Abstract
Photodynamic inactivation (PDI) technology is a promising alternative to antibiotics. This technology is defined as the inhibition of bacterial growth with photosensitizers while irradiated with low-level laser light in the wavelength of 532 ± 2.08 nm. A challenging area in this field is selecting photosensitizers with antibacterial potential. In this paper, to enhance the antibacterial efficiency, the photosensitizers (the selected plant extracts) with a high absorption peak at the selected laser frequency, 532 nm, were prepared. Low-concentration ethanolic plant extracts of Hibiscus sabdariffa and Opuntia ficus-indica were found to exhibit significant antibacterial activity against, Acinetobacter baumannii ATCC 19606 and, Staphylococcus aureus ATCC 33591 as two important human pathogenic bacteria. The effectiveness of these natural photosensitizers was measured by determining their Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values and by performing a time-killing assay in the absence and the presence of laser irradiation. Our results showed that the combination of low-level laser irradiation and the selected photosensitizers had excellent potential for treating in vitro bacterial infections. Therefore, PDI technology has great potential as a viable alternative to traditional antibiotics for combating bacterial infections. This study presents a promising avenue for further exploration of PDI and the use of laser technology in medical science.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicinal plants, rich in bioactive molecules, have intrigued researchers [1,2,3]. In the industry, cost-effective, environmentally sustainable antibacterial skin ointments have been developed [1, 3]. A pressing concern is the rise of antibiotic-resistant infections due to overusing antibiotics. Multidrug-resistant pathogens discredit antibiotics rapidly [4,5,6]. As antibiotics lose effectiveness, international research explores alternatives like photodynamic inactivation (PDI)/photodynamic therapy (PDT) [7,8,9,10,11,12]. The photodynamic action, the therapeutic effect of combining light with chemical matters, was accidentally discovered by German medical researchers Raab and his coworker. Photodynamic inactivation, also known as PDI, is a challenging method that is performed by using the precise cooperation of the key factors, light, oxygen, and photosensitizers that can be targeted the cells of pathogenic bacteria and inactivate or kill them. The most common phenomenon in applying light in the medical field is the absorption process, among other processes such as reflection, transmission, and scattering [13]. So, the other PDI key factors are photosensitive materials, and photosensitizers (PSs), which could optimize the inactivation process by absorption of light in a specific wavelength of light.
The primary mechanism in the PDI method includes the absorption of photons by the photosynthetic molecules, which yields the photophysical and photochemical processes and consequently a photopharmacological effect. The processes initiate when the photosynthetic molecules are excited by the absorption of laser light. The excited energy is dissipated as radiative or non-radiative decay; Radiation decay occurs through fluorescence and phosphorescence effects, and non-radiative decay, includes the inter-system crossing from the singlet excited state to the triplet excited state. The importance of the decay is the production of active pharmaceutical species. Especially type I and II of PDIs processes begin after the phosphorescence decay that led to forming the reactive oxygen species (ROS) such as superoxide (O2• −), hydrogen peroxide (H2O2), hydroxyl radical (OH •), and singlet oxygen (1O2) and also pigment radicals. These species cause high oxidative damage to PSs, which is responsible for damage to bacterial cell membranes and destroying their enzymes and nucleic acids [14].
The selection of PSs is crucial due to their side effects, limiting their usage in the PDI method. Thus, choosing photosensitizers with no adverse health effects and antibacterial properties is highly valuable. Some PDI studies have proposed chemical photosensitizers like porphyrin, phthalocyanines, and chlorine [13, 15,16,17,18,19]. However, the limited availability of chemical photosensitizers without side effects restricts their use with any laser light wavelength. Plant PSs, such as B. orellana, C. longa, C. xanthorrhiza, G. blepharophylla, and H. sabdariffa, containing natural compounds like curcuminoids, alkaloids, and porphyrin, have been historically excellent sources. These plants offer effective therapeutic compounds and serve as alternatives to antibacterial agents for treating human infections. They are also used to inhibit the growth of gram-positive and gram-negative bacteria [20,21,22,23]. Consequently, the best choice for these photosensitizers is using plant pigments, which possess two positive attributes: no harm and antibacterial properties, owing to their bioactive compounds, including phenolic compounds [24,25,26].
Moreover, some studies of the PDI method have been confirmed on Gram-positive and Gram-negative bacteria by using photosensitizers with high-power lasers [27, 28]. However, usage of high-power lasers would be associated with serious damage for long periods, cost, and also side effects. Therefore, low-power lasers have attracted significant interest among researchers due to their cost-effectiveness and very few side effects [29, 30]. Kyungsu Kang and colleagues have proposed utilizing low-level laser and light with natural photosensitizers in the PDI method [14, 31, 32] and, other studies are mentioned [33, 34]. In the other studies, the PDI performance has been improved by selecting the maximum absorption peak of photosensitizers according to the laser wavelength [35].
