Abstract
To analyze the effect of photobiomodulation and dexamethasone on nerve regeneration after a sciatic nerve crushing model. Twenty-six Swiss mice were divided into the following groups: naive; sham; injured, low-level laser therapy (LLLT) (660 nm, 10 J/cm2, 0.6 J, 16.8 J total energy emitted during the 28 days of radiation, 20 s, for 28 days); dexamethasone (Dex) (local injection of 2 mg/kg for 10 consecutive days); and LLLT group associated with Dex (LLLT/Dex), with the same parameters of the other groups. For nerve injury, a portable adjustable pinch was used. The animals were evaluated using the Sciatic Functional Index (SFI) and Sciatic Static Index (SSI). The results obtained were evaluated with Image J™ and Kinovea™. Data and images were obtained at baseline and after 7, 14, 21, and 28 days after surgery. The evaluation of hyperalgesia, using Hargreaves, and behavior through the open field was also performed. In functional and static analysis, all groups presented significant differences when compared to the injured group. In the analysis of the SSI results, the group treated with both LLLT and dexamethasone was more effective in improving the values of this parameter, and in the SFI, the laser-treated group obtained better results. In the evaluation through the open field and the Hargreaves, there was no difference. The application of LLLT and dexamethasone was effective in nerve regeneration according to the results and was more effective when LLLT was associated with dexamethasone than in LLLT alone for the SSI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral nerve injuries result in motor and sensory deficits. It is estimated that the annual incidence of traumatic injuries affecting the peripheral nerves is 3%, affecting 45 cases out of every 100,000, of which 5% are due to automobile accidents [1, 2]. Peripheral nerve injury is one of the major causes of motor disability, leading to reduced functional capacity and resulting in impaired limb function [3,4,5].
Peripheral nerve injuries can be divided into traumatic and non-traumatic. Traumatic injuries include crushing, stretching, avulsion, compression, and partial or total section, and they can lead to functional impairment, resulting in the deficit of nerve impulse transmission in the involved body segment [6]. These lesions damage the integrity of the tissue and lead to significant motor and sensory changes, which, in turn, are classified as neuropraxia, defined by the blockage of nerve conduction; axonotmesis, in which axons and the covering myelin sheaths are damaged but the connective tissue is preserved; or more severely, neurotmesis, which involves disruption of the entire nerve fiber [7, 8].
For the translation between clinical and experimental research, the sciatic nerve of rodents has been the preferred model, due to the ease of handling, the surgical access, and the natural regenerative capacity of these animals [7,8,9,10,11]. Many studies have evaluated the effects of therapeutic modalities on early nerve regeneration, seeking functional improvement. Among them, the most used in clinical and experimental research is photobiomodulation, which modulates the biological responses by stimulating or inhibiting them. Studies have shown positive effects of photobiomodulation in early regeneration, healing of several tissues, functional rehabilitation, and peripheral nerve regeneration [5, 7, 11,12,13,14]. Low-level laser therapy (LLLT) has been used as a phototherapeutic resource in peripheral nerve injury, with positive results [7, 11,12,13,14].
LLLT photobiomodulation stimulates microcirculation through the pre-capillary sphincter paralysis, causing arteriolar, capillary, vasodilatation, and vascular neoformation. Its photons are absorbed by cytochrome C oxidase in the mitochondrial respiratory chain, which increases its activity by increasing the production of adenosine triphosphate (ATP), acting at the cellular level by inducing trophic conditions and inhibiting inflammatory processes, which are necessary for nerve regeneration [7, 11, 12, 15,16,17,18,19]. The physiological effects derived from irradiation contribute to the acceleration of the regenerative process of the peripheral nerve [5, 7, 11, 12, 15, 18]. In addition, the analgesic effects induced by photobiomodulation occur due to the modulation of inflammatory chemical mediators and the synthesis of β-endorphin, which tends to limit the excitability of nociceptive receptors and eliminate allogeneic substances [20, 21].
