Abstract
The aim of this study was to evaluate, in vitro, the bactericidal effect of antimicrobial photodynamic therapy (AmPDT) using phenothiazinium dyes (Toluidine Blue O and methylene blue, 1:1) using different concentrations (100, 50, 25, 12.5, and 6.25 μg/mL) associated to red laser with different energy densities (2.4, 4.8, 7.2, 9.6, and 12 J/cm2) on a strain of Staphylococcus aureus (ATCC 23529). On this study, tests were performed in triplicate and the samples were distributed into 36 test groups: Control and bacterial suspensions were irradiated with the different energy densities, respectively, in the absence of photosensitizer, bacterial suspensions were irradiated with the laser in the different concentrations of the photosensitizer, and finally bacterial suspensions only in the presence of phenothiazinium dye. The pre-irradiation time was 5 min. Therefore, we analyzed the potential of the AmPDT by counting colony-forming units. The logarithm of CFU/mL (log10 CFU/mL) was calculated and the data was analyzed statistically (ANOVA, Tukey’s test, p < 0.05). The results showed that the association 50 and 100 μg/mL with 12 J/cm2 showed the highest percentage of inhibition (100 %). Based upon the present results, it may be concluded that the AmPDT was able to enhance the antimicrobial effect of phenothiazines and both concentration of the compound and energy density are important factors for greater effectiveness of therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resistance to antibiotics is a serious public health problem and different alternative treatments have been tested, including the use of AmPDT [1]. AmPDT is a procedure that may be carried out on both sensitive and antibiotic-resistant bacteria causing inactivation of the strains. This procedure has shown itself advantageous, as it does not induce bacterial selection (resistance observed during the treatment with antibiotics) [2, 3]. Staphylococcus spp. are capable of developing resistance to antibiotics and are opportunistic microorganisms. Resistant strains, such as methicillin-resistant Staphylococcus aureus (MRSA), are a major public health challenge. MRSA is a major concern in nosocomial infections worldwide and is also currently prevalent in residential homes [4, 5]. Staphylococcus is one of the most important causes of nosocomial infections and is often disseminated by medical devices. These microorganisms are protected by a biofilm that causes resistance to phagocytosis, hindering immune system functions and antibiotics activity. Therefore, their virulence is closely related to the biofilm [6].
AmPDT combines the use of a nontoxic photosensitizer combined with a non-ionizing visible light, in which wavelength has to be effective to excite the photosensitizer to a reactive triplet state. This reaction will generate singlet oxygen and superoxide that are highly toxic to the cells reactive oxygen species (ROS). AmPDT has been suggested as a therapeutic option for the treatment of infectious diseases [7]. ROS may damage both the DNA and cell membranes causing loss of cell compartmentation, inactivation of transport systems, and cell death [8, 9]. Up to now phenothiazinium salts, such as Toluidine Blue O (TBO) and methylene blue (MB) are used clinically on antimicrobial treatments [10]. The minimal toxicity of these dyes to human cells, plus their ability to produce high quantum yields of singlet oxygen, has produced a great interest in testing the potential of these photosensitizers as photo-activated antimicrobial agents [11]. The optimized physicochemical properties of photosensitizers as well as specific delivery systems will decide whether AmPDT for MRSA infection could be accepted as an alternative way to traditional antibiotic therapy. After further well-designed preclinical and clinical studies, this novel therapeutic approach for treating MRSA infection could be routinely established in clinical practices [12]. The challenge in AmPDT is to find a therapeutic protocol in which hazardous bacteria maybe efficiently inactivated without harming the surrounding tissue or disturbing the local microenvironment at a given concentration and light dose [12].
It was hypothesized that the use of an efficacious protocol of AmPDT, in vitro, could be transferred to an effective clinical treatment of bacterial infections. Therefore, the aim of this study was to evaluate, in vitro, the bactericidal effect of AmPDT on S. aureus (ATCC 23529 strain) using different concentrations (100, 50, 25, 12.5, and 6.25 μg/mL) of a mixture of two phenothiazinium dyes (50 % MB + 50 % TBO) associated to the use of red laser light at different energy densities (12, 9.6, 7.2, 4.8, and 2.4 J/cm2).
