Abstract
In the application of lasers in dentistry, there is a delicate balance between the benefits gained from laser treatment and the heat-related damage arising from laser irradiation. Hence, it is necessary to understand the different processes associated with the irradiation of lasers on dental materials. To obtain insight for the development of a safe and general-purpose laser for dentistry, the present study examines the physical effects associated with the irradiation of a near-infrared free-electron laser (FEL) on the surface of a commonly used silver dental alloy. The irradiation experiments using a 2900-nm FEL confirmed the formation of a pit in the dental alloy. The pit was formed with one macro-pulse of FEL irradiation, therefore, suggesting the possibility of efficient material processing with an FEL. Additionally, there was only a slight increase in the silver alloy temperature (less than 0.9 °C) despite the long duration of FEL irradiation, thus inferring that fixed prostheses in the oral cavity can be processed by FEL without thermal damage to the surrounding tissue. These results indicate that dental hard tissues and dental materials in the oral cavity can be safely and efficiently processed by the irradiation of a laser, which has the high repetition rate of a femtosecond laser pulse with a wavelength around 2900 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of lasers into dentistry [1], several lasers have been widely applied in operative dentistry, endodontics, periodontics, orthodontics and cosmetic dentistry [2]. The lasers are primarily used in the treatment of dental hard and oral soft tissues. However, the application of lasers has also been expanded to the processing of inorganic dental materials; for example, the removal and surface treatment of inorganic dental materials with laser ablation [3, 4] and the laser welding of dental alloys [5, 6]. It has been suggested that the laser treatment of alloy surfaces contribute to better resin-metal bonding [4]. In addition, super-fine structures acting as laser markers can be generated on dental alloys and resins with lasers. This approach of generating markers on dental prostheses has gained consideration for body identification after natural disasters [7, 8]. However, the increase in temperature accompanying the laser irradiation is one of the significant challenges associated with the use of lasers in dentistry. A temperature increase of 5.5 °C can reduce the vitality of pulpal, while a temperature increase of 10 °C can lead to damaged bone tissue [9,10,11]. These critical threshold temperatures are often exceeded during the laser irradiation of dental hard tissues and dental materials [12,13,14,15,16]. Therefore, to avoid thermal damage stemming from laser irradiation of oral tissues, cooling has been considered necessary during irradiation within the oral cavity [5, 12, 14, 15]. Meanwhile, the application of lasers with ultra-short pulses with duration on the order of femtoseconds has been attempted in dentistry [7, 17]. Because of the very short time scales involved in ablation with a femtosecond laser pulse, it is considered that thermal conduction into neighbouring tissues can be neglected during the laser irradiation [18, 19]. Thus, femtosecond laser allows the precision processing of a workpiece without thermal damage, thereby indicating an advantage over other lasers for the processing of dental tissues and dental materials within the oral cavity [7, 17]. However, the ablation efficiency of femtosecond lasers is significantly lower than that of a microsecond or nanosecond pulse laser. Hence, it is necessary to increase the number of irradiated pulses to achieve satisfactory processing of the dental materials including metals [7, 18, 19].
Free-electron laser (FEL) produced with the help of electrons in synchrotrons are wavelength-tuneable, thereby making them attractive for the next stage of ultra-short laser-based material processing [20]. FEL has a characteristic pulse structure consisting of two types of pulses, namely a macro-pulse and micro-pulse. Each macro-pulse of the FEL has a time duration on the order of a microsecond and consists of a train of micro-pulses, each with duration on the order of a femtosecond. Because of the characteristics of the FEL, it has been demonstrated that irradiation using an FEL can ablate various substances efficiently without thermal damage [20,21,22]. The FEL located at the Laboratory of Electron Beam Research and Application, Nihon University (LEBRA) is a linear-accelerator-based FEL with a variable wavelength ranging between 1600 and 6000 nm within the near-infrared region of the electromagnetic spectrum. The LEBRA-FEL macro-pulses have time duration of about 10–20 μs and is composed of micro-pulses with time duration of about 100 fs [23, 24]. The irradiation experiments using LEBRA-FEL for dental hard tissues have been previously reported [25,26,27,28,29]. Although these studies suggested the efficient and wavelength-dependent ablation of the dental hard tissues, there are no reports that confirm the similar effects of near-infrared FEL irradiation on inorganic dental materials such as dental alloys.