In addition to LLLT, previous experimental studies on sciatic nerve injury demonstrate positive effects of the administration of dexamethasone, an anti-inflammatory glucocorticoid, on the enhancement of motor function in peripheral nervous system lesions. The immunosuppressive effects and the neurotrophic potential of dexamethasone can reduce the infiltration of inflammatory cells and act in the production of inflammatory mediators [22]. Dexamethasone also promotes the regeneration of peripheral nerves by inhibiting the infiltration of CD3-positive cells and regulating GAP-43 expression [23].
The aims of the present study were to analyze the comparative effect of LLLT and dexamethasone on the sciatic nerve regeneration process after crush injury and to test the hypothesis that the associations between LLLT and dexamethasone can improve nerve regeneration when compared to their individual application.
Materials and methods
Twenty-six male adult Swiss mice weighing 30–40 g were obtained from the Central Bioterium of the Federal University of Santa Catarina, Brazil. The experiments were performed in the Laboratory of Autoimmunity and Immunopharmacology, with the animals maintained in collective cages and fed on commercial rations and water ad libitum. All procedures were approved by the Animal Research Ethics Committee (protocol no. PP00956).
The animals were divided into six experimental groups according to the procedure to be performed. The animals were weighed and assigned randomly to six groups as follows:
-
1.
Naive group (n = 3)—not submitted to the surgical procedure.
-
2.
Sham group (n = 3)—submitted to sciatic nerve exposure without crushing and simulation of LLLT irradiation.
-
3.
Injured group (n = 5)—sciatic nerve injury.
-
4.
LLLT group (n = 5)—sciatic nerve injury and LLLT irradiation with 10 J/cm2 fluency, emitted energy (E) = 0.6 J, and exposure time of 20 s.
-
5.
Dex group (n = 5)—local injection of dexamethasone (2 mg/kg).
-
6.
LLLT/Dex group (n = 5)—laser irradiation with 10 J/cm2 fluency, energy emitted (E) = 0.6 J, and exposure time of 20 s associated with local injection of dexamethasone (2 mg/kg).
Procedures
Crushing device
Each animal’s sciatic nerve was smashed by an adjustable pinch of 5000 g and a 0.5-cm squat area that was previously calibrated, which is known for making the crushing process easier and more reliable [10, 11]. After the incision, the sciatic nerve was smashed at a point 5 mm above the three main branches (fibular, tibial, and sural). This device was manufactured at the precision workshop of the University of Sao Paulo, Ribeirao Preto [5].
Surgical procedure
The animals were weighed for calculation of the anesthetic dose, and then they were anesthetized with a mixture of 10% ketamine (Syntec do Brazil Ltda, Rhobifarma™, Hortolândia, São Paulo, Brazil—0.1 ml/100 g body weight) and 2% xylazine (Syntec do Brazil Ltda, Rhobifarma™, Hortolândia, São Paulo, Brazil—0.07 ml/100 g body weight) administered intra-peritoneally. Next, the thigh of the right hind leg of each animal was shaved (disposable razon, Kolplast ci Ltda™, Itupeva, São Paulo, Brazil) and an incision (scalpel number 15, Embramed Indústria e Comércio de Produtos Hospitalares™, São Paulo—SP) was made in the lateral face of this limb. After the incision, the sciatic nerve was smashed at a point 5 mm above the three main branches (fibular, tibial, and sural). A weight of 5000 g was used for 10 min during each smashing procedure. Next, the nerve was relocated and the muscles and skin were sutured (Tecnew™, Quintino, Rio de Janeiro, Brazil).
Photobiomodulation
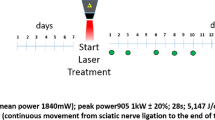
Photobiomodulation was performed through the LLLT applied at a predetermined point in the surgery. The animals were physically contained and LLLT was positioned at a 90° angle to the cutaneous tissue using the point technique with contact [6, 10] immediately after the operation and on 27 consecutive days in the LLLT and LLLT/Dex groups.