Methodology
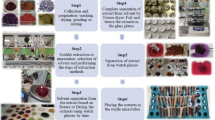
Bacterial strain and culture condition
The bacterial strain used in this study was S. aureus (ATCC 23529) aerobically cultured in blood agar (Merck® Darmstadt, Hessen, Germany) at 37 °C and grown for 24 h. For the experiments, colonies were collected with the aid of a calibrated loop of 100 μL and inoculated into 5 mL of tryptic soy broth (Merck® Darmstadt, Hessen, Germany). For the quantification of colony-forming units (CFU), the suspension was standardized by measuring absorbance using the SpectraMax spectrophotometer (Medical Device) to an optical density of 0.5 McFarland at λ625 nm, corresponding to approximate number 3 × 108 CFU/mL. Subsequently, 10 μL of this suspension were inoculated in 1 mL of TSB (Merck® Darmstadt, Hessen, Germany) in a 24-well culture plate (Falcon®, BD Lab., Franklin Lakes, NJ, USA). After this dilution, each concentration of the photosensitizer was added and irradiated following experimental protocol.
Photosensitizer and light source
A mixture of 50 % of Toluidine Blue O + 50 % methylene blue (A Fórmula Laboratory, Salvador, BA, Brazil) was used for photosensitization of the S. aureus strains. Solutions of different concentrations (100, 50, 25, 12.5, and 6.25 μg/mL) were prepared in sterile phosphate-buffered saline, pH 7.4 and filtering it through a 0.22-μm membrane (Millipore, São Paulo, SP, Brazil). After filtration, the solution was stored in the dark at 4 °C before use. A diode laser (λ660 nm, Twin Flex®, MMOptics, São Carlos, SP, Brazil) was used as the light source (Table 1). The wavelength of the laser corresponded to the maximum absorption of phenothiazinium dye [11].
Antimicrobial photodynamic therapy
Sample distribution is summarized on Table 2. The bacterial suspensions were platted into the 24-well culture plates as shown on Table 2 and incubated in the dark at room temperature for 5 min. After pre-irradiation time (5 min), the bacterial suspensions, with and without photosensitizer, were irradiated according to different energy densities. Immediately after the irradiation, the contents of the wells were mixed before sampling and were seeded in triplicate onto Petri plates divided into four fields containing TSA medium (Merck® Darmstadt, Hessen, Germany) and incubated at 37 °C for 24 h using a calibrated 100 μL loop bacteria. After incubation (24 h), the number of CFU was determined by counting. Statistical analysis was carried out (ANOVA GLM and Tukey’s multiple comparison tests, Graphic Prism® Software 4.0, p < 0.05 was considered statistically significant).
Results
Comparison between the laser and control groups showed no statistical difference (Fig. 1). However, the photosensitizers groups when compared to the control group showed a significant (p < 0.0001) reduction of the count for all given concentration except for the 6.25 μg/mL. The inhibition percentages were as follows: 100 μg/mL (59.4 %), 50 μg/mL (57.0 %), 25 μg/mL (28.0 %), 12.5 μg/mL (17.2 %), and 6.25 μg/mL (0.002 %) (Fig. 2) (Table 3).
Group AmPDT using 6.25 μg/mL showed a significant statistical reduction of the counting in comparison to the control group. The inhibition percentages varied according to the energy density used: 2.4 J/cm2 (2.4 %) (p < 0.01), 4.8 J/cm2 (4.3 %) (p < 0.001), 7.2 J/cm2 (5.6 %) (p < 0.0001), 9.6 J/cm2 (6.0 %) (p < 0.0001), and 12 J/cm2 (8.9 %) (p < 0.0001). Increasing the concentration to 12.5 μg/mL also caused a significant reduction on the bacterial count (p < 0.0001). The inhibition percentages also varied according to the energy density used: 2.4 J/cm2 (22.1 %), 4.8 J/cm2 (22.8 %), 7.2 J/cm2 (25.4 %), 9.6 J/cm2 (29.0 %), and 12 J/cm2 (34.2 %). The use of 25 μg/mL also showed a significant reduction (p < 0.0001). Again, different inhibition percentages were observed: 2.4 J/cm2 (50.6 %), 4.8 J/cm2 (51.7 %), 7.2 J/cm2 (50.8 %), 9.6 J/cm2 (52.2 %), and 12 J/cm2 (53.3 %). Further increase of the concentration to 50 μg/mL also significantly reduced the number of colonies (p < 0.0001) being different inhibition percentages for each energy density: 2.4 J/cm2 (66.1 %), 4.8 J/cm2 (65.7 %), 7.2 J/cm2 (67.8 %), 9.6 J/cm2 (88.6 %), and 12 J/cm2 (100 %). The use of the highest concentration (100 μg/mL) showed the same pattern as in lower ones (p < 0.0001). For this concentration, the inhibition percentages were as follows: 2.4 J/cm2 (68.2 %), 4.8 J/cm2 (77.3 %), 7.2 J/cm2 (74.0 %), 9.6 J/cm2 (95.6 %), and 12 J/cm2 (100 %).
