Abstract
This study aimed to determine the effectiveness of photobiomodulation therapy (PBMT) and cryotherapy, in isolated and combined forms, as muscle recovery techniques after muscle fatigue-inducing protocol. Forty volunteers were randomly divided into five groups: a placebo group (PG); a PBMT group (PBMT); a cryotherapy group (CG); a cryotherapy-PBMT group (CPG); and a PBMT-cryotherapy group (PCG). All subjects performed four sessions at 24-h intervals, during which they submitted to isometric assessment (MVC) and blood collection in the pre-exercise period, and 5 and 60 min post-exercise, while the muscle fatigue induction protocol occurred after the pre-exercise collections. In the remaining sessions performed 24, 48, and 72 h later, only blood collections and MVCs were performed. A single treatment with PBMT and/or cryotherapy was applied after only 2 min of completing the post-5-min MVC test at the first session. In the intragroup comparison, it was found that exercise led to a significant decrease (p < 0.05) in the production of MVC in all groups. Comparing the results of MVCs between groups, we observed significant increases in the MVC capacity of the PBMT, CPG, and PCG volunteers in comparison with both PG and CG (p < 0.05). We observed a significant decrease in the concentrations of the biochemical markers of oxidative damage (TBARS and PC) in all groups and muscle damage (creatine kinase—CK) in the PBMT, PCG, and CPG compared with the PG (p < 0.01). The clinical impact of these findings is clear because they demonstrate that the use of phototherapy is more effective than the use of cryotherapy for muscle recovery, additionally cryotherapy decreases PBMT efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The practice of physical activity promotes health and quality of life, but there is a wide range of inherent risks associated with each type of sport as well as the physical demands that each sport imposes on its practitioners. Every athlete, at either the professional or the amateur level, is subject to injuries; thus, a rapid recovery is always desired to accelerate the return to sports activities. Therefore, therapies such as cryotherapy and phototherapy are used to rehabilitate and prevent injuries. Both therapies aim to decrease the duration of the muscle recovery period in between the game and/or the training sessions.
The use of cold therapy (cryotherapy) is one of the cheapest, most commonly recommended and used forms in the rehabilitation and treatment of acute injuries, pain of musculoskeletal origin, traumatic sports and other types of injuries, postoperative pain and edema, inflammatory processes, and muscle contractures [1]. According to Knight [2], the term cryotherapy means cold therapy, so any use of cold or ice for therapeutic purposes is defined as cryotherapy. Cryotherapy is widely used for muscle recovery after high-intensity exercise, but it can also be used to decrease the pain of musculoskeletal injuries, by reducing local tissue temperature, promoting local vasoconstriction, and decreasing both inflammation and edema [3]. In acute injuries, cold is indicated to minimize the inflammatory process, decrease metabolism and hypoxia secondary to injury, pain, and edema; during rehabilitation, cold decreases pain and muscle spasm enabling early mobilization [2].
Photobiomodulation therapy (PBMT) low-level laser therapy (LLLT) is the application of laser light (1–500 mW) to a pathologic condition and is applied by means of a light (usually low-powered laser and/or light emitting diodes—LEDs, with power between 1 and 500 mW) to a pathological or preventive clinical condition [4–7] and, unlike other procedures with a medical (surgical) laser, PBMT does not have ablative or thermal effects, but rather has photochemical effects in which light is absorbed and induces a chemical change in the tissues [8, 9]. PBMT is generally used to promote tissue regeneration, reduce swelling and inflammation, and relieve pain [5, 10]. The first randomized clinical trial to investigate its effects on disorders of the musculoskeletal system was carried out in the 1980s, in patients with rheumatoid arthritis [9]. Since the publication of this first clinical trial, additional positive effects of phototherapy have been identified in several other pathologies, such as osteoarthritis [11], tendinopathies [12, 13], back pain [14, 15], and neck pain [16, 17]. Thus, phototherapy presented a new form of therapy that has been used to treat muscular pain; however, the mechanisms responsible for the effects observed in clinical trials remain partially unclear [18].