A diode laser of aluminized gallium arsenide (AsGaAl)—Ibramed Equipamentos Médicos™, Laserpulse, Amparo, São Paulo, Brazil—with a wavelength of 660 nm, 10 J/cm2, power of 30 mW, beam area of 0.06 cm2, continuous pulse/continuous wave, energy of 0.6 J per day, and total energy emitted of 16.8 J [7, 11] was used for 28 days. The equipment was calibrated for this research.
Dexamethasone
Dexamethasone (Hypofarma, Ribeirão das Neves, Minas Gerais, Brazil) injection was performed at the lesion site once daily at 2 mg/kg for 10 consecutive days for the Dex and LLLT/Dex groups [23].
Assessment
Sciatic functional index and sciatic static index
Functional gait assessment provides an opportunity to evaluate the specific aspects of nerve regeneration of the sciatic nerve in a non-invasive way [27,28,29,30,31,32].
For the functional evaluation, the Sciatic Functional Index (SFI) proposed by Medinaceli, Freed, and Wyatt in 1982 [30] was used, with a functional gait analysis method that verifies the degree of injury and functional recovery of the sciatic nerve. The SFI consists of obtaining images of the footprint of the animal through the use of a catwalk, where the footprints are transferred to a piece of paper or captured by camera and then analyzed using as parameters the length of the footprint, the spread of the fingers, and the spread intermediate to the fingers [27,28,29] (Fig. 1). The results range from 0 to − 100, which is an indicator of nerve function, where 0 is the absence of dysfunction and − 100 means complete dysfunction, and the results are reliable when compared to those of nerve histological analyses.
For static evaluation, the Sciatic Static Index (SSI) was used, as studies have demonstrated its efficacy in gait evaluation in rats. Takhtfooladi et al. [29] used the SSI to evaluate the animal’s footprints when it is in a static position, verifying that it minimizes the biases due to the speed of the animal at the time of the evaluation. The SSI is also suggested to be more accurate than the SFI through the analysis of the results obtained in the study of Smit et al. [31].
The footprints were recorded by an 8-megapixel camera (Sony™, Minato, Tokyo, Japan), fixed under a transparent acrylic walkway 43 cm long, 5.5 cm high, and 8.7 cm wide, with a wooden casing at the end. The footprint videos were scanned by the Kinovea™ Footprint Imaging (SFI) program. The images were analyzed using the Image J™ program to transform the pixels into millimeters and calculate the predetermined parameters in the assessment of the SFI and SSI. The footprints were obtained preoperatively and after 7, 14, 21, and 28 days from the initial lesion. The images were evaluated by the SFI and SSI formulas [32].
Locomotor activity
The locomotor activity was evaluated in an open field consisting of a wooden box measuring 90 × 90 × 45 cm [24], and the bottom of this arena was divided into 12 identical squares. Mice were treated with dexamethasone or LLLT 30 min before being individually placed in the arena and observed for a period of 5 min. The number of squares crossed with all paws was counted, and the evaluations were performed at 0, 7, 14, 21, and 28 days post-surgery.
Thermal hyperalgesia
The evaluation of thermal hyperalgesia to heat was performed through Hargreaves, as previously described [25, 26], which has an infrared light source (UgoBasile, Comerio, Italy) irradiated directly in the animal’s paw. Initially, the animals were housed in the experiment room for 1 h prior to testing. The right hind paw was allocated just below the ipsilateral paw (which received the algogenic stimulus) and subsequently attached by an operator.
The paw withdrawal latency after the application of the thermal stimulus was automatically measured through a sensor. The time of 20 s was determined as the cut-off in order to avoid possible tissue damage in the paw of the animals. Three response time measurements were performed, which were recorded at 20-min intervals. In order to determine the baseline threshold, all groups were assessed prior to the surgical procedure. The evaluation of thermal hyperalgesia was evaluated on days 0, 14, 21, and 28 post-surgery. We did not perform the evaluation on day 7 because the surgical wound was in the healing phase and previous studies of our research group report the opening of the wound in this period due to the positioning of the animal.
