusions
In order to investigate the environmental impact of gold mining, the evaluation of water quality, speciation, bioavailability and leachability of arsenic and heavy metals in tailing dam sediment were studied using various extraction methods. The concentrations of Al, As, Cu, Mn, Fe, Pb, U and Zn were higher than the concentrations established by Mongolia (MNS 4586: 1998) especially in the monitoring wells and heap leach wells. In general, the control samples from the drinking water well, River Boroo samples and the Borehole (BH) samples all had concentrations of most parameters (except U) within the acceptable levels.
Aqua regia digestion showed that the tailing dam sediment had 4,419 mg/kg of As while the sequential extraction procedure provided evidence that the As was mostly bound with the residual phase (average value of two samples 80%). The soil samples within the vicinity of the mine did not show any elevated concentrations of the heavy metals studied, except for As with the heap leach area having a concentration of 261 mg/kg As. More efficient mitigation process for As should be considered.
The SBET procedure extracted less than 10% of the total As, but As concentration of 288.2 mg/kg exceeded the reference tolerable value of 6 mg/kg recommended by Mongolian soil standards. Although de-ionized water extracted low total arsenic (0.3%), the solution concentration of 1.3 mg/l was higher than the recommended 0.01 mg/l WHO guideline of As in water; therefore, the leaching of arsenic may contaminate ground or surface water in the vicinity of the gold mine.
The information from all the results suggests that the leachability and bioaccessibility of sediment-bound arsenic and heavy metals were governed by the speciation in sediment and changes in speciation may result in simultaneous changes in leachability and bioaccessibility. It is therefore recommended that more treatment to remove As from mine tailing be carried out to safe guard human health, and the continuous monitoring of ground and surface water is also highly recommended.
References
Ahn, J. S., Park, Y. S., Kim, J. Y., & Kim, K. W. (2005). Mineralogical and geochemical characterization of arsenic in an abandoned mine tailings of Korea. Environmental Geochemistry and Health, 27, 147–157.
Borba, R. P., Figueiredo, B. R., & Matschullat, J. (2003). Geochemical distribution of arsenic in waters, sediments and weathered gold mineralized rocks from Iron Quadrangle, Brazil. Environmental Geology, 44, 39–52.
Bose, P., & Sharma, A. (2002). Role of iron in controlling speciation and mobilization of arsenic in subsurface samples. Water Research, 36, 4916–4926.
Bowell, R. J. (1994). Sorption of arsenic by iron oxides and oxyhydroxides in soils. Applied Geochemistry, 9, 279–286.
Bowen, H. J. M. (1979). Environmental chemistry of elements. London: Academic Press.
Bulgan, T. (2008). Chemical analysis of water. Laboratory manual, Central Laboratory of Environment and Meteorology, Ulaanbartar, Mongolia.
Dalai, D., & Dorjbaatar, E. (2008). Environmentally safe tailings storage facility of Boroo gold mine, Mongolia, proceedings of IFOST-2008—3rd international forum on strategic technologies, art. no. 4602892, 623–628.
Devesa-Rey, R., Paradelo, R., Diaz-Fierros, F., & Barral, M. T. (2008). Fractionation and bioavailability of arsenic in the bed sediments of Anllons River (NW Spain). Water Air Soil Pollution, 195, 189–199.
Douay, F., Pruvot, C., Roussel, H., Ciesielski, H., Fourrier, H., Proix, N., et al. (2008). Contamination of urban soils in an area of northern France polluted by dust emissions of two smelters. Water Air Soil Pollution, 188, 247–260.
Espinosa, E., Armienta, M. A., Cruz, O., Aguayo, A., & Ceniceros, N. (2009). Geochemical distribution of arsenic, cadmium, lead and zinc in river sediments affected by tailings in Zimapan, a historical polymetalic mining zone of Mexico. Environmental Geology, 58, 1467–1477.
Fuentes, A., Llorens, M., Saez, J., Soler, A., Aguilar, M. I., Ortuno, J. F., et al. (2004). Simple and sequential extractions of heavy metals from different sewage sludges. Chemosphere, 54, 1039–1047.
Huang, S. S., Liao, Q. L., Hua, M., Wu, X. M., Bi, K. S., Yan, C. Y., et al. (2007). Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere, 67, 2148–2155.
Jarvas, K. E., Gray, A. L., & Hour, R. S. (Eds.). (1992). Sample preparation for ICP-MS. In: Handbook of inductively coupled plasma mass spectrometry (Chap. 7). Glasgow: Blackie.
Johnston, S. E., & Barnard, W. M. (1979). Comparative effectiveness of fourteen solutions for extracting arsenic from four western New York soils. Soil Science Society American Journal, 43, 304.