Metabolism during contractile activity produces reactive oxygen species, which can cause the muscle to develop oxidative stress. This may be a factor associated with a reduction in contractile function and the development of muscle fatigue [7]. Skeletal muscle fatigue is characterized by a deficiency of the muscle’s ability to both generate and maintain force produced during muscle activity. In submaximal activities, skeletal muscle fatigue is denoted as a failure to continue the activity at its initial intensity. The development of muscle fatigue is a complex and multifaceted process involving many physiological and biomechanical elements, including the muscle fiber type, oxidative stress, and both the intensity and duration of the activity [19].
The use of PBMT before and after exercise has shown positive results in slowing skeletal muscle fatigue and improving skeletal muscle recovery in both athletes and non-athletes [4]. Some studies have compared the effects of cryotherapy (ice immersion and application) and PBMT in both rats [20, 21] and humans [22]. However, to our knowledge, no studies have compared the ability of these therapies to act in combination or the effectiveness of such a joint therapeutic intervention for upper limbs.
To address these issues, this study aims to determine the effectiveness of PBMT and cryotherapy, when used in both isolated and combined forms following muscle fatigue, induced by performing high-intensity exercise protocols having a predominance of eccentric contraction.
Methods
Ethical aspects
The study was approved by the Ethics Committee of the University of Caxias do Sul. In accordance with the Declaration of Helsinki, all subjects were advised about the procedure and they signed an informed consent prior to participation in the study (CAEE 31344214.3.3001.5341).
Subjects
Forty volunteers were selected for this study. The number of the participants was calculated using a statistical power of 80% and a significance level of p < 0.05 (or 5%). The individuals were recruited from among healthy physically active male volunteers aged between 19 and 29 years, from the University of Caxias do Sul. Exclusion criteria were any previous musculoskeletal injury in the previous 3 months and the use of any kind of nutritional supplements or pharmacological agents.
Randomization and blinding procedures
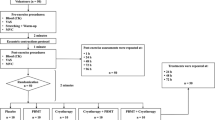
Prior to the study, volunteers were randomly divided into five groups: (1) placebo group (PG); (2) PBMT group (PBMT); (3) cryotherapy group (CG); (4) cryotherapy-PBMT group (CPG); and (5) PBMT-cryotherapy group (PCG). Individuals of all groups attended the Institute of Sports Medicine for four sessions, with 24-h intervals. On the first day, they were subjected to a muscle fatigue-inducing protocol (MFIP) and blood collection during the pre-exercise period, 5 min post-exercise, and 60 min post-exercise. In the remaining sessions performed 24, 48, and 72 h later, blood collection and isometric evaluation in the isokinetic dynamometer were repeated. To ensure the blind nature of the study, the researchers responsible for verbal stimulation during the performance of the isokinetic dynamometry protocol had no knowledge about the allocation of the volunteers in the groups, thereby ensuring impartiality during the evaluations. A single researcher, responsible for randomization and programming the PBMT equipment, was aware of the correct allocation of the volunteers in the groups.
Exercise protocol
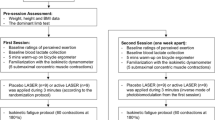
Initially, the volunteers were subjected to kinanthropometric measurements (body mass and height) and information about DOMS through the 100-mm visual analog scale (VAS). Next, the volunteers were properly positioned with their non-dominant upper limb in the position of evaluation by the isokinetic dynamometer Biodex System 4 Pro (Biodex Medical Systems, USA) according to the resolutions provided by the manufacturer for evaluating the forearm flexion movements. In the other sessions (evaluation points of muscle recovery), the measurements and positioning of the equipment remained the same.
The first part of the protocol on the isokinetic dynamometer consisted of determining the maximum isometric torque of the elbow flexors (biceps brachii). To this end, three maximal voluntary contractions (MVCs) were performed in the isometric mode, in the position of 45° of elbow flexion, having a 5-s duration and a 5-s interval between contractions. During the MVCs, a constant and standardized verbal stimulus was presented by the researchers. The maximum isometric torque value reached in the MVCs was considered the maximum capacity of power generation of the volunteer prior to exercise (PRE-MVC).