Statistical analysis
The sample size was defined by the formula: n = {[(zalfa + zbeta) × s]/sigma}/2, were the alpha value was set at 0.05. Thus, the zalpha value based on the z value table for bi-flow distribution is 1.96; the beta value was set at 0.10. Thus, the zeta value based on the single-valued z value table is 1.28; the value of the difference between the averages is 45% (based on experimental data from our research group). The standard deviation value as being on average is 35% (based on experimental data from our research group).
The results were expressed as mean ± standard error of the mean. The normality was assessed through Shapiro–Wilk test and was analyzed by ANOVA followed by the Tukey post hoc test. The values of p ˂ 0.05 were considered to show significant differences between the means. GraphPad Prism® 6.0 software (San Diego, CA, USA) was used.
Results
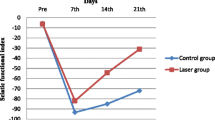
The animals with nerve crush injury (injured group, LLLT group, Dex group, LLLT/Dex group) did not present postoperative complications such as autotomy and dehiscence. The results presented in Fig. 2 show that in the intragroup assessment for the SFI and SSI, all groups presented significant differences in the day 7 to 28 comparison. In all analyses, there was a difference between baseline and day 7, characterizing the effectiveness of nerve crushing. In the analysis of the results obtained in the SSI, we observed that the group treated with LLLT/Dex showed significant improvements in nerve regeneration from the 14th day of treatment. In the analysis of the data obtained in the SFI, we observed that the group treated with only LLLT obtained significant results from the 14th day.
In Fig. 3, it is possible to verify that the LLLT/Dex group presented values closer to zero on the 14th day when compared to the other groups, indicative of early nerve regeneration through the SSI. The LLLT group and the Dex group had positive effects on regeneration. In the LLLT group, these effects appeared from the 14th day, with a regeneration peak on the 21st day, and the Dex group showed regenerative potential from the 21st day, but with unsatisfactory results when compared to the other groups. However, the group that presented the most evident early regeneration was LLLT/Dex.
In Fig. 4, the LLLT group presented values close to zero from the 14th day, approaching the basal values and presenting better results functionally when compared to the other groups (p < 0.05). The Dex and LLLT/Dex groups had positive effects on sciatic nerve regeneration from the 14th day, but the values obtained by the functional analysis when compared to the LLLT group did not show effective results.
In the evaluation through the open field and in the thermal hyperalgesia to heat (Fig. 5), the data did not present significant differences in any group.
Discussion
Peripheral nerve injury due to sciatic nerve crushing in rodents has been widely used in experimental research because it causes rupture of nerve fibers without rupture of most of the structures of nerve support, causing an axonotmesis, and thus facilitating post-injury nervous regeneration [33,34,35]. The widespread use of an experimental lesion by crushing the sciatic nerve has led to the development of several experimental methods capable of generating such an injury, such as the use of calibrated pinch, dead weight, tourniquet, and even sutures with surgical thread, where the lesion is directly proportional to the load applied [36].
The crush injury using portable pinch was selected in this study because it allows standardization of the lesion intensity, besides being a simple technique that preserves the nerve support structure and does not require surgical repair. The equipment provides results similar to dead weight equipment, widely used in clinical research and with high applicability; however, the adjustable gripper has simpler application and handling compared to equipment and dead weight. As a result, the crushing clamp was designed for use in loco, without the need of transportation and adaptation of the animal to the equipment [33]. In the present study, the experience of the group in the management beyond the technique used demonstrated good results of the procedure related to nerve damage.
Studies show that the SFI has a clear correlation with the morphologic and morphometric evaluation of the nerve and is a quantitative, reliable, and reproducible method to evaluate the process of peripheral nerve regeneration, providing a numerical value to the function and allowing statistical analysis of the results [27,28,29,30,31,32].
It was observed during the first seven postoperative days that the animals showed difficulties in walking, presenting a flexor pattern of the paw, adduction of the metatarsals, support of the tread in the heel region, and inability to perform push-off in the weight discharge of one paw for the other, which entailed a more elongated appearance, compatible with severe dysfunction of the sciatic nerve. In the present study, the success of the surgery model was evident due to the evolution of the groups operated on the 7th day and with difference when compared to the baseline in the intragroup evaluation and in the same period in the intergroup evaluation for both the SSI and the SFI.
