Abstract
The aim of this study was to evaluate the effect of photobiomodulation therapy (PBMT) on histone 3 acetylation (acH3) and NF-κB expression during oral ulcer healing. A total of 48 male Wistar rats were divided into control group (CG) and PBMT group (n = 24 each). Traumatic ulcers were created in the dorsum of the rats’ tongue with a punch tool. Irradiation with InGaAlP laser, 660 nm, 40 mW, 0.04 cm2 spot size, 4 J/cm2, 4 s, and 0.16 J per spot was performed once a day in close contact for 10 consecutive days. CG received only daily handling. Rats were euthanized on days 3, 5, and 10 (n = 8) and were monitored daily to assess wound status. Immunohistochemical analysis for acH3 and NF-κB detection was performed. One thousand epithelial cells were counted, and mean acH3- and NF-κB-positive cells were calculated and compared between the groups. PBMT accelerated the repair of oral ulcers. On day 3, PBMT showed significantly higher means for acH3- and NF-κB-positive cells than CG. On day 5, no difference was observed between the groups concerning both markers. On day 10, PBMT presented lower acH3 and NF-κB means than the control group. We concluded that PBMT stimulates keratinocyte migration in the early stage of oral wound healing and keratinocyte differentiation at the final stage by modulating histone acetylation and NF-κB expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral ulcers are common in the dental clinic routine. They are characterized by the loss of the epithelial barrier and exposure of the underlying connective tissue resulting in symptoms such as pain or soreness that can impact patients’ quality of life [1]. The healing process of ulcers follows physiological mechanisms to repair the wound area, involving several events that begin with hemostasis followed by three main overlapping phases, inflammation, proliferation, and remodeling [2,3,4]. This multi-step process occurs through the combined action of several cell types and a cascade of biochemical reactions [5, 6]. Moreover, the mechanisms involved in wound healing have, in part, an epigenetic origin, which aims to activate the repair machinery regulated at the transcription and post-transcription levels [7].
Histones are proteins that support the packaging of DNA and are associated with important functions, including gene expression regulation in several types of tissues [8]. The acetylation process occurs through the addition of acetyl groups by the enzymes histone acetyltransferases (HATs) to lysine residues located in the histone tails. During deacetylation, the opposite event of acetylation, acetyl groups are removed by histone deacetylase (HDACs) [9]. The balance between acetylation and deacetylation of histones regulates several biological processes, including wound healing [4], by controlling important events such as transcription, nuclear translocation, and cytoskeletal architecture modifications. Evidence shows that the nuclear transcription factor NF-κB is also regulated by HDAC enzymes [10]. The NF-κB has been considered an important pro-inflammatory signaling pathway acting as a key regulator of inflammatory gene transcription. It plays important roles in adaptive immune defense, secretion of antimicrobial peptides, cytokine and chemokine release, leukocyte recruitment, and cell survival [10,11,12].
Several treatment protocols have been proposed to improve the healing process of oral ulcers and alleviate pain. The most commonly prescribed treatments are analgesics, antibiotics, antiseptics, immunomodulators and anti-inflammatories, herbal medicines, and specific local treatments, such as surgical removal, debridement, chemical cauterization, low-density ultrasound, and photobiomodulation therapy (PBMT) [3, 13,14,15,16,17].
PBMT promotes the increase of cellular metabolism, which can induce various tissue effects, such as analgesic, anti-inflammatory, and reparative [16,17,18,19]. In addition, our group has demonstrated that PBMT is capable of accelerating keratinocyte migration [3, 20]. During the process of wound healing, the formation of a new epithelial lining is extremely important to restore the physical barrier and prevent wound contamination [21]. However, the effects of PBMT on epigenetic mechanisms involved in re-epithelization of oral mucosa wounds are completely unknown. Thus, the aim of this study was to evaluate the effect of PBMT on histone acetylation and NF-κB in keratinocytes during oral wound healing in rats.