In this study, we aim to understand the different processes accompanying the laser irradiation on inorganic dental materials, to help in the development of a safe and general-purpose laser for dentistry. The processes investigated as a part of this study include the changes in the surface morphology, alloy composition and temperature change as a function of LEBRA-FEL irradiation with a wavelength of 2900 nm. A silver alloy was selected as the inorganic dental material for the laser irradiation experiments, as silver-based dental alloys are commonly used in dentistry and are the likely target for laser processing in the future. The slight increase in temperature and the minimal change in composition of the silver alloy during the irradiation indicate the usefulness and safety of the FEL in processing substances within the oral cavity. Any safe and general purpose laser for dental application must be able to process both dental hard tissues and materials without thermal damage. As dental enamel and dentin are effectively etched by a near-infrared FEL [25, 28], the experimental verification of the same effect for FEL irradiation on inorganic dental materials is key to the development of one general-purpose laser in dentistry.
Materials and methods
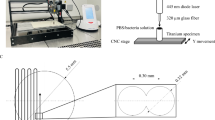
Sample preparation
In the present study, a dental alloy (Kinpara S12, Ishifuku Metal Industry Co., Ltd., Tokyo, Japan) was employed for the FEL irradiation experiments. The composition of the alloy was as follows: 49.5% silver, 20% palladium, 14.4% copper, 12% gold and less than 1% of iridium, zinc and indium. This silver alloy is generally used in making dental crowns, bridges and inlays. For the FEL irradiation experiments, a rectangular alloy plate with dimensions of 6-mm by 12-mm and a thickness of about 1.2 mm was prepared.
FEL irradiation
The structure and features of LEBRA-FEL has been described previously [23, 24]. In brief, the macro-pulse of LEBRA-FEL has a duration of about 10 μs and a repetition rate of 2 Hz. Each macro-pulse consists of a train of micro-pulses with duration of about 100 fs. The interval between two consecutive micro-pulses is 350 ps, which corresponds to the radio frequency (2856 MHz) employed in the linear accelerator at LEBRA. In this study, the wavelength of the LEBRA-FEL was 2900 nm.
The alloy plate was mounted on an automated XYZ stage, with the irradiation position controlled with the help of a computer program. The FEL was focused to a spot size with a diameter of about 0.1 mm with a calcium fluoride convex lens placed before the alloy plate. In a typical experiment, each spot on the silver alloy was irradiated with one FEL macro-pulse. The number of macro-pulses for each spot for temperature-dependence measurements was five.. As the FEL power fluctuated pulse-to-pulse, the laser power of each macro-pulse was monitored with the help of a power meter during the entire course of the experiment. To mimic, typical dental clinical environments, all irradiation experiments were performed at room temperature in air.
The FEL-irradiated alloy plates were characterised under a binocular microscope (Nikon, Tokyo Japan), a digital optical microscope (VHX-2000, Keyence Co., Osaka, Japan) and a scanning electron microscope (SEM; S2700, Hitachi Co., Ltd., Tokyo, Japan). The depth of the pits formed by FEL irradiation was measured with a laser profile micrometre (VF7510, Keyence Co.).
Measurement of composition changes by X-ray diffraction analysis
The changes in the dental alloy composition after FEL irradiation was characterized with X-ray diffraction (XRD). The XRD data was recorded from (1) a pristine spot on the alloy plate with no FEL irradiation, (2) the inside wall of a pit formed by FEL irradiation and (3) the rim surrounding the pit. For XRD measurement from the inner wall of the pit, the irradiated alloy plate was cut longitudinally through the centre of a pit. Subsequently, the XRD pattern was collected using a two-dimensional imaging plate detector and CuKα radiation from a rotating-anode X-ray generator (RINT-RAPID CMF, Rigaku Co. Ltd., Tokyo, Japan). The measured two-dimensional diffraction pattern was converted into a conventional powder pattern using the 2DP software (Rigaku Co. Ltd.). The XRD analysis was performed using the PDXL software (Rigaku Co. Ltd.).
Temperature measurement during FEL irradiation
To examine thermal effects from the FEL irradiation, the temperature of the alloy plate was continuously recorded during the experiment for a duration of 30 min. In this experiment, an alloy plate was packed in a plastic case with heat insulators (Aeroflex USA Inc., TN, USA). The plastic case had a window for the laser irradiation. The temperature of the dental alloy was measured with a filmed platinum film resistance thermometer (Netsushin Co., Ltd., Saitama, Japan). The insulating material for the plastic case was obtained from Aeroflex USA Inc., TN, USA. The thermometer was tightly attached to the bottom side of the alloy plate. The changes in temperature before, during and after the FEL irradiation was continuously recorded every 0.1 s with a data acquisition unit (MX100, Yokogawa Electric Co., Ltd., Tokyo, Japan). During irradiation, the alloy plate was moved with the automated stage while maintaining the laser in focus to the surface of the alloy plate. The stage was programmed to move horizontally on the same irradiation line by 0.1 mm every 3 s. After every 3 min, the stage returned to the starting position of the next irradiation line which shifted vertically by 0.1 mm from the previous line. The FEL irradiation on the alloy was continued during all the stage movements.