The sham was used to ensure that the manipulation and exposure in the nerve does not result in considerable losses/alterations of the analyzed parameters; this is clear at the results in which the naive (not submitted to the surgical procedure) showed similar results to the sham (sciatic nerve exposure without smashing) and the injured (sciatic nerve injury, only) showed the worst results.
In the evaluation through the open field and the thermal hyperalgesia to heat, the results were not satisfactory, indicating that the methods do not configure outcome variables to evaluate the regeneration of the sciatic nerve. In the analysis of the thermal hyperalgesia to heat with the Hargreaves device, the analysis on the 7th day was not performed, because a pilot study showed high potential of dehiscence of the surgical wound due to the handling of the animal. Therefore, measurements were performed only at 0, 14, 21, and 28 days post-surgery.
LLLT has biological effects on the acceleration of the bone repair process, collagen synthesis, fibroblast proliferation, and wound healing [15, 16, 34, 35]. In addition, the photobiomodulation regenerative events in the peripheral nerve can acting as modulator of neurotrophic factors and revealing its anti-inflammatory effects through the reduction of inflammatory cells and expression of cytokines [15, 16, 34]. In the present study, the laser was applied to the skin and carried on the muscular tissue, where there is a possibility of increases in levels of nerve growth factor. The authors then evaluated the SFI and SSI [7, 10,11,12]. In this study, the groups that underwent intervention showed an improvement in nerve regeneration, especially with the use of laser alone or associated with dexamethasone.
Different parameters such as wavelength, energy density, pulse mode, and power are used to stimulate and accelerate the functional recovery of peripheral nerves, so different authors have investigated which parameters are most effective in nerve regeneration. Barbosa et al. [7] evaluated the degree of functionality inferred by the SFI and the effect of LLLT (660 nm) in rats submitted to sciatic nerve crush injury. The animals were irradiated with a yield of 10 J/cm2, emitted energy of 0.6 J, power of 30 mW, beam area of 0.06 cm2, and continuous mode and exposure time of 20 s. Barbosa et al. [11] verified the effect of two LLLT wavelengths (660 and 830 nm) on sciatic nerve regeneration in rats and found that LLLT of 660 nm with the same parameters provided better functional recovery compared to that of the other groups. These data from previous studies of our group demonstrate that these parameters are effective in nerve regeneration in the experimental model. In the present study, we observed the effect of LLLT alone and when associated with dexamethasone.
Marcolino et al. [10] investigated the effects of LLLT (830 nm, power of 30 mW, beam area of 0.116 cm2) on the functional recovery of the gait of rats after sciatic nerve crushing. The animals were irradiated with a laser diode of 830 nm, an energy density of 40 J/cm2, and energy emitted of 4.64 J in a single point during 21 consecutive days. After analyzing the results, there were significant improvements in the SFI on the 7th and 14th postoperative days, but there were no significant differences between the groups after the 21st postoperative day, which is compatible with the results obtained in the present study. However, our results showed significant differences after the 21st day. The fact that the lesion model was performed in mice may interfere with these findings.
In addition to the SFI variable, Bervar [32] used static posture through the SSI to evaluate the functional loss and recovery of rats submitted to sciatic nerve injury, demonstrating reliability of the use of this variable when compared to the SFI. The author verified that static analysis is technically easier to perform and is a viable method to quantify functional loss and recovery after sciatic nerve injury. These data corroborate our findings, since the SSI, in addition to quantifying the nerve damage, is a simple and efficient tool to capture the image and calculate the score when compared to the SFI.
