Abstract
Photobiomodulation is a widely used tool in regenerative medicine thanks to its ability to modulate a plethora of physiological responses. Wound re-epithelialization is strictly regulated by locally produced chemical mediators, such as nitric oxide (NO), a highly reactive free radical generated by the nitric oxide synthase (NOS) enzymatic family. In this study, it has been hypothesized that a 980-nm low-level laser stimulation could increase NO production in human keratinocytes and that such event might be directly related to the re-epithelialization process. Human keratinocytes were irradiated with increasing energy outputs (10–75 J) in the absence or presence of L-NAME, a NOS inhibitor. Laser stimulation induced an increase in NO production, resulting in an energy-dependent increase in both keratinocytes proliferation and re-epithelialization ability. The direct link between increased NO production and the observed physiological responses was confirmed by their inhibition in L-NAME pre-treated samples. Since NO production increase is a quick event, it is conceivable that it is due to an increase in existing NOS activity rather than to a de novo protein synthesis. For this reason, it could be hypothesized that photobiomodulation-derived NO positive effects on keratinocytes behavior might rely on a near infrared mediated increase in NOS conformational stability and cofactors as well as substrate binding ability, finally resulting in an increased enzymatic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different inflammatory mediators produced both locally or systemically are involved in the initiation and regulation of wound healing response. Among the pro-inflammatory mediators produces by keratinocytes after skin wound, there is nitric oxide (NO), a small molecule involved in different physiological as well as pathological processes.
NO is a highly reactive and diffusible free radical gas physiologically produced by all organisms, ranging from bacteria to humans. In mammals, it is produced at cellular level by the transformation of the amino acid L-arginine to L-citrulline in the presence of oxygen. This reaction is catalyzed by the nitric oxide synthase (NOS) enzymatic family, which is composed of three isoforms, two of which are constitutively expressed while the other is inducible. The two constitutive isoforms were originally characterized in neurons (nNOS) and endothelial cells (eNOS): such enzymes are not only restricted to the originally described localization, but also have been reported to display a wider expression. The third NOS isoform (iNOS) is generally not expressed in resting cell but can be synthesized upon cell activation [1]. In particular, neuronal isoform is known to be constitutively expressed also in keratinocytes, where it is the mainly responsible for the basal NO release that is involved in various networks of physiological signaling, such as nonspecific host defense during wound healing and epidermal cell proliferation. [2, 3]
NO production can be stimulated by a variety of endogenous and exogenous stimuli. Among the exogenous stimulations able to induce nitric oxide production, there is near infrared radiation used for photobiomodulation applications [4]. Although the skin is naturally exposed to light, it is able to respond to red and near infrared wavelengths used with therapeutic intent. Phototherapy or photobiomodulation refers to the delivery of low levels of energy, thus not emitting heat, sound, or vibrations, to alter biological activities [5, 6]. Currently, laser therapy is widely used in the medical practice for a variety of applications ranging from photodynamic therapies aimed to kill cancer and bacterial cells to photobiomodulation, aimed to promote cell growth and wound healing. Photobiomodulation positive effects on wound healing are due to its ability to modulate wound environment, through the modulation of several cellular responses, resulting in an increase in ATP levels, as well as in specific chemical mediators release [7]. Among the second messengers involved in wound healing and which production is known to be modulated by laser stimulation, there are reactive oxygen species (ROS) and NO. Both ROS and NO induce different cellular responses depending on their concentrations: subtoxic (picomolar to nanomolar range) concentrations generally stimulate cell proliferation, migration and survival, while higher (micromolar) concentrations induce cell cycle arrest, apoptosis, and senescence [2, 3, 8,9,10]. In particular, it is known that NO could influence cellular physiology by complexing heme or iron-sulfur containing proteins, resulting in metabolic processes activation or inhibition. Among the biological targets of NO, guanylate cyclase is of particular interest, as cGMP is known to be an important regulator of cell growth and differentiation as well as of cell migration [2, 12].