On the other hand, in relation to energy densities, in using 2.4 J/cm2, a significant difference on the counting was observed for all concentrations (p < 0.0001) in comparison to the control group, except for the concentration of 6.25 μg/mL, that the inhibition percentage was 2.4 %, as previously reported. Increasing the energy density to 4.8 J/cm2, 7.2 J/cm2, or 9.6 J/cm2 shows the same pattern with a significant reduction on the counting for all concentrations (p < 0.0001), except for 6.25 μg/mL, that the inhibition percentages for each energy density were, respectively, 4.3, 5.6, and 6.0 %, as previously reported too. Finally, using 12 J/cm2 significantly reduced the inhibition percentages for all concentrations (p < 0.0001), with the following percentages of inhibition: 100 μg/mL (100 %), 50 μg/mL (100 %), 25 μg/mL (53.3 %), 12.5 μg/mL (34.2 %), and 6.25 μg/mL (8.9 %) (Fig. 3).
Discussion
The use of phenothiazinium dyes as photosensitizers after irradiation with visible light has been shown in several previous studies. However, the results of AmPDT are different according to the cell conditions (density, culture medium, Gram-positive or -negative bacteria, species, physiological state, etc.), to the photosensitizer (concentration, period of incubation), and light (type, energy density, wavelength, etc.) [12–14].
AmPDT is a promising therapy and presenting positive results even against resistant microbial strains, however, is essential to establish an appropriate protocol for its usage clinically. Thus, based on this demand, the present study was carried out aiming to verify the efficacy of several protocols associated with the use of different power densities and concentrations of the compound. Therefore, after evaluation of these data, it was possible to set appropriate conditions for the in vivo study; such concerns about the ideal conditions can also be observed in the study by Tonon et al., in 2015 [15], which shows the need to establish the optimal protocol for AmPDT used curcumin against the Streptococcus mutans.
The choice of concentrations was based from previous studies in the literature, which indicated that the concentration of 100 μg/mL of phenothiazine is effective in photodynamic therapy protocols [16]. In the study by Garcia et al., concentrations above 100 μg/mL were considered not effective. It was then decided to test lower concentrations with a maximum concentration of 100 μg/mL. The concentrations below 100 μg/mL used in this study were obtained from a serial dilution, thereby obtaining four minor concentrations. Besides, the effect of the concentration of the compound on the photodynamic effect also depends on the energy density as shown by Tonon et al. in [15].
On the present investigation, it was opted to use a log transformation of the data. When using this transformation, one must remember that the result of the transformation corresponds to the geometric mean and not to the averaged mean and most studies on this specific topic did not use log transformation. It is also important to observe that a fundamental problem is that there is little value in comparing the variability of original versus log-transformed data because they are on totally different scales. This would make a tricky comparison of the results of the present investigation with previous reports on the literature.
This may be the cause of the lack of a significant statistical difference between laser-irradiated strains and their controls as the sole use of the laser light at different energy densities did not cause any statistically significant changes on the bacterial count (Fig. 1) under the conditions of the present study when both adequate irradiation protocol and culture conditions were used.
The literature is controversial concerning the effects of laser on bacterial growth. The stimulation or inhibition of photoreceptor functions, which is part of the cellular respiratory chain, determines the magnitude of cell proliferation or inhibition. The irradiation dose and the energy density are the most important parameters in photobiomodulation [17, 18].
According to Chan and Lai [19], it is obvious that the bactericidal effect is wavelength dependent. In relation to the use of phenothiazines or porphyrins, the existence of effective absorption of light when using wavelengths above λ600 ηm [20]is known; therefore, in the present study, we used a diode laser emitting light at λ660 ηm as the light source as this wavelength corresponds to the maximum absorption of the phenothiazinium dye.