In this paper, we attempted to improve the PDI efficiency and investigated the enhancement of the antibacterial properties of the selected photosensitizers on Gram-positive and Gram-negative bacteria. To this aim, the correct and accurate selection of natural photosensitizers was the priority of the research. Therefore, we purposefully started to survey the plant extracts that have excellent antibacterial properties in addition to a high absorption peak according to our low-power laser. Also, gas chromatography-mass spectrometry (GC/MS) was performed to study the chemical composition of the selected great natural photosensitizers including H. sabdariffa and O. ficus-indica. To evaluate the antibacterial properties of the natural pigments, well diffusion was performed to determine the zone of inhibition. Also, we have investigated the effectiveness of utilizing a 532 nm low-level laser in accompanying the selected pigments as good photosensitizers by determining the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) and also the time-killing assay in the absence and the presence of the low power laser.
Materials and methods
Collection and extraction
This paper evaluated two bacterial groups, Staphylococcus aureus ATCC 33591, and Acinetobacter baumannii ATCC 19606, which are two important Gram-positive and Gram-negative human pathogenic bacteria, respectively.
Plant extracts were selected based on their previous demonstrated effectiveness at inhibiting bacterial growth against both Gram-positive and Gram-negative bacteria [36,37,38,39]. These studied groups were Leaf and branch of Hibiscus sabdariffa, the fruits of Capsicum annuum group, Purple leaves of B.oleracea, Cornus mas, and Opuntia ficus-indica as it is shown in Table 1. The choice of plant species and their potential impact on human health was carefully considered, as these plants have been examined by global organizations, such as the International Union for Conservation of Nature (IUCN) and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), to ensure they are not on the endangered red list and are not threatened with extinction. The plant species used in this study H. sabdariffa, purple leaves of B. Olrtacea, Capsicum annuum group, Cornus mas, Opuntia, were all collected in a responsible manner, identified and confirmed taxonomically by the herbarium of the Islamic Azad University, Central Tehran Branch of Iran, and are presented alongside their useful parts in Table 1. Of these plant species, H. sabdariffa, purple leaves of B. Olrtacea, and Capsicum annuum group, are safe for human consumption, while Cornus mas is classified as Least Concern (LC) and Opuntia is Data Deficient (DD). In subsequent sections, low concentrations of these extracts were studied for their effect on Gram-positive and -negative bacteria.
Plant samples collection and extract preparation
In this paper, we used five samples, namely, H. sabdariffa, purple leaves of B. olrtacea, Capsicum annuum group, Cornus mas, and Opuntia, were mainly purchased from a local market in Kelardasht, Iran. All the samples, except H. sabdariffa, were sourced from various regions in Iran, as indicated in Table 1. The species used were confirmed by Central-Tehran University herbarium.
To prepare the samples, the shade drying method [51] was used. The plant parts were washed and then dried in the shade at room temperature to prevent the reduction of effective ingredients [52]. After drying, all plants were ground, and fresh fruits were washed and sliced to increase the level of solvent contact with plant materials, which optimizes compound extraction [52].
Preparation of plant extracts by Soxhlet and maceration methods
This study employed two extraction techniques, maceration and Soxhlet, details of which can be found in Table 2.
-
a.
Soxhlet extraction Method
The Soxhlet extraction method involved adding 5 g of dried plant material and 40 g of sliced fruit to 100 mL of 70% ethanol in D.W. (v/v) and extracting the mixture at a temperature of 70℃ for 2 h using a Soxhlet device (Barn stead/Electrotbermae, UK).
-
b.
Maceration Method
The maceration method, started with dispersing 40 g of the samples in 50 mL of 70% ethanol in D.W. (v/v). The samples were then kept in a dark place for two weeks, followed by shaking in the dark at room temperature for 24 h.
Also, the extracts were filtered using Whatman #3 paper filters, and solvent removal was carried out using a Rotary device (Heidolph Instruments, Germany) with a rotational speed of 136 rpm and a heating bath temperature of 30 °C for 20 min. For the extracts that were not completely dried, a freezer dryer [53] (Martin Christ, Alpha 2–4 LD plus, German) was used for 48 h (Table 3).
The solvent extraction processes for the maceration and Soxhlet methods are depicted schematically in Fig. 1.
Finally, the spectroscopic method was used to confirm the presence of some compounds of active ingredients in plant extracts [54]. Specifically, the absorption spectra of control samples were compared with the absorption spectra of the extracts, and each extract's spectroscopy was repeated several times to ensure accuracy. Tables 4 and 5 presents the measured absorption spectrum of the extracts and the spectral fingerprints of the extract compounds used in previous studies.