As keratinocyte proliferation and migration represent an essential step in the re-epithelialization process during wound healing, the aim of the study was to investigate if, in human keratinocytes, near infrared laser stimulation could induce NO production and if this second messenger could be involved in in vitro re-epithelialization process.
Materials and methods
Cell culture
Human spontaneously immortalized keratinocytes (HaCaT) cells [13] were purchased from Cell Lines Service GmbH (Eppelheim, Germany). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Euroclone, Milan, Italy) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Euroclone), 100 U/ml penicillin (Euroclone), 100 mg/l streptomycin (Euroclone), and 2 mM glutamine (Euroclone) in a humidified incubator with 5% CO2 and 95% air at 37 °C.
Laser irradiation
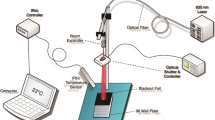
Cells were irradiated using a DMT Giotto laser equipment (DMT srl, Lissone, Italy). Laser stimulation was performed in the continuous mode, with the light source positioned vertically above each well (distance between the light source and the bottom of the well = 9.7 cm), in order to irradiate the whole well area (1.54 cm2). Laser irradiation was performed at 980 nm wavelength, using a 600-μm optical fiber and setting the power output to 1 W (649,35 mW/cm2) as previously described [14]. Before laser irradiation, in each well, a small volume (300 μl) of complete culture medium without phenol red (Euroclone) was added and the culture multiwells, with the lids off, were irradiated for 0 (control condition), 10, 25, 50, and 75 s, corresponding to an energy stimulation of 0 (control condition), 10, 25, 50, and 75 J and to an energy density (spatial average energy fluence) of 0 (control condition), 6.5, 16.23, 32.47, and 48.7 J/cm2, respectively. In both cell proliferation and in vitro scratch assays, laser stimulation was performed twice at 24-h intervals and 24 h after the last irradiation cells were fixed and analyzed.
Nitrite assay
NO production was evaluated indirectly by Griess method (Promega, Milan, Italy), following manufacturer’s instructions. Griess assay, in fact, allow the quantification of nitrite, one of the two primary, stable, and non-volatile breakdown products of NO. Briefly, 5 × 104 cells/well were seeded in a 24-wells culture plate and allowed to adhere overnight. Non adherent cells were removed by gentle wash in PBS and cell culture medium was substituted with complete medium without phenol red. Cells were irradiated as previously described and incubated for 1 h before assay, and in some experiments, before each laser stimulation, cells were pre-treated for 30 min with 10 mM L-NAME (ω-nitro-L-arginine methyl ester, Sigma Aldrich, Saint. Louis, MO, USA). NO production was evaluated by mixing 50 μl of each supernatant with an equal volume of Griess reagent following the manufacturer’s protocol. At the end of the assay, the absorbance of the resulting solution was read at 570 nm using a microplate reader (Victor X4, Perkin-Elmer, Waltham, MA, USA).
Cell proliferation
HaCaT cells were seeded at an initial density of 5 × 104 cells/well in 24-wells cell culture plates and allowed to adhere overnight. Non adherent cells were removed by gentle wash in phosphate buffer (PBS, pH = 7.4). In control experiments, cells were irradiated as previously described, while in experiments aimed to investigate NO involvement in laser-induced cell proliferation, before each laser stimulation, cells were pre-treated for 30 min with 10 mM L-NAME (ω-nitro-L-arginine methyl ester, Sigma Aldrich, Saint. Louis, MO, USA) [15]. Twenty-four hours after the last stimulation, cell proliferation was evaluated by means of MTT assay, an experimental approach based on tetrazolium salts reduction [16, 17]. At the end of the experiment, cells were incubated in cell culture medium without phenol red containing 0.5 mg/ml MTT (Thermo Fisher Scientific, Waltham, MA, USA) for 4 h at 37 °C to allow formazan salts precipitation. The resulting insoluble purple precipitate was then dissolved in DMSO (Carlo Erba Reagents, Cornaredo, Italy) and the absorbance was read at 570 nm using a microplate reader (Victor X4, Perkin-Elmer, Waltham, MA, USA).