After determining the PRE-MVC, a period of 180 s of rest before the MFIP by eccentric exercise was allowed. After the rest period, the MFIP was initiated, consisting of five sets of 10 eccentric/concentric contractions of the elbow flexors separated by 30 s. The contractions were performed with an amplitude of 90° and speed of 90°.seg−1 for the eccentric contractions and 180°.seg−1 for concentric contractions. The guidance provided to the volunteers was to employ the highest strength possible to execute the elbow flexion movement and resist the elbow extension movement imposed by the dynamometer from the first to the last repetition.
Exactly 30 s after the MFIP, volunteers were subjected to a new MVC following the parameters of the MVCs prior to MFIP in relation to the limb’s position, the duration, and the verbal stimulation provided by the researchers. The value found in this isolated MVC will be considered the maximum capacity of power generation of the volunteer after the exercise (POST-MVC). The MVC will be evaluated 24 (MVC24), 48 (MVC48), and 72 (MVC72) hours after the execution of MFIP.
PBMT and cryotherapy
A single treatment with PBMT and/or cryotherapy was applied 2 min after the completion of the post-exercise MVC test. For the application of PBMT (Table 1), we used a cluster of 69 LEDs (34 red LEDs and 35 infrared LEDs), with 660 and 850 nm, 10 mW (red) and 30 mW (infrared) output power (each diode), manufactured by THOR® Photomedicine (London, UK). The application of PBMT was held with the cluster in direct contact with the skin, on the muscle belly of the biceps, as illustrated in Fig. 1. Thus, volunteers received phototherapy with the 41.7-J dose (30 s of irradiation) or 0 J—placebo (3 seconds of irradiation, but without effective irradiation). The choice of these parameters was based in a previous study that used this same PBMT device and observed positive outcomes in performance enhancement and in biochemical markers of recovery [23]. Cryotherapy was performed on the muscle belly of the biceps, with the patient lying down, using thermal bags containing ice cubes, and fixed on the segment with compression. As for duration of cryotherapy, there is good [24, 25] and fair evidence [26] that support cryotherapy should not exceed 20 min. Therefore, the application of ice was limited to 20 min total. The PBMT device was calibrated before and after data acquisition and the equipment showed the same power output in both calibrations. The optical power was measured using a Newport multifunction optical meter model 1835 C. The stability of the laser during the laser irradiation was measured collecting light with a partial reflect (4%).
Blood samples and biochemical assays
Blood samples were collected by a qualified nurse blinded to group allocation and were obtained from an antecubital vein before exercise and exactly 5 min, 60 min, 24 h, 48 h, and 72 h after the end of the exercise protocol. Blood was centrifuged at 2700×g for 10 min at 4 °C. Serum was immediately pipetted into Eppendorf tubes and stored at −80 °C until analysis. Lipid damages were measured spectrophotometrically (Shimadzu spectrophotometer Model UV-1700, Shimadzu®, Japan) by determining thiobarbituric acid reactive substances (TBARS), as previously described by Wills [27]. Results were expressed as nanomole per milliliter. The oxidative damage to proteins was assessed by determining carbonyl groups based on the reaction with 2,4-dinitrophenylhydrazine (DNPH), as previously described by Levine et al. [28]. Results were expressed as DNPH nanomole per milligram of protein. Total protein levels were evaluated using the Total Protein kit from Labtest® (Protein Kit, Labtest Diagnostica S.A., Brazil). Creatine kinase (CK) activity was measured by using a commercial kit (CK—Labtest®, Brazil). CK catalyzes the dephosphorylation of creatine phosphate to produce adenosine if thiotriphosphate, which reacts with glucose in the presence of hexokinase forming glucose-6-phosphate. Glucose-6-phosphate is acted on by glucose-6-phosphate dehydrogenase, is oxidized to phosphogluconate, and reduces NADP+ to NADPH. The rate of increase in absorbance at 340 nm is proportional to CK activity in the sample. Results were expressed as units per liter.