The spectroscopy of each extract was repeated several times to ensure accuracy.
GC/MS analysis
Gas chromatography-Mass spectroscopy (GC–MS) is an effective technique to detect the composition of volatile components of herbal samples origin. The GC–MS analysis of the selected great natural samples of H. sabdariffa [55], and Opuntia [56] were carried out on an Agilent Technologies, USA) GC model 7890 B equipped with a mass selective detector model MSD model 5977 A in the electron ionization mode at 70 eV. All method was the same for the two samples. For both samples, 1 μl of each sample volume was used for injection, with a split ratio of 1:50 and operating temperature set at 260 °C. The carrier gas utilized was high-purity helium with a flow rate of 0.46 mL/min, and volatiles were separated using a capillary column on a DB5-MS column (30 m length, 0.25 mm inner diameter, and 0.25 μm film, J&W Scientific, Santa Clara, CA, USA). The column temperature was held at 50 °C for 4 min, followed by an increase to 240 °C with a 10 °C/min rate and a hold for 2 min. The GC analysis and the column temperature programming were in the same situation. Then, the GC column effluent was introduced into the MS source, while ion source and interface temperatures were set at 200 °C and 240 °C, respectively. MS parameters were set to 70 eV (E1), electron multiplier voltage 1800 eV, mass range 35–550 amu, the event time 0.15 s, and the scan speed 5000. Standard matters in the references revealed the quartz index, NIST, and WILEY library database was used to find out mass spectrum components.
Antibacterial activity evaluation
In this research, Staphylococcus aureus ATCC 33591, and Acinetobacter baumannii ATCC 19606 were evaluated as two critical types of human pathogenic bacteria, classified as Gram-positive and Gram-negative, respectively.
Well diffusion method
To examine the antibacterial activity of the extracts, a diffusion test was used on Muller-Hinton Agar and Blood Agar mediums, according to Clinical and Laboratory Standards Institute (CLSI) guidelines [57]. Initially, a bacterial suspension with a turbidity of 0.5 McFarland in sterile normal saline was prepared and then plated. Sterile Pasteur pipettes were utilized to create the wells, into which 30 μL of each extract solution was added. The plates were then incubated at 37 \(^\circ{\rm C}\) for 20–22 h. In subsequent steps, the most potent plant extracts exhibiting a broad zone of inhibition around the wells were evaluated.
Determination of MIC and MBC without and with laser irradiation
The antimicrobial efficacy of the extracts was evaluated using the standard broth microdilution method as recommended by the Clinical and Laboratory Standards Institute (CLSI) [58]. The experiment was conducted using Mueller–Hinton broth as the test medium. Two-fold serial dilutions of each extract were prepared, and a final volume of 100 µL was added to wells containing 100 µL of MHB. A bacterial suspension was prepared in sterile normal saline and added to each well to achieve a final concentration of -106 colony forming units (CFU)/mL. The microplate was incubated at 37 \(^\circ{\rm C}\) for 20–22 h. The minimum inhibitory concentration (MIC) was determined as the lowest concentration of the extract that could inhibit visible growth of the bacteria being tested. The minimum bactericidal concentration (MBC) was determined by culturing 100 µL of the MIC well and all wells that showed no growth on Muller-Hinton agar. After 20–22 h of incubation at 37 °C, the MBC values were recorded as the lowest concentrations that could kill all bacterial cells, represented by the absence of bacterial colonies.
Selecting the good photosensitizers
We leveraged a spectrophotometer (Cecil Spectrophotometer (SE9500), UK) to determine the absorption spectra of the extracts within the 200–900 nm UV–Visible range in order to select the most effective photosensitizers.
Selecting the relevant light source and setup
The methodology in the article involves tailoring laser wavelengths based on the absorption peaks of plant wavelengths, optimizing absorption for maximal bacterial inactivation during Photodynamic Therapy (PDT). Low-power laser was used to minimize damage risks and conduct trials to identify the most effective inhibition strategies. Additionally, factors such as photosensitizer type and concentration, as well as light output power and duration, to select the ideal wavelength for photoinactivation were considered. This meticulous approach ensures both efficacy and safety in bacterial inactivation within antibacterial plant species. To leverage its therapeutic backgrounds and benefits, we utilized a low-level laser [59], which was specifically selected based on the photosensitizers' absorption peaks. The technical specifications for the laser entailed a wavelength of 532 nm, a bandwidth of laser: 4.16 nm, continuous mode of irradiation, a power output of 20 mW, irradiation durations of 6, 12, 18, and 20 min, a 5 cm distance to target, a laser spot size of 1 mm2, a light intensity of 25 mW/cm2, and energy for each times periods, 9, 18, 27, and 30 J/cm2, relatively.