Ziago et al. [15] investigated the morphological and morphometric effects of three energy densities (4.10 and 50 J/cm2) on nerve regeneration from sciatic nerve crush injury. The device used was Twin Laser™ (780 nm, 40 mW, 0.04 cm2). Thirty Wistar rats (200–250 g) were randomly divided into five groups of six animals each (C, injured group; L0, with lesion and without irradiation; L4, nerve lesion with LLLT 4 J/cm2; L10, lesion of the nerve with LLLT 10 J/cm2; L50, nerve damage with LLLT 50 J/cm2). The animals received six irradiations applied for 2 days at three points of the injured nerve region: the proximal, distal, and central regions of the lesion. Fifteen days after surgery, the animals were euthanized and the sciatic nerve was collected for morphological and morphometric analysis, where the authors verified that the LLLT induced improvement in the lesion state at all the energy densities used; however, the energy density of 10 J/cm2 was the most effective treatment to improve regeneration of the sciatic nerve after crushing, obtaining positive results with the application of this energy density and thus corroborating our findings.
Feng and Yuan [23] investigated the effects of dexamethasone on functional and histological changes after sciatic nerve crush injury, where animals randomly divided into groups were treated with different doses of dexamethasone (0.5, 1, and 2 mg/kg) for 10 consecutive days and evaluated through the SFI, obtaining satisfactory results when compared to the injured group and sham group. However, the dose of 2 mg/kg was more effective in comparison to that of the other groups; therefore, we followed this methodology, without any intercurrence in the application of the substance. Mohammadi et al. [37] also found positive effects of the application of dexamethasone on the acceleration of neural regeneration, concluding that it was effective for the treatment of nerve damage by sciatic nerve crushing. These data corroborate our finding because of the positive effects of the use of dexamethasone alone and optimized when associated with LLLT.
In the researches that relate the use of photobiomodulation and the nerve injury, there is no standardization of the parameters used, and few studies have been carried out to verify the effect of dexamethasone on the peripheral nerve lesion, making it difficult to compare the results and the understanding of the involved mechanisms. Thus, new studies are needed to verify the use of LLLT and dexamethasone in nerve injury, and to compare their effects when associated at different application times and doses. Additionally, new clinical trials are needed with individuals with lesions in the peripheral nervous system to translate the effects of LLLT to the human model.
Conclusion
In the sample and in the procedures used, the AsGaAl (660 nm) and dexamethasone laser were found to be effective in the early functional recovery of the sciatic nerve of mice. However, the variables used showed that in the SFI, the group treated with LLLT alone obtained better results when compared to the other groups; for the SSI, the group treated with LLLT/Dex obtained better results in the intergroup comparison.
References
Simon NG, Spinner RJ, Kline DG, Kliot M (2016) Advances in the neurological and neurosurgical management of peripheral nerve trauma. J Neurol Neurosurg Psychiatry 87:198–208
Stratton JA, Kumar R, Sinha S, Shah P, Stykel M, Shapira Y, Midha R, Biernaskie J (2017) Purification and characterization of Schwann cells from adult human skin and nerve. Eneuro 4:307–316
Rateb EE, Amin SN, El-Tlabawy N, Rashed LA, El-Attar S (2017) Effect of melatonin supplemented at the light or dark period on recovery of sciatic nerve injury in rats. EXCLI J 16:138–150
Anders JJ, Moges H, Wu X, Erbele ID, Alberico SL, Saidu EK, Smith JT, Pryor BA (2014) In vitro and in vivo optimization of infrared laser treatment for injured peripheral nerves. Lasers Surg Med 46:34–35