In vitro scratch assay
Cell migration has been evaluated by in vitro scratch assay [18]. HaCaT cells were seeded in 24-wells cell culture plates and grown in complete medium to reach a confluent monolayer. Cell monolayers were then mechanically scratched with a yellow tip (diameter = 2 mm) and cell debris were removed by gentle was with fresh medium. Some control samples were immediately fixed in 3.7 formaldehyde, 3% sucrose solution in PBS to fix the initial scratch width (t0, time zero) while other samples were irradiated in the presence or absence of L-NAME as previously described. At the end of the experiments, treated samples were fixed and stained with a 0.1% crystal violet solution in 20% methanol. Images of the stained samples were digitally acquired to evaluate wound closure, considering the values referred to time zero as 0%. Wound width was measured using ImageJ software and wound closure was expressed as mean values ± standard deviation (SD).
Statistical analysis
One-way ANOVA followed by Dunnett’s post hoc tests were done for statistical analysis. Statistical procedures were performed with the Prism 4.0 statistical software (GraphPad Software Inc., CA, USA). Probability values of p < 0.05 were considered statistically significant.
Results
Near infrared laser irradiation induce NO production in HaCaT cells
Human keratinocytes constitutively express nNOS, and produce basal amounts of NO [19]. As NOS expression and activity can be upregulated by a plethora of stimuli, in this study, the ability of 980-nm near infrared laser to increase NO production in HaCaT cells has been evaluated. As shown in Fig. 1, near infrared laser stimulation was able to stimulate NO release by cultured human keratinocytes, as indirectly demonstrated by Griess assay. NO production has been evaluated 1 h after laser stimulation and results showed a linear correlation between energy intensity and radical production. The correlation between laser stimulation and NO production is confirmed by pre-treating cells with L-NAME that result in a complete block of NO production. The increase in NO levels compared to basal condition became statistically significant starting from 50-J stimulation (p < 0.05) and reached the maximum value at the higher intensity tested (75 J, p < 0.001).
NO production. Quantification of NO production in presence (dark gray bars) or absence (light gray bars) of L-NAME by means of Griess assay 60 min after laser stimulation. *p < 0.05 0 J (not irradiated control) vs, 50 J stimulation; ***p < 0.001 0 J (not irradiated control) vs. 75 J stimulation; ###p < 0.001 no L-NAME (50–75 J) vs. L-NAME (50–75 J)
NO effects on cell proliferation
Near infrared (980 nm) stimulation induced a sustained increase in human keratinocyte proliferation at each intensity (Fig. 2, p < 0.001). The observed increase in cell proliferation was statistically significant starting from 10-J intensity and reach the maximum value at 50-J intensity. In order to demonstrate NO production involvement in promoting cell growth after photobiomodulation stimulation, cells were pre-treated with 10 mM L-NAME before each irradiation. As shown in Fig. 2, L-NAME pre-treatment successfully inhibited laser-induced increase in cell growth in each test condition, reducing cellular proliferation to basal (not irradiated) levels.
NO effects on re-epithelialization
The observed laser-induced increase in NO production was also able to affect in vitro wound closure. As shown in Fig. 3, near infrared stimulation was able to increase HaCaT cells re-epithelialization ability. Laser-induced wound closure became statistically significant starting from 10-J stimulation. As observed in cell prolifreration assays, L-NAME was also able to decrease laser induced re-epithelialization: starting from 25-J stimulation, cell pre-treatment with the inhibitor resulted in a significant decrease in keratinocytes migration, with a wound closure rate similar to that observed for not irradiated samples (p < 0.001).