Materials and methods
Animal model
All experiments were performed according to the Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the ethics committee of Porto Alegre Clinics Hospital (Brazil) under process number 14-0534. Forty-eight male Wistar rats weighing 250 to 300 g were kept under standard temperature conditions (20 to 24 °C) and 12-h light/dark cycle with food and water ad libitum. The animals were randomly divided into two experimental groups of 24 animals each: control group, which was not treated but the animals were handled daily similar to the treated groups (0 J/cm2), and photobiomodulation group (PBMT), treated with low power laser therapy.
Under aseptic conditions, the animals were anesthetized with intraperitoneal administration of ketamine (0.1 mL/100 g) and xylazine (0.05 mL/100 g). Traumatic ulcers measuring 3 mm in diameter were made on the dorsal surface of the tongue by punch biopsy. The lesions were performed by a single investigator. Randomization of the animals was done after the wounds were performed. Eight rats from each group were euthanized using an isoflurane anesthetic overdose on days 3, 5, and 10, and the tongues were removed for posterior analyses.
Laser irradiation
Photobiomodulation was performed using a continuous wave diode laser (InGaAlP, MM Optics Ltd., São Carlos, SP, Brazil) with a spot size of 0.04 cm2, at a wavelength of 660 nm, and power of 40 mW applied by contact to the spot. The power of the apparatus was confirmed every day prior to application using a power meter. Irradiation was performed perpendicular to the mucosa in two spots 3 mm apart on the opposite margins of the ulcer for 4 s. Thus, the energy density provided was 4 J/cm2 (energy per spot of 0.16 J, total energy of 0.32 J). A single investigator (same that produce the wounds) applied the laser to the wounds once a day, starting immediately after the wound creation and for 10 days. During laser application, the animals were kept under isoflurane inhalation anesthesia. The control group was handled under identical conditions, but with the laser equipment turned off.
Histopathological analysis
The tongues were fixed in 10% buffered formalin solution for 48 h. After washing with water, the specimens were dehydrated and embedded in paraffin. Slices 5-μm thick were obtained and stained with hematoxylin–eosin. The analyses of experimental groups in each evaluation day (3, 5 and 10) were performed, followed by a semi-quantitative analysis. The evaluation was performed by pathologist blinded to clinical procedures. The degree of re-epithelialization was determined by a classification system (0 to 4), as described previously: grade 0—re-epithelialization at the wound margins; grade 1—re-epithelialization in less than half the wound; grade 2—re-epithelialization in more than half of the wound; grade 3—re-epithelialization of the entire wound with irregular thickness; and grade 4—re-epithelialization of the entire wound with normal thickness [3].
Immunohistochemistry analysis
Histological sections of 4 μm were deparaffinized in an oven at 60 °C for 12 h, followed by xylene baths and alcohol hydration. The slides were immersed in 0.3% hydrogen peroxide solution in methanol to block endogenous peroxidase, subjected to antigenic recovery in water bath, and subsequently incubated with the primary antibodies acH3 (lys9) (clone C5B11, 1:500, Cell Signaling, Danvers, MA, USA) and NF-κB p65 (clone L8F6, 1:400, Cell Signaling). Reactions were performed using a chromogen solution containing 0.03% of 3-31-diaminobenzidine (Dako, Santa Clara, CA, USA) and counterstained with Mayer’s hematoxylin solution. All reactions were accompanied by positive controls according to the manufacturer’s instructions.