Andraus RAC, Maia LP, Souza Lino AD, Fernandes KBP, De Matos Gomes MV, de Jesus Guirro RR, Barbieri CH (2017) LLLT actives MMP-2 and increases muscle mechanical resistance after nerve sciatic rat regeneration. Lasers Med Sci 32:771–778
Possamai F, Pacheco DR, Santos TS, Andre ES (2012) Repercussões morfológicas e funcionais do exercício sobre a regeneração nervosa periférica. Fisioter Mov 25:617–627
Barbosa RI, Marcolino AM, Guirro RRJ, Mazzer N, Barbieri CH, Fonseca MCR (2010a) Efeito do laser de baixa intensidade (660 nm) na lesão do nervo Ciático em ratos. Fisioter Pesq 17:294–299
Wang CZ, Chen YJ, Wang YH, Yeh ML, Huang MH, Ho ML, Liang JI, Chen CH (2014) Low-level laser irradiation improves functional recovery and nerve regeneration in sciatic nerve crush rat injury model. PLoS One 9:1–11
Altun I, Çiralik H (2015) Histopathological effects of tissue adhesives on experimental peripheral nerve transection model in rats. J Coreano Neurosurg Soc 58:504–507
Marcolino AM, Barbosa RI, Neves LMS, Vinas TS, Duarte DTB, Mazzer N, Fonseca MCR (2009) Low-intensity laser (830 nm) functional to recover of the sciatic nerve in rats. Acta Ortop Bras 18:207–211
Barbosa RI, Marcolino AM, Guirro RRJ, Mazzer N, Barbieri CH, Fonseca MCR (2010b) Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci 25:423–430
Song JW, Li K, Liang ZH, Dai C, Shen XF, Gong YZ, Wang S, Hu XY, Wang Z (2017) Low-level laser facilitates alternatively activated macrophage/microglia polarization and promotes functional recovery after crush spinal cord injury in rats. Sci Rep 7:1–13
Holanda VM, Chavantes MC, Wu X, Anders JJ (2017) The mechanistic basis for photobiomodulation therapy of neuropathic pain by near infrared laser light. Lasers Surg Med 49:516–524
Paolillo FR, Arena R, Dutra DB, de Cassia Marqueti Durigan R, de Araujo HS, de Souza HC, Parizotto NA, Cipriano G Jr, Chiappa G, Borghi-Silva A (2014) Low-level laser therapy associated with high intensity resistance training on cardiac autonomic control of heart rate and skeletal muscle remodeling in wistar rats. Lasers Surg Med 46:796–803
Ziago EK, Fazan VP, Iyomasa MM, Sousa LG, Yamauchi PY, da Silva EA, Borie E, Fuentes R, Dias FJ (2017) Analysis of the variation in low-level laser energy density on the crushed sciatic nerves of rats: a morphological, quantitative, and morphometric study. Lasers Med Sci 32:369–378
Tomazoni SS, Frigo L, dos Reis Ferreira TC, Casalechi HL, Teixeira S, de Almeida P, Muscara MN, Marcos RL, Serra AJ, de Carvalho PTC, Leal-Junior ECP (2017) Effects of photobiomodulation therapy and topical non-steroidal anti-inflammatory drug on skeletal muscle injury induced by contusion in rats—part 2: biochemical aspects. Lasers Med Sci 32:1879–1887
Buchaim DV, Rodrigues Ade C, Buchaim RL, Barraviera B, Junior RS, Junior GM, Bueno CR, Roque DD, Dias DV, Dare LR, Andreo JC (2016) The new heterologous fibrin sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med Sci 31:965–972
Fallah A, Mirzaei A, Gutknecht N (2017) Clinical effectiveness of low-level laser treatment on peripheral somatosensory neuropathy. Lasers Med Sci 32:721–728
Ökmen BM, Ökmen K (2017) Comparison of photobiomodulation therapy and suprascapular nerve-pulsed radiofrequency in chronic shoulder pain: a randomized controlled, single-blind, clinical trial. Lasers Med Sci 32:1719–1726
De Andrade ALM, Bossini PS, de Souza ALMDC, Sanchez AD, Parizotto NA (2017) Effect of photobiomodulation therapy (808 nm) in the control of neuropathic pain in mice. Lasers Med Sci 32:865–872
Rocha IR, Ciena AP, Rosa AS, Martins DO, Chacur M (2017) Photobiostimulation reverses allodynia and peripheral nerve damage in streptozotocin-induced type 1 diabetes. Lasers Med Sci 32:495–501
Sun H, Yang T, Li Q, Zhu Z, Wang L, Bai G, Li D, Li Q, Wang W (2012) Dexamethasone and vitamin B12 synergisticallypromote peripheral nerve regeneration in rats by upregulating theexpression of brain-derived neurotrophic factor. Arch Med Sc 8:924–930