NO effects on cell migration. HaCaT cells migration was evaluated by means of scratch assay in the presence (dark gray bars) or absence (light gray bars) of L-NAME. #p < 0.05 0 J (not irradiated control) vs. 50 J stimulation; ##p < 0.01 0 J (not irradiated control) vs. 10, 25, and 75 J stimulation; ***p < 0.001 L-NAME pre-treated cells vs. control cells (no L-NAME) in the same conditions
Discussion
The epidermal epithelium represents an important barrier between the organism and the surrounding environment, thus assuring the protection from physical, chemical, and microbial damage. Considering this important barrier function of epidermis, when an injury occurs, it is necessary to re-establish tissue integrity as fast and efficiently as possible, through the re-epithelialization process [20].
Skin is known to well respond to red and near infrared wavelengths used for therapeutic purposes, mainly in the form of low-level laser therapy approach. A key feature of photobiomodulation is represented by the low level of energy used, resulting in wavelength and radiant exposure-dependent non-thermal responses that are known to modulate several biological processes, including cell growth, proliferation, and differentiation, as well as reducing pain and inflammation [4,5,6,7].
The molecular mechanisms associated with such beneficial photobiomodulation effects have not been fully elucidated, but it appears that it acts at molecular, cellular, as well as tissue level [3, 4]. From a biological point of view, photobiomodulation effects rely on the absorption of red and near infrared radiation by plasma membrane bound photoacceptors as well as mitochondrial chromophores, such as cytochrome c oxidase, and its conversion into metabolic energy [3,4,5,6, 9].
Moreover, many studies highlighted the ability of photobiomodulation to induce a shift in the overall cellular redox potential, thus increasing ROS generation, finally resulting in the activation of several intracellular signaling pathways, such as nucleic acid synthesis, enzyme activation, and cell cycle progression [8, 21].
In particular, it has been reported that photobiomodulation could directly modulate skin redox levels by interfering with antioxidant enzymes and NOS. Moreover, according to some authors, photobiomodulation also results in an increase in intracellular calcium levels, a condition known to increase NOS activity in endothelial cells [3, 22].
When a skin injury occurs, the main cellular population to be affected is represented by keratinocytes, that, through cell proliferation and migration responses, assure wound closure [12, 19]. It is now well-recognized that laser stimulation could positively modulate local wound environment mainly thanks to its photochemical effects at mitochondrial level, resulting in an increase in cellular energy levels as well as in the stimulation of specific chemical mediators release [7]. Among the second messengers involved in the wound healing process, there is NO, which could act as an autocrine or paracrine messenger, playing a key role in nonspecific host defense [2, 10].
Results obtained in this study show that laser stimulation induces a dose-dependent increase in NO production, cell proliferation, and re-epithelialization in cultured keratinocytes. The observed increase in NO production results from an increase in NOS biosynthetic activity: cell pre-treatment with L-NAME, a competitive inhibitor of both constitutive and inducible NOS isoforms [23, 24], in fact, resulted in a strong reduction of laser-induced NO production, cell proliferation, and re-epithelialization.
According to the existing literature on NO biological significance, such results can be related to the known biphasic effects of nitric oxide on cell behavior, with low radical concentrations displaying a positive effect while higher concentrations result in cell damage and even death [2, 10, 11, 19]. It is known that, at the molecular level, NO ability to act as a second messenger relies on its ability to induce guanylate cyclase activity, finally resulting in cGMP production, a key regulator of both cell proliferation and migration, both involved in wound re-epithelialization process [2, 12, 25,26,27]. Moreover, NO-induced cGMP production is involved in protein kinase G (PKG) activation, a downstream signaling pathway of crucial importance in mediating the effects of low levels of NO production, which seems to occur with constitutive NOS activation [19].