One previously calibrated observer performed the quantitative immunohistochemical analysis of acH3 (lys9) and NF-κB p65 expression at the epithelial tissue. The calibration process was performed in two stages. Initially, two observers evaluated the slides using a conventional light microscope: the one who performed the quantitative analysis (AFG) and an experienced investigator with previous experience in immunohistochemical quantitative analysis (MDM). In this moment, cell positivity and capture area were defined. The second stage was carried out after the image captures. The observer (AFG) performed two analyses of the same slide at a 1-week interval. This process was carried out in 10 slides aiming to confirm the reproducibility of the observer. Only nuclear brown staining was considered positive. High-power fields (× 400 magnification) of the migratory epithelial tongue or epithelial tissue recovering the wound area were captured using a conventional light microscope (CX41RF model; Olympus Latin America, Inc., Miami, FL, USA) The slides were evaluated and photographed under an Olympus BX 50 microscope in a × 400 magnification. The images were analyzed using the ImageJ program (NIH, Bethesda, MD, USA). One hundred cells were counted, and the percentage of positive cells was calculated.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Differences between the groups in degree of re-epithelialization and percentage of acH3- and NF-κB-positive cells were evaluated by the Student’s t test. The level of significance was 5% (P < 0.05).
Results
PBMT accelerated re-epithelialization in oral lesions
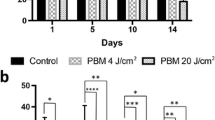
The group submitted to PBMT showed a higher degree of re-epithelialization of the wound when compared to the control group on days 3 and 5 (p = 0.01 and p = 0.002) (Fig. 1). On day 10, no difference was observed since all groups had epithelium covering the entire wound.
PBMT stimulated H3 acetylation during the early stages of wound healing
The epithelium in both PBMT and control groups had a large number of acH3-positive cells. However, on day 3, acH3 was significantly higher in the PBMT group than in the control group (p = 0.04) (Fig. 2); on day 5, no significant difference was observed between the groups; and on day 10, acH3-positive cells were significantly lower in the PBMT group (p = 0.05) (Fig. 2).
PBMT stimulated NF-κB in the initial phases of re-epithelialization
The effect of NF-κB in the re-epithelialization process was evaluated by p65 nuclear labeling, since the nucleus location indicates whether the protein is active. On days 3 and 5, the PBMT group showed an increase in this pathway compared to the control group (p = 0.02; p = 0.03) (Fig. 3), showing that PBMT caused NF-κB activation in epithelial cells at the initial stages of tissue repair. On day 10, a decrease in NF-κB in the PBMT group (p = 0.001) was observed in relation to the control group (Fig. 3).
Figure 4 summarizes the main findings of the present study showing the epigenetic modulation of epithelial tissue during oral mucosal repair (initial and final periods) and the association with NF-κB activation.
Summary of the study main findings. The control group in the initial repair stage (day 3) had a larger wound area accompanied by lower expression of acH3 (more condensed chromatin) and lower NF-kB activity. In the final repair stage (day 10), the control group had more relaxed chromatin and higher NF-kB activity. Wounds treated with PBMT in the initial repair phase presented a higher degree of re-epithelialization accompanied by more relaxed chromatin by the action of acH3 and higher NF-kB activity. This configuration of chromatin allows for greater gene transcription that together with the activation of NF-κB stimulated the migration of oral keratinocytes. In the final phase of the repair, the PTBM group had thicker and more organized epithelium. At this time, PTBM induced a decrease in acH3 expression leading to chromatin compaction and lower NF-kB activity in the epithelial cell nucleus. These events may be associated with terminal differentiation of keratinocytes
Discussion