Feng X, Yuan W (2015) Dexamethasone enhanced functional recovery after sciatic nerve crush injury in rats. Biomed Res Int 2015:627923
Meymandi MS, Sepehri G, Abdolsamadi M, Shaabani M, Heravi G, Yazdanpanah O, Agthaei MM (2017) The effects of co-administration of pregabalin and vitamin E on neuropathic pain induced by partial sciatic nerve ligation in male rats. Inflammopharmacology 25:237–246
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Fernandes ES, Russell FA, Alawi KM, Sand C, Liang L, Salamon R, Bodkin JV, Aubdool AA, Arno M, Gentry C, Smillie SJ, Bevan S, Keeble JE, Malcangio M, Brain SD (2016) Environmental cold exposure increases blood flow and affects pain sensitivity in the knee joints of CFA-induced arthritic mice in a TRPA1-dependent manner. Arthristis Res Ther 18:7
Marcolino AM, Barbosa RI, das Neves LM, Mazzer N, de Jesus Guirro RR, de Cássica Registro Fonseca M (2013) Assessment of functionalrecovery of sciatic nerve in rats submitted to low-level laser therapy 35 with different fluences: an experimental study. J Hand Microsurg 5:49–53
Reis FA, Belchior ACG, Nicolau RA, Fonseca TS, Carvalho PTC (2008) Effect of gallium-aluminum-arsenide laser therapy (660Nm) on recovery of the sciatic nerve in rats following neurotmesis lesion and epineural anastomosis: functional analysis. Rev Bras Fisioter 3:215–221
Takhtfooladi MA, Jahanbakhsh F, Takhtfooladi HA, Yousefi K, Allahverdi A (2015) Effect of low-level laser therapy (685 nm, 3 J/cm2) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 30:1047–1052
De Medinaceli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 3:634–643
Smit X, Van Neck JW, Ebeli MJ, Hovius SE (2004) Static footprint analysis: a time-saving functional evaluation of nerve repair in rats. Scand J Plast Reconstr Surg Hand Surg 38:321–325
Bervar M (2000) Video analysis of standing: an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods 102:109–116
Monte-Raso VV, Moro CA, Mazzer N, Fonseca MCR, Fazan VPS, Barbieri G, Barbieri CH (2009) A new adjustable pinch designed for producing crush nerve injuries in the sciatic nerve of rats. Acta Ortop Bras 17:236–238
Akgul T, Gulsoy M, Gulcur HO (2013) Effects of early and delayed laser application on nerve regeneration. Lasers Med Sci 29:351–357
Fredoni M, Ghatrehsamani M, Abdollahifar MA, Bayat S, Bayat M (2017) Evaluation of the effects of photobiomodulation on vertebras in two rat models of experimental osteoporosis. Lasers Med Sci
Pachioni CAS, Mazzer N, Barbieri CH, Fazan VPS, Padovani CR, Moro CA, Silva CAA (2006) Lesão por Esmagamento do Nervo Isquiático de Ratos: Estudo da Vascularização. Acta Ortop Bras 14:203–207
Mohammadi R, Azad-Tirgan M, Amini K (2013) Dexamethasone topically accelerates peripheral nerve repair and target organ reinnervation: a transected sciatic nerve model in rat. Injury 44:565–569
Acknowledgements
The authors would like to thank the CNPQ (National Research and Technology Development Council, Brazil), Rafael Cypriano Dutra (Laboratory of Autoimmunity and Immunopharmacology of Federal University of Santa Catarina, Brazil), and Nilton Mazzer (University of São Paulo, Brazil) for the support of this research.
Role of funding source
This project have had financial support from the Laboratory of Autoimmunity and Immunopharmacology of Federal University of Santa Catarina and State of São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP), Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were approved by the Animal Research Ethics Committee of Federal University of Santa Catarina (protocol no. PP00956).
Rights and permissions
About this article
Cite this article
de Souza, L.G., Marcolino, A.M., Kuriki, H.U. et al. Comparative effect of photobiomodulation associated with dexamethasone after sciatic nerve injury model. Lasers Med Sci 33, 1341–1349 (2018). https://doi.org/10.1007/s10103-018-2494-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2494-9