Keratinocytes are known to constitutively express nNOS and to be able to express iNOS after appropriate stimulation and it has been reported that photobiomodulation could affect NOS gene expression in endothelial cells [19, 28]. Since the increase in NO levels is not immediate, thus suggesting a direct role of light stimulation in NO mobilization from cellular stores, but occurs in a quick time lapse (1-h after the stimulation), it can be hypothesized that the observed increase in NO levels could be due to an increase in the existing enzymes’ activity rather than to a de novo protein synthesis, a physiological response that needs a longer time to take place [29]. The hypothesized increase in existing nNOS and iNOS activity could be due to a photobiomodulation-mediated increase in the enzymes’ conformational stability. In fact, it is known that the absorption of the infrared radiation excites vibrational transitions of molecules and that the strength of absorption increases with the increasing polarity of the vibrating bonds, with basically all polar bonds contributing to infrared absorption [30, 31].
All the three known NOS isoforms are, in their active form, homodimers composed of an N-terminal oxygenase domain and a C-terminal reductase domain separate by a recognition sequence for calmodulin. Oxygenase domain binds heme, tetrahydrobiopterin (H4B), and L-arginine, thus representing the active site for NO synthesis. On the other hand, reductase domain binds FAD, FMN and NADPH, assuring the electron flow essential to NO synthesis.
From a structural point of view, dimerization process take place in different ways in constitutive or inducible isoforms. In iNOS, dimerization is promoted by heme incorporation and stable incorporation of H4B in the obtained dimer. On the other hand, in nNOS, while heme incorporation is essential for dimer assembly, H4B may not be essential, although it can stabilize the dimer once formed [32, 33]. Considering the complex structural assembly of these enzymes, in light of the observed results, it is possible to speculate that near infrared radiation is able to increase the stability of NOS dimers by altering the vibrational energy of the chemical bonds linking the essential cofactors to the core of the oxygenase as well as reductase domains.
Conclusion
In conclusion, data presented in this study support a strong relationship between photobiomodulation and in vitro wound re-epithelialization, a process involving an increased NO availability at cellular level. Moreover, it can be hypothesized that the observed increase in NO dependent re-epithelialization after photobiomodulation is due to an increase in existing enzymes stability and activity rather than to a de novo protein synthesis and that this effect could be mediated by near infrared radiation absorption by the chemical bonds essential for substrate and cofactors linking to the active enzyme.
References
Tripathi P, Tripathi P, Kashyap I, Sing V (2007) The role of nitric oxide in inflammatory reactions. FEMS Immunol Med Microbiol 51:443–452
Heck DE, Laskin DL, Gardner CR, Laskin JD (1992) Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. J Biol Chem 267:21277–21280
Roméro-Graillet C, Aberdam E, Clément M, Ortonne JP, Ballotti R (1997) Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J Clin Invest 99:635–642
Moriyama Y, Nguyen J, Akens M, Moriyama EH, Lilge L (2009) In vivo effects of low level laser therapy on inducible nitric oxide synthase. Lasers Surg Med 41:227–231
Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR (2013) Low level laser (light) therapy (LLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 32:41–52
AlGhamdi KM, Kumar A, Moussa NA (2012) Low level laser therapy: a useful techniques for enhancing theproliferation of various cultured cells. Lasers Med Sci 27:237–249
Silva JC, Lacava ZG, Kuckelhaus S, Silva LP, Neto LF, Sauro EE, Tedesco AC (2004) Evaluation of the use of low level laser and photosensitizer drugs in healing. Lasers Surg Med 34:451–457
Migliario M, Pittarella P, Fanuli M, Rizzi M, Renò F (2014) Laser-induced osteoblast proliferation is mediated by ROS production. Lasers Med Sci 29:1463–1467