Statistical analysis
Data from the exercise protocol, oxidative stress, and muscle damage markers were expressed as mean and standard deviation (±SD) and tested statistically by an ANOVA and post hoc Tukey-Kramer and the significance level was set a p < 0.05. The software used was SPSS 18.0 for Windows.
Results
Volunteers in this study were 25.30 years old (±3.32), weighed 77.98 kg (±11.43), with a height of 176.55 cm (±5.55). The results of the MVCs (mean ± SD) containing the muscle recovery protocols are presented in Table 2 and Fig. 2. Initially, we observed that there were no significant differences between the groups in the pre-exercise evaluations in all the variables analyzed (MVC, TBARS, PC, CK, and DOMS). In an intragroup statistical test (pre and post comparison), it was found that exercise led to a significant decrease (p < 0.05) in the production of MVC after the fatigue protocol in all groups. Comparing the results of MVCs and DOMS between the groups, we observed that after treatment (from 1 to 72 h after), we obtained significant increases in the MVC capacity and decrease in DOMS (Fig. 3) of the volunteers who received treatment with PBMT, CPG, and PCG, compared with the PG and CG (p < 0.05). The CG showed no differences compared to the PG.
The concentrations of the biochemical marker of oxidative damage to lipids, as shown in Table 3, indicate that after treatment (from 1 to 72 h after), we obtained a significant decrease in TBARS concentrations in PBMT, CPG, and PCG, compared with the PG (p < 0.01). In the CG, we observed a significant decrease in TBARS concentrations at 1 h (p < 0.01), 48 h (p < 0.05), and 72 h (p < 0.01) after treatment. In addition, our results of the concentrations of the biochemical marker of oxidative damage to proteins indicate that after treatment (from 1 to 72 h after), we obtained a significant decrease in PC concentrations in the PBMT, CG and PCG, compared with the PG (p < 0.01) as shown in Table 4. In the CPG, we observed a significant decrease in PC concentrations in 24 to 72 h (p < 0.01) after treatment.
From the results found in the concentrations of the biochemical marker of muscle damage (CK) presented in Table 5 and Fig. 4, we can see that after treatment (from 1 to 72 h after), we obtained a significant decrease in CK concentrations in the PBMT, compared with the PG (p < 0.01). The PCG and CPG groups presented a significant decrease in CK concentrations in 48 and 72 h after treatment (p < 0.05 and p < 0.01, respectively).
Discussion
In the rehabilitation process, many therapeutic options are used in an associated manner, one after another, with virtually no recovery interval between their uses. The verification of its actual effects is rare and we found only one study when we searched the effects of PBMT and cryotherapy association in humans. To our knowledge, this is the first time that the synergistic effects of cryotherapy and PBMT have been tested in order to improve the performance of exercise and post-exercise muscle recovery for upper limbs. Some authors [7, 23] have shown the efficacy of both the PBMT dose and the duration of cryotherapy used in this study.
It is known that high-intensity exercises are associated with hyperthermia, energy depletion, muscle injury, oxidative stress, inflammation, and fatigue that lead to decreased performance due to both fatigue and the start of delayed-onset muscle soreness [29]. Prevention and treatment of such afflictions are important tools for the maintenance of exercise programs. The use of non-steroidal anti-inflammatory drugs, stretching, compression therapy, ultrasound, acupuncture, deep massage, nutritional supplements, antioxidants, and electrical stimulation have all been tested, with varying degrees of success, to reduce the symptoms of muscle injury, fatigue, and delayed-onset muscle soreness [29–34]. However, there is no consensus regarding the most appropriate method to prevent delayed-onset muscle pain and muscle injury effectively. Many studies [4, 7] have demonstrated the protective effects of PBMT when applied prior to exercise; thus, we used phototherapy and cryotherapy with resources to assist the muscle recovery process, and applied the modalities subsequent to performing MFIP.