PBMT has been widely used in the dental clinical practice to treat oral ulcers because it accelerates healing and alleviates pain. The mechanisms involved in this process include modulation of chemical mediators, stimulation of cell proliferation and migration, regulation of angiogenesis, and collagen remodeling [19, 20, 22]. However, other cellular and molecular processes involved in wound healing should be studied to better understand the action of PBMT. In the present study, we evaluated the impact of PBMT on oral ulcers repair and its action on acH3 and NF-κB in keratinocytes. Our results showed that PBMT accelerates the repair of ulcers by activating epigenetic mechanisms such as histone 3 lysine 9 acetylation and the NFkB pathway in early healing stages and decreasing their expression at advanced stages. Histopathological analysis of the wounds showed that PBMT accelerated the repair of oral ulcers in rat tongue. Most lesions submitted to PBMT demonstrated total epithelial covering of the wound at day 3. These results corroborate the positive effect of PBMT previously described in oral wound healing in vitro [17, 20, 23], animal models [3, 19, 24] and clinical studies [25, 26]. Sperandio et al. (2015) [24] evaluated in vitro growth and differentiation of skin keratinocytes and in vivo wound healing response when treated with PBMT. These authors observed improvement of epithelial healing (more epithelial proliferation and maturation) using different energy densities protocol (3, 6 and 12 J/cm2), 660 nm, 100 mW). In addition, other studies demonstrated that PBMT activated TGF-β1 [23, 27] and mTOR signaling [20] promoting epithelial cell migration and wound closure. In animal models, Wagner et al. (2013) [3] using 4 J/cm2 observed a reduction of clinical and histopathological aspects in oral tongue wounds on the initial days of repair. Also, the clinical improvement of oral ulcers with PBMT appears to be well established to treat different oral lesions such as traumatic ulcer, aphthous lesion, oral mucositis, periimplantitis etc. [16, 17, 23, 25, 26]. Antunes et al. (2018) [25] using similar PBMT parameters that we used in the present study (660 nm, 100 mW, 4 J/cm2) observed an increased expression of seven SPRR gene family members in samples of oral mucosa of oncological patients submitted to PBMT compared to placebo group. These genes are known to be induced during differentiation of human epidermal keratinocytes which explain the epidermal regeneration mediated by PBMT. Although the literature has demonstrated the effect of PBMT on some cellular and molecular pathways to date, no study has evaluated the possible involvement of epigenetic mechanisms with the laser-stimulated re-epithelialization.
Epigenetics plays a key role in tissue healing, controlling cellular activity and regulating gene repair. Epigenetic regulatory mechanisms are important in the maintenance of cellular phenotypes in organs and tissues, and act in pathological conditions. Wound healing occurs through a dynamic and complex process of cell proliferation, migration and differentiation, which are dependent on epigenetic signaling. The acetylation and deacetylation of histones, regulated by HAT and HDACs enzymes, are part of the epigenetic mechanism. Acetylation is associated with gene activation, as the open chromatin of the DNA (acetylated) gives access to transcription factors. Deacetylation is associated with gene suppression, as the closed chromatin (deacetylated) does not allow access to these factors. Histones 3 and 4 are the main targets of acetylation and methylation, which have roles in the regulation of gene expression by recruiting other proteins [28, 29, 30, 31, 32]. Our study is the first to evaluate epigenetic mechanisms in oral tissue repair submitted to PBMT, demonstrating its capacity to increase the acetylation of histone 3 in the initial stages of the repair process (D3) of oral wounds. Therefore, we suggest that PBMT promotes the increase of chromatin relaxation via histone 3 acetylation, activating the transcription of genes involved in tissue repair. In another tissue repair model, an increase in total protein acetylation was observed in the wound area through α-tubulin and histone 3 Lysine 9, improving the clinical conditions of the lesion [7]. Our results showed that the PBMT group presented lower histone acetylation compared to the control group on the last day of analysis (D10). There is evidence that the activation of HDACs is essential for the final differentiation of the epidermis [33, 34]. Thus, we consider that the lower expression of acetyl histone 3 at the final stages of repair indicates an imbalance in favor of deacetylation (HDACs), which is associated with a greater keratinocyte differentiation in the laser-treated group. The epithelium of the laser-treated animals had thickness and cellular characteristics more similar to the normal mucosa compared to the control group at the final stage.