Results
Morphological changes due to FEL irradiation
Figure 1 shows the morphological changes on the dental alloy surface after one macro-pulse irradiation of the 2900-nm FEL. The irradiation of just one FEL macro-pulse resulted in the production of a pit on the alloy plate (Fig 1a, b). In the case of the pit displayed in Fig. 1a, the energy of the irradiated macro-pulse was 3.9 mJ, the average energy of micro-pulses constituting the macro-pulse was estimated at 0.14 μJ; Therefore, the laser density of the focused FEL spot was about 50 J/cm2 for the macro-pulse and about 1.7 mJ/cm2 for the micro-pulse. The pits were surrounded by a rim with droplets (see Fig. 1b), which seems to have been formed by the melting and resolidification of the alloy. Additionally, the alloy surface surrounding the pit was discoloured.
Morphological changes in the dental alloy surface after a single macro-pulse irradiation of the 2900-nm FEL. a Digital microscope image displaying the formation of pit and discolouration of the alloy surface surrounding the pit, b SEM images of a pit formed by FEL irradiation, c SEM image of the circular dent formed by FEL irradiation at energy of 2.6 mJ/macro-pulse. Scale bars in (a) is 50 μm and those in (b) and (c) represent a length of 10 μm
Figure 2 shows the plot of pit depth as a function of the FEL energy for 19 irradiation experiments. In our experiments, the energy of the FEL macro-pulse was varied between 1.3–4.1 mJ/macro-pulse. The pit formation was observed when the FEL irradiation exceeded 3.2 mJ/macro-pulse. Beyond the threshold FEL energy of 3.2 mJ/macro-pulse, there appears to be correlation between the pit depth and the FEL energy input on the alloy surface. When the FEL energy was below 3.2 mJ/macro-pulse, there was no pit formation and circular dents on the surface were observed (see Fig. 1c).
Composition change due to FEL irradiation
The XRD patterns collected from the non-irradiated alloy surface, the inside wall of a pit formed by FEL irradiation, and the discoloured surface surrounding the pit on the dental alloy plate are displayed in Fig. 3. The XRD pattern of the discoloured surface is clearly different from the patterns of the other areas. The peaks corresponding to Ag and Au–Cu–Zn alloy were clearly observed in XRD patterns of the non-irradiated area and can be considered as the reference XRD pattern. The XRD pattern collected from the inside wall of a pit was nearly identical to the reference XRD pattern collected from the non-irradiated alloy surface. Meanwhile, the XRD pattern of the tarnished surface surrounding the pit reveals peaks matching to In–Pd alloy. Although, we speculated that the surface discoloration might be due to the heat-induced formation of oxides, there were no peaks related to oxides in this XRD pattern.
XRD patterns of the dental alloy irradiated by the FEL. The grey line, black dots and red line correspond to patterns collected from the non-irradiated area, the inside wall of a pit formed by FEL irradiation, and the discoloured surface surrounding the pit, respectively. Circles and asterisks indicate diffraction peaks of Ag and Au–Cu–Zn, respectively
Temperature change during FEL irradiation
The graph in Fig. 4 shows the temperature of the dental alloy and the FEL energy per macro-pulse as function of time. In this case, we recorded the temperature before, during and after the FEL irradiation. The alloy surface was irradiated with FEL in this experiment between 300 and 2100 s time stamps on the graph. The average FEL energy during the irradiation was 3.8 mJ/macro-pulse. During this experiment, the ambient temperature was between 24.8 and 25.1 °C. The total increase in temperature of the dental alloy during the entire course of this experiment was below 0.9 °C. It was remarkable to observe only a slight temperature increase, considering the alloy was not specifically cooled. The temperature fluctuations with a periodicity of about 3 min observed in the graph correlated well with the programmed movements of the automated stage on which the dental alloy was loaded. The irregular decreases in temperature change observed after 1500 s can be correlated to the decrease of the FEL energy, which is due to the instability of the linear accelerator. The temperature of the alloy returned to its initial value, approximately 5 min after the irradiation was stopped.