We noted that PBMT has considerable potential not only for the prevention of muscle fatigue and damage caused by high-intensity exercises, but also can also improve performance conditions when applied post-exercise, in order to attain the goal of muscle recovery. Skeletal muscle is designed to withstand mechanical and metabolic overloads up to a certain limit. When stimulated, it rapidly reaches its maximum contraction load and increases oxygen flow up to 100%, which may lead to increased oxidative stress [35]. It is known that this phenomenon accompanies skeletal contractile activity [36] and may cause a decrease in the contractile function of the muscle groups involved and produce fatigue [37].
Cryotherapy has been widely used in sports to both prevent muscle injury and improve recovery [30, 38]. Therefore, it is not surprising that cryotherapy, despite not having demonstrated any effect on maintaining or increasing MVC after use in isolation, has demonstrated some effect on the reduction of markers of oxidative damage to lipids and proteins, probably through their known effects on vasoconstriction, reduction in muscle temperature, and inflammatory activity [39, 40]. This implies that the oxidative damage to lipids and proteins generated by the ischemia-reperfusion process may have been reduced by cryotherapy.
Associated with these findings, it is important to highlight that cryotherapy had no influence on maintaining the MVC capacity, having a behavior similar to the placebo group from the time of its application. Previous studies report that cryotherapy can reduce nerve conduction velocity by not only changing the perception of pain but also interfering with the recruitment of motor units [41]. In contrast, the groups that received the PBMT application exhibited a significant improvement in MVC after 60 min after the application of the muscle recovery protocol. The results obtained by cryotherapy in reducing markers of oxidative damage to lipids and proteins have also been reached in the group that received only the application of PBMT; moreover, this group had a significant decrease in the marker of muscle damage (CK), which was not observed in the cryotherapy group. Similar results have been reported in studies conducted in animals [20, 21, 42], where the use of cold water immersion and cryotherapy proved ineffective in providing effective muscle recovery, while the use of PBMT was capable of improving muscle condition 24 h after exercise.
Another interesting factor is the fact that joint application of therapeutic interventions has not shown much relevance, as indicated by the results achieved with joint application. For example, regardless of the application order, combined application of therapeutic interventions is not seen to be more effective than the individual application of PBMT and it corroborates with recent literature [43].
Recently, Albuquerque-Pontes et al. [44] have demonstrated that one single irradiation with PBMT is capable of increasing the activity of cytochrome c-oxidase in intact skeletal muscle tissue up to 24 h after irradiation, and this up-regulation is dependent of dose and wavelength. Furthermore, the combined use of three wavelengths is beneficial for this aim [45–50]. This study [44] shows that PBMT plays a leading role in the self-regulation of mitochondrial activity by increasing the mitochondrial respiratory chain. This, in turn, consequently increases the production of ATP in muscle cells and leads to the reduction of oxidative stress and subsequently to the production of reactive oxygen species (ROS). It is important to emphasize that, in this study, uninjured animal muscles were irradiated.
The results observed lead to the discussion of using cryotherapy as a tool to speed recovery; therefore, more studies need to be conducted to confirm this hypothesis. However, the clinical impact of these findings is obvious because they demonstrate that the use of PBMT is more effective than the use of cryotherapy for muscle recovery. It is worth remembering that only one session of each mode was performed, that is, the potential shown by PBMT may be even higher if treatment is continued throughout the same week.
Conclusion
Based on the above results and discussion, this study demonstrates that the application of cryotherapy associated with PBMT does not improve the effects of the application of PBMT, so the isolated application of PBMT seems to be the best option to improve muscle recovery in both the short and long term. In contrast, the use of cryotherapy in isolation was unable to provide muscle recovery. Additional field studies should be performed to optimize dose parameters for differences in elite and recreational athletic recovery and to examine the long-term effects of PBMT.