In relation to the dosimetry, the present study tested five different energy densities for light (12, 9.6, 7.2, 4.8, and 2.4 J/cm2) using the same parameters of cell and photosensitizer, precisely in order to obtain an efficacious protocol of AmPDT, in vitro, that could be transferred to an effective clinical treatment of bacterial infections.
Actually, phenothiazinium dyes are used clinically for antimicrobial treatments, because the minimal toxicity to human cells and their ability to produce high quantum of singlet oxygen [10, 11]. The results of the present study, in relation to the action of photosensitizer against S. aureus, demonstrated, for all concentrations used (100, 50, 25, and 12.5 μg/mL) when compared to the control group, a statistically significant decrease (p < 0.0001) on microorganisms counts, except when using the concentration of 6.25 μg/mL. This demonstrated that the sole use of the dye in concentrations higher than or equal to 12.5 μg/mL resulted in a significant reduction on bacterial counts in comparison to the control group. However, a previous study [21] tested the toxicity of phenothiazinium dyes against methicillin-resistant S. aureus (ATCC 25923) and multi-drug resistant Escherichia coli (ATCC 25922) showing that concentration of the photosensitizer did not have antimicrobial toxicity when incubated in the dark with any of the organisms for 30 min, showing no significant difference when compared with the control groups (p > 0.05).
Several previous studies on the effects of photodynamic therapy on bacterial growth that the both bactericidal or bacteriostatic are related to the absorption of the laser light by chromophores causing conformational changes in certain molecules, generating free radicals and reactive oxygen species which will promote the rupture of bacterial membranes [22–24].
In the present study, the results of the AmPDT showed that the use of the laser light increased the effectiveness of the dye as seen when comparing with groups kept in the dark. Using the same concentration of photosensitizer, it was also observed that increasing the energy density resulted in increased bactericidal effects, except when using a concentration of 25 μg/mL that showed a significant reduction (p < 0.0001) for all conditions in comparison to the control group. However, comparing different energy densities between them, a significant increase on inhibition percentage was not detected: 2.4 J/cm2 (50.6 %), 4.8 J/cm2 (51.7 %), 7.2 J/cm2 (50.8 %), 9.6 J/cm2 (52.2 %), and 12 J/cm2 (53.3 %).
Although it did not show the 100 % of inhibition compared to the control group, the combination of concentration 12.5 μg/mL with the laser energy density of 12 J/cm2 should be highlighted as the sole use of the photosensitizer showed inhibition on 17.2 % and the association with the light doubled the inhibition (34.2 %). The concentrations of 50 and 100 μg/mL used solely resulted in a significant reduction on bacterial counts in around 57 % (50 μg/mL) and 59.6 % (100 μg/mL), but the association with the laser energy density of 12 J/cm2 showed 100 % inhibition when compared to the control group in both cases. For this reason, it considered the use of lower concentration (50 μg/mL) since they showed the same result.
Conclusion
Based upon the present results, it may be concluded that the AmPDT was able to enhance the antimicrobial effect of phenothiazines and both concentrations of the compound and energy density are important factors for greater effectiveness of the therapy.
References
Tegos GP, Masago K, Aziz F, Higginbotham AA, Stermitz FR, Hamblin MR (2008) Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob Agents Chemother 52:3202–3209. doi:10.1128/aac.00006-08
Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA (1998) Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett 160:177–81. doi:10.1111/j.1574-6968.1998.tb12908.x
Lauro FM, Pretto P, Covolo L, Jori G, Bertoloni G (2002) Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene–polylysine conjugates. Photochem Photobiol Sci 1:468–470. doi:10.1039/b200977C
Ayliffe GAJ, Casewell MSC, Cookson BD, Cox RA, Duckworth GJ, French GL, Griffiths-Jones AG, Heathcock R, Humphreys H, Keane CT, Marples RR, Shanson DC, Slack R, Tebbs E (1998) Revised guidelines for the control of methicillin-resistant Staphylococcus aureus infection in hospitals. J Hosp Infect 39:253–290. doi:10.1016/S0195-6701(98)90293-6