The NF-κB transcription factor is a key part of the inflammatory response, and plays an important role in immune defense [35, 36]. In its inactive form, this factor is located in the cytoplasm of cells coupled to its inhibitor IkB. Different stimuli may lead to the activation of NF-kB through ubiquitination followed by degradation of IkB, causing its release and translocation to the nucleus of the cell, stimulating gene transcription. In our study, PBMT increased the percentage of cells expressing nuclear NF-κB at the initial stages of repair (D3). NFκB is generally recognized for its proinflammatory role through the activation of proinflammatory cytokines [37, 38]; however, recent studies have demonstrated the importance of NFκB activation during the re-epithelialization process. Na et al. (2016) demonstrated in vitro that the migration of skin keratinocytes is dependent on the activation of NF-kB [39]. This group also demonstrated the importance of NF-kB activation for wound closure in an animal model. This transcription factor acts through the activation of NOTCH1 that stimulates the migration of keratinocytes [40], which may explain the early reepithelialization observed in the group treated with PBMT. In addition, our group has demonstrated in an animal model of chemo-induced mucositis that PBMT leads to an early wound closure through the activation of the NF-kB signaling pathway. The increased production of reactive oxygen species by PBMT seems to activate directly or indirectly NF-kB [41]. Similarly to acH3, at D10, a reduction of NF-kB in the PBMT group was observed. Adams et al. (2006) demonstrated that the reduction of NF-kB activation in keratinocytes is accompanied by increased expression of genes associated with the final differentiation of these cells, such as cytokeratin 10 [42].
Conclusion
Our study was the first to show the ability of PBMT to modulate epigenetic events during the repair of oral ulcers by affecting histone 3 lysine 9 acetylation and NF-κB expression. In early stages of repair, PBMT activates epithelial migration and, in late stages, it stimulates final differentiation of oral epithelial cells. Our study contributes with the elucidation of the yet unexplored domain of epigenetics related to PBMT. Nevertheless, other studies analyzing with greater detail these mechanisms (e.g., through mechanistic assays in cell culture) should be performed to better understand how the laser interacts in this important area of regulation of gene expression.
References
Scully C, Felix DH (2005) Oral medicine-update for the dental practitioner. Aphthous and other common ulcers. Br Dent J 199(5):259–264
de Mendonça RJ, Coutinho-Netto J (2009) Cellular aspects of wound healing. An Bras Dermatol 84(3):257–262
Wagner VP, Meuer L, Martins MAT, Danilevicz CK, Magnusson AS, Marques MM, Filho MS, Squarize CH, Martins MD (2013) Influence of different energy densities of laser phototherapy on oral wound healing. J Biomed Opt 18(2):128002
Dongong TI, Meirong LU, Xiaobing FU, Weindon H (2014) Causes and consequences of epigenetic regulation in wound healing. Wound Rep Reg 22(3):305–312
Gonzalez AC, Costa TF, Andrade ZA, Medrado AR (2016) Wound healing - a literature review. An Bras Dermatol 91(5):614–620
Maver T, Maver U, Stana Kleinschek K, Smrke DM, Kreft S (2015) A review of herbal medicines in wound healing. Int J Dermatol 54(7):740–751
Spallota F, Cencioni C, Straino S, Sbardella G, Castellano S, Capogrossi MC, Martelli F, Gaetano C (2013) Enhancement of lysine acetylation accelerates wound repair. Communicative & Integrative Biology 6(5):e25466
Martins MD, Castilhos R (2013) Histones: Controlling Tumor Signaling Circuitry. J Carcinog Mutagen 1(5):1–12