References
Hohenauer E, Taeymans J, Baeyens JP, Clarys P, Clijsen R (2015) The effect of post-exercise cryotherapy on recovery characteristics: a systematic review and meta-analysis. PLoS One 28(10):e0139028
Knight KL (1995) Cryotherapy in sport injury management. Human Kinetics, Champaign
Jakeman JR, Macrae R, Eston R (2009) A single 10-min bout of cold-water immersion therapy after strenuous plyometric exercise has no beneficial effect on recovery from the symptoms of exercise-induced muscle damage. Ergonomics 52:456–460
Leal Junior ECP, Vanin AA, Miranda EF, Carvalho PTC, Dal Corso S, Bjordal JM (2015) Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci 30:925–939
de Almeida P, Lopes-Martins RA, De Marchi T, Tomazoni SS, Albertini R, Corrêa JC, Rossi RP, Machado GP, da Silva DP, Bjordal JM, Leal JEC (2012) Red (660nm) and infrared (830 nm) low-level laser therapy in skeletal muscle fatigue in humans: what is better? Lasers Med Sci 27:453–458
Leal JEC, Lopes-Martins RA, Frigo L, De Marchi T, Rossi RP, de Godoi V, Tomazoni SS, da Silva DP, Basso M, Lotti FP, Corsetti FV, Iversen VV, Bjordal JM (2010) Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to post-exercise recovery. J Orthop Sports PhysTher 40:524–532
De Marchi T, Leal JEC, Bortoli C et al (2012) Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status and oxidative stress. Lasers Med Sci 27:231–236
Huang YY, Chen AC, Carroll JD, Hamblin MR (2009) Biphasic dose response in low-level light therapy. Dose Response 7:358–383
Karu TI (1987) Photobiological fundamentals of low-power laser therapy. IEEE J Quantum Electron 23:1703–1719
Goldman JA, Chiapella J, Casey H, Bass N, Graham J, McClatchey W, Dronavalli RV, Brown R, Bennett WJ, Miller SB, Wilson CH, Pearson B, Haun C, Persinski L, Huey H, Muckerheide M (1980) Laser therapy of rheumatoid arthritis. Lasers Surg Med 1:93–101
Hegedus B, Viharos L, Gervain M, Gálfi M (2009) The effect of low-level laser in knee osteoarthritis: a double-blind, randomized, placebo-controlled trial. Photomed Laser Surg 27:577–584
Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA (2006) Photoradiation in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg 24:158–168
Stergioulas A, Stergioula M, Aarskog R, Lopes-Martins RA, Bjordal JM (2008) Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic achilles tendinopathy. Am J Sports Med 36:881–887
Basford JR, Sheffield CG, Harmsen WS (1999) Laser therapy: a randomized, controlled trial of the effects of low-intensity Nd:YAG laser irradiation on musculoskeletal back pain. Arch Phys Med Rehabil 80:647–652
Konstantinovic LM, Cutovic MR, Milovanovic AN, Jovic SJ, Dragin AS, Letic MD, Miler VM (2010) Low-level laser therapy for acute neck pain with radiculopathy: a double-blind placebo-controlled randomized study. Pain Med 11:1169–1178
Chow RT, Johnson MI, Lopes-Martins RA et al (2009) Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 374:1897–1908
Chow RT, Heller GZ, Barnsley L (2006) The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain 124:201–210
Lopes-Martins RA, Marcos RL, Leonardo PS et al (2006) Effect of low level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical simulation in rats. J Appl Physiol 101:283–288
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74:49–94
Camargo MZ, Siqueira CP, Preti MC, Nakamura FY, de Lima FM, Dias IF, Toginho F, Dde O, Ramos Sde P (2012) Effects of light emitting diode (LED) therapy and cold water immersion therapy on exercise-induced muscle damage in rats. Lasers Med Sci 27:1051–1058
da Costa Santos VB, de Paula Ramos S, Milanez VF, Corrêa JC, de Andrade Alves RI, Dias IF, Nakamura FY (2014) LED therapy or cryotherapy between exercise intervals in Wistar rats: anti-inflammatory and ergogenic effects. Lasers Med Sci 29:599–605