Kabata-Pendias, A., & Pendias, H. (1984). Trace elements in soils and plants. Boca Raton: CRC Press.
Keon, N. E., Swartz, C. H., Brabander, D. J., Harvey, C., & Hemond, H. F. (2001). Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environmental Science Technology, 35, 2778–2784.
Kim, J. Y., Kim, K. W., Ahn, J. S., & Lee, C. H. (2005). Investigation and risk assessment modeling of As and other heavy metals contamination around five abandoned metal mines in Korea. Environmental Geochemistry and Health, 27, 193–203.
Kim, J. Y., Kim, K. W., Lee, J. U., Lee, J. S., & Cook, J. (2002). Assessment of As and heavy metal contamination in the vicinity of Duckum Au-Ag mine, Korea. Environmental Geochemistry and Health, 24, 215–227.
Kim, K. W., Lee, H. K., & Yoo, B. C. (1998). The environmental impact of gold mines in the Yugu-Kwangcheon Au-Ag metallogenic province, Republic of Korea. Environmental Technology, 19, 291–298.
Krysiak, A., & Karczewska, A. (2007). Arsenic extractability in the areas of former arsenic mining and smelting, SW Poland. Science of the Total Environment, 379, 190–200.
Laxen, D. P. H., & Harrison, R. M. (1981). Cleaning methods for polyethene containers prior to the determination of trace metals in freshwater samples. Analytical Chemistry, 53, 345.
Lee, S. W., Lee, B. T., Kim, J. Y., Kim, K. W., & Lee, J. S. (2006). Human risk assessment for heavy metal and As contamination in the abandoned metal mine areas, Korea. Environmental Monitoring and Assessment, 119, 233–244.
Liu, F., De Cristofaro, A., & Violante, A. (2001). Effect of pH, phosphate and oxalate on the adsorption/desorption of arsenate on/from goethite. Soil Science, 166, 197–208.
MNS 4586:1998 Water quality standard, Mongolia.
MNS 4943:2000 Effluent standard, Mongolia.
MNS 900:2005 Environment. Health protection. Safety. Drinking water. Hygienically requirements, assessment of the quality and safety, Mongolia.
MNS 5850:2008 Soil quality. Maximum allowable concentrations of soil pollutants and elements, Mongolia.
O’Neil, P. (1995). Arsenic. In B. J. Alloway (Ed.), Heavy metals in soils (pp. 83–99). Glasgow: Blackie.
Oliveira, A. S., Bocio, A., Trevilato, B. T. M., Takayanagui, A. M. M., Domingo, J. L., & Segura-Munoz, S. I. (2007). Heavy metals in untreated/treated urban effluent and sludge from a biological wastewater treatment plant. Environmental Science and Pollution Research, 14(7), 483–489.
Ramsey, M. H., Thompson, M., & Banerjee, E. K. (1987). Realistic assessment of analytical data quality from inductively coupled plasma atomic emission spectrometry. Analysis Proceedings, 24, 260–265.
Ruiz-Chancho, M. J., Lopez-Sanchez, J. F., & Rubio, R. (2007). Analytical speciation as a tool to assess arsenic behavior in soils polluted by mining. Analytical and Bioanalytical Chemistry, 387, 627–635.
Thalenhorst, H., & Farquharson, P. (2004). Technical report on the Boroo Gold Mine Mongolia for CENTERRA GOLD INC. centerragold.com/media/…/boroo_techreport_may13_final.pdf.
Ure, A. M. (1995). Methods of analysis for heavy metals in soils. In B. J. Alloway (Ed.), Heavy metals in soils (pp. 58–102). Glasgow: Chapman and Hall.
Wenzel, W. W., Kirchbaumer, N., Prohaska, T., Stingeder, G., Lombi, E., & Adriano, D. C. (2001). Arsenic fractionation in soils using an improved sequential extraction procedure. Analytica Chimica Acta, 436, 309–323.
WHO. (2006). Guidelines for drinking water quality (3rd ed.). World Health Organization.
Yellishetty, M., Ranjith, P. G., & Kumar, D. L. (2009). Metal concentrations and metal mobility in unsaturated mine wastes in mining areas of Goa, India, Resources. Conservation & Recycling, 53, 379–385.
Acknowledgments
This study was supported by United Nations University (UNU) and Gwangju Institute of Science and Technology (GIST) Joint Programme on Science and Technology for Sustainability.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inam, E., Khantotong, S., Kim, KW. et al. Geochemical distribution of trace element concentrations in the vicinity of Boroo gold mine, Selenge Province, Mongolia. Environ Geochem Health 33 (Suppl 1), 57–69 (2011). https://doi.org/10.1007/s10653-010-9347-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-010-9347-1