Wade PA, Kikyo N (2002) Chromatin remodeling in nuclear cloning. Eur J Biochem 269(9):2284–2287
Zhou R, Gong A-Y, Chen D, Myler RE, Eischeid AN, Chen XM (2013) Histone deacetylases and NF-kB signaling coordinate expression of CX3CL1 in epithelial cells in response to microbial challenge by suppressing miR-424 and miR-503. Plos One 8(5):e65153
Lawrence T (2009) The nuclear factor NFk-B pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):1–10
Aupperle KR, Bennett BL, Boyle DL, Tak PP, Manning AM, Firestein GS (1999) NF-kappa B regulation by I kappa B kinase in primary fibroblast-like synoviocytes. J Immunol 163(1):427–433
Scully C, Shotts R (2000) Mouth ulcers and other causes of orofacial soreness and pain. BMJ 321(7254):162–165
Field EA, Allan RB (2003) Review article: oral ulceration – etiopathogenesis, clinical diagnosis and management in the gastrointestinal clinic. Aliment Pharmacol Ther 18(10):949–962
Martins MD, Fernandes KPS, Pavesi VC, França MC, Mesquita-Ferrari RA, Bussadori SK (2011) Healing properties of papain-based gel on oral ulcers. Braz J Oral Sci 10(1):120–123
Bourguignon-filho AM Feitosa ACR, Beltrão GC, Pagnoncelli RM (2005) Utilização do laser de baixa intensidade no processo de cicatrização tecidual. Revisão de literatura. Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial 46(1): 37-43
Eduardo FP, Mehnert TU, Monezi TA, Zezzel DM (2007) Cultured epithelial cells response to phototherapy. Lasers Surg Med 39(4):365–272
Baptista J, Martins MD, Pavesi VCS, Bussadori SK, Fernandes KPS, Pinto Júnior DS, Ferrari RAM (2011) Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomedicine and Laser Surgery 29(1):11–17
Wagner VP, Curra M, Webber LP, Nor C, Matte U, Meuer L, Martins MD (2016) Photobiomodulation regulates cytokine release and new blood vessel formation during oral wound healing in rats. Lasers Med Sci 31(4):665–671
Pellicioli AC, Martins MD, Dillenburg CS, Marques MM, Squarize CH, Castilho RM (2014) Laser phototherapy accelerates oral keratinocyte migration through the modulation of the mammalian target of rapamycin signaling pathway. J Biomed Opt 19(2):028002
Castilho RM, Squarize CH, Gutkind JS (2013) Exploiting Pl3K/Mtor signaling to accelerate epithelial wound healing. Oral Dis 19(6):551–558
Corazza AV, Jorge J, Kurachi C, Bagnato VS (2007) Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg 25(2):102–106
Tang E, Khan I, Andreana S, Arany PR (2017) Laser-activated transforming growth factor-β1 induces human β-defensin 2: implications for laser therapies for periodontitis and peri-implantitis. J Periodontal Res 52(3):360–367
Sperandio FF, Simões A, Corrêa L, Aranha AC, Giudice FS, Hamblin MR, Sousa SC (2015) Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J Biophotonics 8(10):795–803
Antunes HS, Wajnberg G, Pinho MB, Jorge NAN, de Moraes JLM, Stefanoff CG, Herchenhorn D, Araújo CMM, Viégas CMP, Rampini MP, Dias FL, de Araujo-Souza PS, Passetti F, Ferreira CG (2018) cDNA microarray analysis of human keratinocytes cells of patients submitted to chemoradiotherapy and oral photobiomodulation therapy: pilot study. Lasers Med Sci 33(1):11–18
Marín-Conde F, Castellanos-Cosano L, Pachón-Ibañez J, Serrera-Figallo MA, Gutiérrez-Pérez JL, Torres-Lagares D (2018) Photobiomodulation with low-level laser therapy reduces oral mucositis caused by head and neck radio-chemotherapy: prospective randomized controlled trial. Int J Oral Maxillofac Surg S0901-5027(18):30475–30472
Engel KW, Khan I, Arany PR (2016) Cell lineage responses to photobiomodulation therapy. J Biophotonics 9(11-12):1148–1156 z
Huynha NC-N, Evertsb V, Ampornaramveth RS (2017) Histone deacetylases and their roles in mineralized tissue regeneration. Bone Rep 16(7):33–40
Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, Oakley F, Burt AD, Wilson CL, Anstee QM, Barter MJ, Masson S, Elsharkawy AM, Mann DA, Mann J (2012) Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med 18(9):1369–1377
Mann J, Mann DA (2013) Epigenetic regulation of wound healing and fibrosis. Curr Opin Rheumatol 25(1):101–107
Lewis CJ, Mardaryev AN, Sharov AA, Fessing MY, Botchkarev VA (2014) The epigenetic regulation of wound healing. Adv Wound Care 3(7):468–474
Feinberg AP (2010) Genome-scale approaches to the epigenetics of common human disease. Virchows Arch 456(1):13–21
Robertson ED, Weir L, Romanowska M, Leigh IM, Panteleyev AA (2012) ARNT controls the expression of epidermal differentiation genes through HDAC- and EGFR-dependent pathways. J Cell Sci 125(Pt 14):3320–3332
LeBoeuf M, Terrell A, Trivedi S, Sinha S, Epstein JA, Olson EN, Morrisey EE, Millar SE (2010) Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev Cell 19(6):807–818
Chen AC-H, Arany PR, Huang Y-Y, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR (2011) Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 6(7):e22453
Egan LJ, de LA, Lehrman ED, Myhre GM, Eckmann L, Kagnoff MF (2003) Nuclear fator NF-kB activation promotes restitution of wounded intestinal epithelial monolayers. Am J Physiol Cell Physiol 285(5):C1028–C1035
Badr CE, Niers JM, Tjon-Kon-Fat LA, Noske DP, Wurdinger T, Tannous BA (2009) Real-time monitoring of nuclear factor kappaB activity in cultured cells and in animal models. Mol. Imaging 8(5):278–290
Rahmana I, Marwickb J, Kirkhamc P (2004) Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kB and pro-inflammatory gene expression. Biochem Pharmacol 68(6):1255–1267
Na J, Lee K, Na W, Shin J-Y, Lee M-J, Yune TY, Lee HK, Jung H-S, Kim WS, Ju B-G (2016) Histone H3K27 Demethylase JMJD3 in cooperation with NF-kB regulates keratinocyte wound healing. J Investig Dermatol 136(4):847–858
Na J, Shin JY, Jeong H, Lee JY, Kim BJ, Kim WS, Yune TY, Ju B-G (2017) JMJD3 and NF-κB-dependent activation of Notch1 gene is required for keratinocyte migration during skin wound healing. Sci Rep N7(1):6494
Curra M, Pellicioli ACA, Kretzmann Filho NA, Ochs G, Matte U, Sant’Ana Filho M, Martins MAT, Martins MD (2015) Photobiomodulation reduces oral mucositis by modulating NF-kB. J Biomed Opt 20(12):125008
Adams S, Pankow S, Werner S, Munz B (2007) Regulation of NF-kB activity and keratinocyte differentiation by the RIP4 protein: Implications for cutaneous wound repair. J Investig Dermatol 127(3):538–544
Acknowledgments
The authors are grateful to Marta Justina Giotti Cioato and Flavia Rejane Giusti for technical support.
Funding
This study was funded by the Postgraduate Research Group of Porto Alegre Clinics Hospital (GPPG/FIPE: 2014-0534), Brazilian National Council for Scientific and Technological Development (CNPq student scholarship), Children’s Cancer Institute (student scholarship), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil(CAPES)-finance code 001. Manoela Domingues Martins is a research fellow funded by the Brazilian National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of Porto Alegre Clinics Hospital (Brazil) under process number 14-0534. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed involving animals were in accordance with the ethical standards of the institution, which the study was conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Farias Gabriel, A., Wagner, V.P., Correa, C. et al. Photobiomodulation therapy modulates epigenetic events and NF-κB expression in oral epithelial wound healing. Lasers Med Sci 34, 1465–1472 (2019). https://doi.org/10.1007/s10103-019-02745-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02745-0