Gould IM (2006) Community-acquired MRSA: can we control it? Lancet 368:824–826. doi:10.1016/S0140-6736(06)69303-3
Sharma M, Visai L, Bragheri F, Cristiani I, Gupta PK, Speziale P (2008) Toluidine Blue-mediated photodynamic effects on staphylococcal biofilms. Antimicrob Agents Chemother 52:299–305. doi:10.1128/AAC.00988-07
Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy. Part one. Photosensitizers, photochemistry and cellular localization. Photodiag Photodyn Ther 1:79–93. doi:10.1016/S1572-1000(05)00007-4
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42:13–28. doi:10.1093/jac/42.1.13
Hamblin MR, Hasan T (2004) Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3:436–450. doi:10.1039/b311900a
Wainwright M (2003) The use of dyes in modern biomedicine. Biotech Histochem 78:147–155. doi:10.1080/10520290310001602404
Wainwright M (2005) The development of phenothiazinium photosensitisers. Photodiagn Photodyn Ther 2:263–272. doi:10.1016/S1572-1000(05)00110-9
Fu X, Fang Y, Yao M (2013) Antimicrobial photodynamic therapy for methicillin-resistant Staphylococcus aureus infection. BioMed Res Intern 2013:1–9. doi:10.1155/2013/159157
Demidova TN, Hamblin MR (2005) Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother 49:2329–35. doi:10.1128/AAC.49.6.2329-2335.2005
Kashef N, Abadi GRS, Djavid GE. Phototoxicity of phenothiazinium dyes against methicillin-resistant Staphylococcus aureus and multi-drug resistant Escherichia coli. Photodiagnosis and Photodynamic Therapy 9:11-15. doi: 10.1016/j.pdpdt.2011.11.004
Tonon CC, Paschoal MA, Correia M, Spolidório DMP, Bagnato VS, Giusti JSM, Santos-Pinto L (2015) Comparative effects of photodynamic therapy mediated by curcumin on standard and clinical isolate of Streptococcus mutans. J Contemparary Dental Pratice 16:1–6. doi:10.5005/jp-journals-10024-1626
Garcia VG, Longo M, Gualberto Júnior EC, Bosco AF, Nagata MJ, Ervolino E, Theodoro LH (2014) Effect of the concentration of phenothiazine photosensitizers in antimicrobial photodynamic therapy on bone loss and the immune inflammatory response of induced periodontitis in rats. J Periodontal Res 49:584–94. doi:10.1111/jre.12138
Kipshidze N, Nikolaychik V, Keelan MH, Shankar LR, Khanna A, Kornowski R, Leon M, Moses J (2001) Low-power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med 28:355–364. doi:10.1002/lsm.1062
Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D (2009) Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg 27:221–226. doi:10.1089/pho.2008.2413
Chan Y, Lai C (2003) Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med Sci 18:51–55. doi:10.1007/s10103-002-0243-5
Maisch T (2007) Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci 22:83–91. doi:10.1007/s10103-006-0409-7
Kashef N, Abadia GRS, Djavid GE (2012) Phototoxicity of phenothiazinium dyes against methicillin-resistant Staphylococcus aureus and multi-drug resistant Escherichia coli. Photodiagn Photodyn Ther 9:11–15. doi:10.1016/j.pdpdt.2011.11.004
DeSimone NA, Hristiansen C, Dore D (1999) Bactericidal effect of 0.95-mw helium-neon and 5-mw indiumgallium-aluminum-phosphate laser irradiation at exposure times of 30, 60, and 120 seconds on photosensitized Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Phys Ther 79:839–846
Kawamoto K, Senda N, Shimada K, Itol K, Hirano Y, Murai S (2000) Antibacterial effect of yellow He-Ne laser irradiation with crystal violet solution on Porphyromonas gingivalis: an evaluation using experimental rat model involving subcutaneous abscess. Lasers Med Sci 15:257–262. doi:10.1007/PL00011325
Karu TI (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B 49:1–17. doi:10.1016/S1011-1344(98)00219-X
Acknowledgments
This study was supported by the National Program of Post-Doctoral Fellowship (Edital CAPES-PNPD/2011) through a Post-Doctoral Fellowship Grant (LGPS); Bahia State Research Foundation – FAPESB through a Post-Doctoral Fellowship Grant (SCPSO); and Brazilian National Research Council – CNPq through a Research Grant (ALBP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Monteiro, J.S.C., de Oliveira, S.C.P.S., Pires Santos, G.M. et al. Effectiveness of antimicrobial photodynamic therapy (AmPDT) on Staphylococcus aureus using phenothiazine compound with red laser. Lasers Med Sci 32, 29–34 (2017). https://doi.org/10.1007/s10103-016-2079-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2079-4