Temperature change in the dental alloy due to FEL irradiation as a function of time. The total irradiation period was between 300 and 2100 s. A temperature change of 0 °C is the lowest temperature change of the dental alloy before irradiation. The graph plotted was corrected for the variation of room temperature during the experiment. The grey dots denote the FEL energy per macro-pulse, with the energy values depicted in the y-axis on the right side of the graph
Discussion
The present study confirms the formation of pits by the irradiation of a 2900-nm FEL on silver-based dental alloy. To our knowledge, this is the first report to demonstrate the use of near-infrared FEL irradiation to process the surface of dental metal alloys. The morphological changes of the dental alloy due to FEL irradiation were comparable to those observed previously in laser irradiation experiments on metals [7, 19, 30]. Ichikawa et al. [7] have evaluated the feasibility of using a 800-nm Ti:sapphire laser with a pulse length of 150 fs to record personal information on dental materials like titanium and Ag–Pt–Au dental alloys. In their experiments, shallow pits with depth of 0.2 μm on titanium plates were observed upon the irradiation of Ti:sapphire femtosecond laser pulse with a power density of 4 J/cm2, and they could create clear dots marked on the dental alloy by increasing the number of irradiation pulses. Similarly, Leitz et al. [19] have demonstrated the high-precision processing of materials at low ablation efficiency using a 800-nm Ti:sapphire femtosecond laser. In our LEBRA-FEL irradiation experiments, the formation of a deeper pit with a maximum depth of 87 μm was achieved by the irradiation of just one macro-pulse (see Fig. 2). Although the power density of each micro-pulse in the irradiated macro-pulse was estimated to be less than 2 mJ/cm2, one macro-pulse of LEBRA-FEL consists of a train of micro-pulses with a radio frequency of 2856 MHz. Thus, one macro-pulse with a duration of 10 μs contains roughly 28,600 μ-pulses, each with duration of 100 fs. Furthermore, the peak power of LEBRA-FEL was estimated to exceed 1 MW [23, 24]. This characteristic feature of FEL will allow more efficient formation of an apparent pit on the dental alloy than that obtained using the Ti:sapphire femtosecond laser. Also, the ablation rates of the workpiece can be increased by increasing the repetition rate of the macro-pulse; in the case of LEBRA-FEL, the macro-pulse repetition rate of 12.5 Hz is theoretically possible [24]. In addition, our results suggest a correlation between the FEL energy and pit depth (see Fig. 2). Laser ablation occurs when the laser intensity exceeds a certain threshold value [31]. In case of the FEL irradiation on dental alloy, the threshold value of energy appears to be above 3 mJ/macro-pulse. Therefore, the surface of dental alloy can be processed as intended by controlling the laser power and pulse frequency.
One of the most important results from this study is the low increase in temperature of the dental alloy during FEL irradiation. Thermal effects accompanying laser irradiation on dental tissues and dental materials have been studied for several lasers. In general, laser irradiation can lead to a significant increase in temperature, which adversely affects dental tissues and bone [12,13,14,15,16]. For example, Hirota and Furumoto [13] reported that Er:YAG and CO2 laser irradiation of dental enamel for a few seconds raised the temperature by up to 1600 °C. Therefore, water and/or air cooling is essential to reduce the heat generated by laser irradiation [5, 12, 14, 15]. However, despite the absence of specific cooling and a long duration of FEL irradiation (30 min), the temperature increase was well below the critical thresholds for pulp (5.5 °C) [9] and bone (10 °C) [10, 11] (Fig. 4). Chichkov et al. [18] indicated that thermal conduction into the sample due to irradiation of 780-nm femtosecond laser can be neglected owing to the extremely short interaction time of the sample with the laser. Our result demonstrates the reduction of the temperature increase in dental metal processing using an FEL that contains a train of femtosecond micro-pulses. This characteristic of FEL irradiation may be eventually available for the processing of fixed prostheses within the oral cavity.
Meanwhile, the FEL irradiation resulted in burrs and discoloration of the alloy surface surrounding a pit (Figs. 1a and 5a). When considering the processing of dental prostheses, such as laser marking for body identification, it is important to understand the properties of the tarnished surface in terms of safety and aesthetics. The XRD analysis revealed the presence of peaks related to In–Pd alloy in the discoloured surface (Fig. 3). Although the mechanism involving the production of In–Pd alloy by FEL irradiation is not clear, the composition changes of the irradiated alloy do not pose any appreciable health risk. In addition, we polished the surface of the irradiated alloy with dental metal polisher (Silicon Point M3, Shofu, Inc., Japan). The images of the irradiated surface of the alloy before and after polishing are shown in Fig. 5. From the figure, it is evident that the discoloured surface and burrs can be easily removed with general polishing. This indicates that the formation of burrs and the discolouration of the dental alloy by FEL irradiation do not constitute an appreciable barrier to the clinical application of the FEL.