Leal Junior EC, de Godoi V, Mancalossi JL et al (2011) Comparison between cold water immersion therapy (CWIT) and light emitting diode therapy (LEDT) in short-term skeletal muscle recovery after high-intensity exercise in athletes: preliminary results. Lasers Med Sci 26:493–501
Leal Junior EC, Lopes-Martins RA, Rossi RP, De Marchi T, Baroni BM, de Godoi V, Marcos RL, Ramos L, Bjordal JM (2009) Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers Surg Med 41:572–577
Graham CA, Stevenson J (2000) Frozen chips: an unusual cause of severe frostbite injury. Br J Sports Med 34:382–383
Moeller JL, Monroe J, McKeag DB (1997) Cryotherapy-induced common peroneal nerve palsy. Clin J Sport Med 7:212–216
Bassett FH 3rd, Kirkpatrick JS, Engelhardt DL, Malone TR (1992) Cryotherapy-induced nerve injury. Am J Sports Med 20:516–518
Wills ED (1996) Mechanism of lipid peroxide formation in animal tissues. Biochem J 99:667–676
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Petrofsky JS, Khowailed IA, Lee H, Berk L, Bains GS, Akerkar S, Shah J, Al-Dabbak F, Laymon MS (2015) Cold vs. heat after exercise—is there a clear winner for muscle soreness. J Strength Cond Res 29:3245–3252
Leeder J, Gissane C, van Someren K, Gregson W, Howatson G (2012) Cold water immersion and recovery from strenuous exercise: a meta-analysis. Br J Sports Med 46:233–240
Cheung K, Hume P, Maxwell L (2003) Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med 33:145–164
Zainuddin Z, Newton M, Sacco P, Nosaka K (2005) Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. J Athl Train 40:174–180
Stay JC, Richard MD, Draper DO, Schulthies SS, Durrant E (1998) Pulsed ultrasound fails to diminish delayed-onset muscle soreness symptoms. J Athl Train 33:341–346
Arent SM, Pellegrino JK, Williams CA, Difabio DA, Greenwood JC (2010) Nutritional supplementation, performance, and oxidative stress in college soccer players. J Strength Cond Res 24:1117–1124
Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL (2000) Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc 32:1576–1581
Fukuda S, Nojima J, Motoki Y, Yamaguti K, Nakatomi Y, Okawa N, Fujiwara K, Watanabe Y, Kuratsune H (2016) Um biomarcador potencial de fadiga : o estresse oxidativo e anti- oxidativo atividade. Biol Psychol 118:88–93
Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS (1992) Reactive oxygen in skeletal muscle. I. intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 73:1797–1804
Ascensão A, Leite M, Rebelo AN, Magalhäes S, Magalhäes J (2011) Effects of cold water immersion on the recovery of physical performance and muscle damage following a one-off soccer match. J Sports Sci 29:217–225
Bailey DM, Erith SJ, Griffin PJ, Dowson A, Brewer DS, Gant N, Williams C (2007) Influence of cold-water immersion on indices of muscle damage following prolonged intermittent shuttle running. J Sports Sci 25:1163–1170
Pournot H, Bieuzen F, Duffield R, Lepretre PM, Cozzolino C, Hausswirth C (2011) Short term effects of various water immersions on recovery from exhaustive intermittent exercise. Eur J Appl Physiol 111:1287–1295
García-Manso JM, Rodríguez-Matoso D, Rodríguez-Ruiz D, Sarmiento S, de Saa Y, Calderón J (2011) Effect of cold-water immersion on skeletal muscle contractile properties in soccer players. Am J Phys Med Rehabil 90:356–363
de Almeida P, Tomazoni SS, Frigo L, de Carvalho PT, Vanin AA, Santos LA, Albuquerque-Pontes GM, De Marchi T, Tairova O, Marcos RL, Lopes-Martins RÁ, Leal-Junior EC (2014) What is the best treatment to decrease pro-inflammatory cytokine release in acute skeletal muscle injury induced by trauma in rats: low-level laser therapy, diclofenac, or cryotherapy? Lasers Med Sci 29:653–658