Huang YY, Sharma SK, Hamblin MR (2011) Biphasic dose response in low level light therapy—an update. Dose-Response 9:602–618
Napoli C, Paolisso G, Casamassimi A, Al-Omaran M, Varbieri M, Sommese L, Infante T, Ugnarro LJ (2013) Effects of nitric oxide on cell proliferation. Novel insights J Am Coll Cardiol 62:89–95
Villalobo A (2007) Enhanced cell proliferation induced by nitric oxide. Dynamic Cell Biol 1:1–5
Zhan R, Yang S, He W, Wang F, Tan J, Zhou J, Yang S, Yao Z, Wu J, Luo G (2015) Nitric oxide enhances keratinocyte cell migration by regulating rho GTPase via cGMP-PKG signalling. PlosOne 10:e0121551. https://doi.org/10.1371/journal.pone.0121551
Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771
Rizzi M, Migliario M, Rocchetti V, Tonello S, Renò F (2016) Near-infrared laser increases MDPC-23 odontoblast-like cells proliferation by activating redox sensitive pathways. J Photochem Photobiol B 164:283–288
Molinari C, Rizzi M, Squarzanti DF, Pittarella P, Vacca G, Renò F (2013) 1α,25-dihydroxycholecalciferol (vitamin D3) induces NO-dependent endothelial cell proliferation and migration in a three dimensional matrix. Cell Physiol Biochem 31:815–822
Berridge MV, Herst PM, Tan AS (2005) Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotech Ann Rev 11:127–152
Berridge MV, Tan AS (1993) Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys 303:474–482
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333
Cals-Grierson MM, Ormerod AD (2004) Nitric oxide function in the skin. Nitric Oxide 10:179–193
Koivisto L, Häkkinen L, Larjava H (2012) Re-epithelialization of wounds. Endod Top 24:59–93
Farivar S, Malekshahabi T, Shiari R (2014) Biological effects of low level laser therapy. J Lasers Med Sci 5:58–62
Santuzzi CH, Buss HF, Pedrosa DF, Oliverira Vieira Moniz Freire M, Nogueira BV, Silva Gonçalves WL (2011) Combined use of low level laser therapy and cyclooxygenase-2 selective inhibition on skin incisional wound reepithelialization in mice: a preclinical study. An Bras Dermatol 86:278–283
Wang H, Leigh J (2006) Effects of nitric oxide synthase inhibitor ω-nitro-L-arginine methyl ester, on silica-induced inflammatory reaction and apoptosis. Particle Fibre Toxicol 3:14
Conners W, Whitebeck C, Chicester P, Legget R, Lin ADY, Johnson A, Kogan B, Levin R, Mannikarottu A (2005) L-NAME, a nitric oxide synthase inhibitor, diminishes oxidative damage in urinary bladder partial outlet obstruction. Am J Physiol – Renal Physiol 290:F357–F363
Deliconstantinos G, Vulliotou V, Stravrides C (1995) Release by ultraviolet B (u.V.B) radiation of nitric oxide (NO) from human keratinocytes: a potential role for nitric oxide in erythema production. Br J Pharm 114:1257–1265
Dhaunsi GS, Ozand PT (2004) Nitric oxide promotes mitogen-induced DNA synthesis in human dermal fibroblasts through cGMP. Clin Exp Pharm Phys 31:46–49
Rizk M, Witte MB, Barbul A (2004) Nitric oxide and wound healing. World J Sur 28:301–306
Szymczyszyn A, Doroszko A, Szahidewicz-Krupska E, Rola P, Gutherc R, Jasiczek J, Mazur G, Derkacz A (2016) Effect of the transdermal low level laser therapy on endothelial function. Lasers Med Sci 31:1301–1307
Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A (2005) Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg 23:3–9
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta 1767:1073–1101
Barth A (2000) The infrared absorption of amino acid side chains. Progress Biophys Mol Biol 74:141–173
Stuehr DJ (1999) Mammalian nitric oxide synthases. Biochim Biophys Acta 1411:217–230
Zhou L, Zhu DY (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation and clinical implications. Nitric Oxide 20:223–230
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable to the present submission
Informed consent
Not applicable to the present submission
Rights and permissions
About this article
Cite this article
Rizzi, M., Migliario, M., Tonello, S. et al. Photobiomodulation induces in vitro re-epithelialization via nitric oxide production. Lasers Med Sci 33, 1003–1008 (2018). https://doi.org/10.1007/s10103-018-2443-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2443-7