The previous irradiation reports using the LEBRA-FEL focused on hard dental tissues [25,26,27,28,29], confirming pit formations on enamel and dentin due to irradiation. Our results demonstrate the ability of FEL to process dental alloys in addition to the dental hard tissues. As the FEL irradiation ensures efficient processing of dental tissues and materials, along with the reduction of thermal damage to surrounding tissues, it is expected that an FEL-like laser, which has a high repetition rate of a femtosecond laser, can be used safely to process several substances within the oral cavity. Interestingly, the wavelength of the FEL used in this study, 2900 nm, is almost similar to that used in dental tissue processing, 2940 nm [25,26,27,28,29]. This indicates that an FEL with a single wavelength close to 2900 nm can be employed for the processing of both dental tissues and dental alloy. The use of a single-wavelength femtosecond laser for processing several materials can be considered important for the future development of one general purpose laser for deployment in an office-type environment.
References
Stern RH, Sognnaes RF (1964) Laser beam effect on dental hard tissue. J Dent Res 43:873
Sharma N, Williams C, Angadi P, Jethlia H (2013) Application of lasers in dentistry. Res Rev J Dent Sci 1:22–25
Siniaeva ML, Siniavsky MN, Pashinin VP, Mamedov AA, Konov VI, Kononenko VV (2009) Laser ablation of dental materials using a microsecond Nd: YAG laser. Laser Phys 19:1056–1060
sadat Madani A, Astaneh PA, Shahabi S, Nakhaei MR, Bagheri HG, Chiniforush N (2013) Influence of different power outputs of intraoral Nd:YAG laser on shear bond strength of a resin cement to nickel-chromium dental alloy. Lasers Med Sci 28:229–234
Shibuya I, Nishiyama N, Hayakawa T, Koishi I, Nemoto K (2004) Intraoral laser welding—part 1. Effect of laser irradiation on temperature rise. J J Dent Mater 23:495–500 (in Japanese)
Fornaini C, Merigo E, Cernavin I, Lòpez de Castro G, Vescovi P (2012, 2012) Intraoral laser welding (ILW) in implant prosthetic dentistry: case report. Case Rep Dent:939141. doi:10.1155/2012/839141
Ichikawa T, Hayasaki Y, Fujita K, Nagao K, Murata M, Kawano T, Chen J (2006) Femtosecond pulse laser-oriented recording on dental prostheses: a trial introduction. Dent Mater J 25:733–736
Naito Y, Meinar AN, Iwawaki Y, Kashiwabara T, Goto T, Ito T, Sakuma T, Ichikawa T (2013) Recording of individual identification information on dental prostheses using fluorescent material and ultraviolet light. Int J Prosthodont 26:172–174
Zach L, Cohen G (1965) Pulp response to externally applied heat. Oral Surg Oral Med Oral Pathol 19:515–530
Eriksson AR, Albrektsson T (1983) Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent 50:101–107
Eriksson RA, Albrektsson T (1984) The effect of heat on bone regeneration: an experimental study in the rabbit using the bone growth chamber. J Oral Maxillofac Surg 42:705–711
Armengol V, Jean A, Marion D (2000) Temperature rise during Er: YAG and Nd: YAP laser ablation of dentin. J Endodontics 26:138–141
Hirota F, Furumoto K (2003) Temperature rise caused by laser (CO 2, Nd: YAG, Er: YAG) irradiation of teeth. Int Congr Ser 1248:301–304
Colucci V, do Amaral FL, Pécora JD, Palma-Dibb RG, Corona SA (2009) Water flow on erbium:yttrium-aluminum-garnet laser irradiation: effects on dental tissues. Lasers Med Sci 24:811–818
Geminiani A, Caton JG, Romanos GE (2011) Temperature increase during CO2 and Er:YAG irradiation on implant surfaces. Implant Dent 20:379–382
Leja C, Geminiani A, Caton J, Romanos GE (2013) Thermodynamic effects of laser irradiation of implants placed in bone: an in vitro study. Lasers Med Sci 28:1435–1440