de Paiva PR, Tomazoni SS, Johnson DS, Vanin AA, Albuquerque-Pontes GM, Machado CD, Casalechi HL, de Carvalho PT, Leal-Junior EC (2016) Photobiomodulation therapy and/or cryotherapy in skeletal muscle restitution, what is better? A randomized, double-blinded, placebo-controlled clinical trial. Lasers Med Sci 31:1925–1933
Albuquerque-Pontes GM, Vieira RP, Tomazoni SS, Caires CO, Nemeth V, Vanin AA, Santos LA, Pinto HD, Marcos RL, Bjordal JM, de Carvalho PDT, Leal-Junior EC (2015) Effect of pre-irradiation with different doses, wavelengths, and application intervals of low-level laser therapy on cytochrome c oxidase activity in intact skeletal muscle of rats. Lasers Med Sci 30:59–66
Santos LA, Marcos RL, Tomazoni SS, Vanin AA, Antonialli FC, Grandinetti V.dos S, Albuquerque-Pontes GM, de Paiva PR, Lopes-Martins RÁ, de Carvalho PDT, Bjordal JM, Leal-Junior EC (2014) Effects of pre-irradiation of low-level laser therapy with different doses and wavelengths in skeletal muscle performance, fatigue, and skeletal muscle damage induced by tetanic contractions in rats. Lasers Med Sci 29:1617–1626
Antonialli FC, De Marchi T, Tomazoni SS, Vanin AA, dos Santos Grandinetti V, de Paiva PR, Pinto HD, Miranda EF, de Tarso Camillo de Carvalho P, Leal-Junior EC (2014) Phototherapy in skeletal muscle performance and recovery after exercise: effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med Sci 29:1967–1976
Grandinétti V.dos S, Miranda EF, Johnson DS, de Paiva PR, Tomazoni SS, Vanin AA, Albuquerque-Pontes GM, Frigo L, Marcos RL, de Carvalho PDT, Leal-Junior EC (2015) The thermal impact of phototherapy with concurrent super-pulsed lasers and red and infrared LEDs on human skin. Lasers Med Sci 30:1575–1581
Miranda EF, Vanin AA, Tomazoni SS, Grandinetti V.dos S, de Paiva PR, Machado CS d, Monteiro KK, Casalechi HL, de Tarso PD, Carvalho C, Leal-Junior EC (2016) Using pre-exercise photobiomodulation therapy combining super-pulsed lasers and light-emitting diodes to improve performance in progressive cardiopulmonary exercise tests. J Athl Train 51:129–135
Vanin AA, Miranda EF, Machado CS, de Paiva PR, Albuquerque-Pontes GM, Casalechi HL, de Tarso Camillo de Carvalho P, Leal-Junior EC (2016) What is the best moment to apply phototherapy when associated to a strength training program? a randomized, double-blinded, placebo-controlled trial: phototherapy in association to strength training. Lasers Med Sci 31:1555–1564
Pinto HD, Vanin AA, Miranda EF, Tomazoni SS, Johnson DS, Albuquerque-Pontes GM, Junior AIO, Grandinetti VD, Casalechi HL, de Carvalho PT, Leal Junior EC (2016) Photobiomodulation therapy improves performance and accelerates recovery of high-level rugby players in field test: a randomized, crossover, double-blind, placebo-controlled clinical study. J Strength Cond Res 30:3329–3338
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Professor Ernesto Cesar Pinto Leal-Junior receives research support from Multi Radiance Medical (Solon, OH - USA), a laser device manufacturer. Multi Radiance Medical had no role in the planning of this study, and the laser device used was not theirs. They had no influence on study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that they have no conflict of interests.
Ethical aspects
The study was approved by the Ethics Committee of the University of Caxias do Sul. In accordance with the Declaration of Helsinki, all subjects were advised about the procedure and they signed an informed consent prior to participation in the study (CAEE 31344214.3.3001.5341).
Rights and permissions
About this article
Cite this article
De Marchi, T., Schmitt, V.M., Machado, G.P. et al. Does photobiomodulation therapy is better than cryotherapy in muscle recovery after a high-intensity exercise? A randomized, double-blind, placebo-controlled clinical trial. Lasers Med Sci 32, 429–437 (2017). https://doi.org/10.1007/s10103-016-2139-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2139-9