Ji L, Li L, Devlin H, Liu Z, Jiao J, Whitehead D (2012) Ti: sapphire femtosecond laser ablation of dental enamel, dentine, and cementum. Lasers Med Sci 24:197–204
Chichkov BN, Momma C, Nolte S, von Alvensleben F, Tünnermann A (1996) Femtosecond, picosecond and nanosecond laser ablation of solids. Appl Phys A Mater Sci Process 63:109–115
Leitz KH, Redlingshöfer B, Reg Y, Otto A, Schmidt M (2011) Metal ablation with short and ultrashort laser pulses. Phys Procedia 12:230–238
Nishimura A, Yamaushi T, Minehara E (2004) Demonstration of material processing using JAERI-FEL. In: Minehara EJ, Hajima R, Sawamura M (eds) Free electron lasers 2003. Elsevier, Amsterdam, pp II57–II58
Junbiao Z, Yonggui L, Nianqing L, Guoqing Z, Minkai W, Gan W, Xuepin Y, Yuying H, Wei H, Yanmei D, Xuejun G (2001) Primary experimental studies on mid-infrared FEL irradiation on dental substances at BFEL. Nuclear Instr Meth A 475:630–634
Spector N, Reinisch L, Spector J, Ellis DL (2002) Free-electron laser and heat-conducting templates: a study of reducing cutaneous lateral thermal damage. Lasers Surg Med 30:117–122
Tanaka T, Hayakawa K, Hayakawa Y, Mori A, Nogami K, Sato I, Yokoyama K (2004) Tunability and power characteristics of the LEBRA infrared FEL. Proc 2004 FEL Conf: 247–250
Nakao K, Hayakawa K, Hayakawa Y, Inagaki M, Nogami K, Sakai T, Tanaka T (2012) Pulse structure measurement of near-infrared FEL in burst-mode operation of LEBRA linac. Proc FEL 2012:472–474
Sakae T, Sato Y, Numata Y, Suwa T, Hayakawa T, Suzuki K, Kuwada T, Hayakawa K, Hayakawa Y, Tanaka T, Sato I (2007) Thermal ablation of FEL irradiation using gypsum as an indicator. Lasers Med Sci 22:15–20
Sakae T, Sato Y, Tanimoto Y, Higa M, Oinuma H, Kozawa Y, Okada H, Yamamoto H, Hayakawa T, Nemoto K, Sakai T, Nogami K, Mori A, Kuwada T, Hayakawa Y, Tanaka T, Hayakawa K, Sato I (2005) Pit formation in human enamel and dentin irradiated using the 2.94 μm LEBRA-free electron laser. Int J Oral Med Sci 4:8–13
Nemoto S, Iwai H, Suzuki H, Kamiya N, Iwai H, Iki K, Ikemi T (2010) Increased temperature and morphological change of bovine dentin irradiated by a free electron laser. Jpn J Conserv Dent 53:419–427 (in Japanese)
Nemoto S (2012) Dentin abration with free electron laser. Jpn J Conserv Dent 55:185–194 (in Japanese)
Sakae T, Hayakawa K, Hayakawa Y, Inagaki M, Kuwada T, Nakao K, Nogami K, Sato I, Tanaka T, Kii T, Ohgaki H, Zen H (2012) Pit Formation on Dental Hard Tissues Using Two Different Free Electron Laser Sources, LEBRA-FEL and KU-FEL. Proc FEL 2012:563–565
Stasic J, Trtica M, Gakovic B, Petrovic S, Batani D, Desai T, Panjan P (2009) Surface modifications of AISI 1045 steel created by high intensity 1064 and 532nm picosecond Nd: YAG laser pulses. Appl Surf Sci 255:4474–4478
Hashida M, Semerok AF, Gobert O, Petite G, Izawa Y (2002) Ablation threshold dependence on pulse duration for copper. Appl Surf Sci 197:862–867
Acknowledgements
We are grateful to Prof. Norihiro Nishiyama and former Prof. Takuji Ikemi of the Nihon University School of Density at Matsudo for their technical advice, and to all the staff of LEBRA for operating the machinery and assisting in the experiments. We also thank all the staff of the Department of Crown Bridge Prosthodontics for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Role of funding source
No outside funding was received for this study.
Ethical approval
The authors have nothing to disclose.
Informed consent
The authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Kuwada-Kusunose, T., Kusunose, A., Wakami, M. et al. Evaluation of irradiation effects of near-infrared free-electron-laser of silver alloy for dental application. Lasers Med Sci 32, 1349–1355 (2017). https://doi.org/10.1007/s10103-017-2251-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2251-5