We fixed laser setups and aligned them vertically, covering the sub-microplate using a black mask to hinder reflected light from reaching the neighboring wells during laser irradiation. As the laser beam was divergent, we employed a plano-convex lens to focus it. Additionally, to maintain a sterile environment, we conducted laser irradiations under laminar flow hood conditions.
MIC determination
The minimum inhibitory concentration MIC test was done the same as the previous one using the standard broth dilution method according to CLSI [58]. Negative control (without bacteria) and positive control (without photosensitizer) wells were implemented in the sterile microplate's eleventh and twelfth positions, respectively, in each row. The exposure time of approximately 6, 12, 18 and 20 min was applied to the wells. Next, the microplates were incubated for 20–22 h after the laser exposure. The MBC values were estimated applying the process outlined in Sect. "GC/MS analysis".
Time-killing assay
To determine the colony-forming units (CFUs), 100 μL from the control well (which contained bacteria without extracts) and the two wells preceding the MBC well and the actual MBC well (which included both bacteria and herbal sample) were serially diluted in sterile normal saline into 9 consecutive tubes (0.1 mL of well content and 0.9 mL of diluent). Next, 100 μL from the tubes 7, 8, and 9 were transferred to Muller-Hinton agar plates, then incubated for bacterial growth overnight. After 18–24 h, the colonies were counted for CFU/mL using naked-eye observation. Each experiment was repeated thrice to ensure accuracy."
Data analysis
Tests were performed thrice to promote accuracy. The standard deviation (mean ± standard deviation) for each test were calculated using the descriptive statistics frequency method available in the SPSS (Statistical package for the social sciences) package. One-sample t-tests were carried out to compare antibiotic zone inhibition and medicinal plants effectiveness against bacteria. Similarly, the t-test was used to compare MIC tests outcomes for each sample in the presence and absence of laser irradiation. It's important to mention that a p-value of less than 0.05 was considered statistically significant.
Results and discussion
The results of spectroscopy measurements
There has been a lot of research on the healing properties of plants due to the presence of certain chemical structures. In this study, the selection of the plants was based on the presence of bioactive compounds including phenolic and non-phenolic compounds. The information about the color, bioactive compounds, fingerprints, and pharmacology of the plants can be found in Table 3. Plant color is dependent on the type of pigments present, with carotenoids being responsible for yellows, oranges, and reds in plants such as the Capsicum annuum group, as indicated in Table 3. Bioactive compounds found in plants can lead to various pharmacological activities including antibacterial properties, as shown in the pharmacological section of Table 3. One of the most important groups of bioactive compounds found in plants is phenolic compounds. These compounds play critical roles in human health mainly as antioxidants, anti-allergic, anti-inflammatory, anticancer, antihypertensive, and antimicrobial agents [60].
They are secondary metabolites of plants are characterized by the presence of aromatic ring(s) bearing one or more hydroxyl moieties, and can be divided into different groups based on the number of phenolic rings [24,25,26]. For the extraction of both phenolic compounds and non-phenolic from the plants studied in this paper, the maceration and soxhlet methods were employed. Ethanol extraction was chosen due to its simplicity and effectiveness, as well as its boiling point of 78.37 °C which prevents the reduction of effective ingredients [52].
To select the best photosensitizers, the spectroscopy of most plant extracts was conducted in the range of 200–700 nm, with the rest being measured in the range of 400–700 nm, using a spectrophotometer. Additionally, the bioactive ingredients of the extracts were confirmed by comparing their absorption peaks of the extracts with their respective fingerprints, which are presented in Table 3.
As presented in Table 3, all extracts have high absorption in the ultraviolet region at 200–300 nm, indicating the presence of phenolic compounds such as phenols, tannins, and flavonoids. Non-phenolic compounds including alkaloids and coumarin, stachydrine, steroids, β-caryophyllene, allicin, and ascorbic acid, are listed in the phytochemical section of Table 3. Furthermore, the absorption peaks of the bioactive ingredients are directly correlated with the concentration of the non-phenolic compounds. The absorption peaks at about 535 nm for Purple leaves of B. olrtacea, H. sabdariffa, Opuntia, C. mas, and Capsicum annuum group have shown the presence of compounds such as anthocyanin and Carotenoid pigments. The presence of bioactive compounds in the examined plants was confirmed through spectral analysis, which revealed the presence of compounds such as anthocyanin and carotenoid pigments. These findings are consistent with the available fingerprints of the plants from previous studies, as presented in Table 3.
The selected plants possess therapeutic potential due to the presence of the bioactive compounds, especially phenolic structures [61], which exhibit significant antimicrobial properties.
The drying and extraction of the plant species used in this study were performed following the guidelines of valid references cited in this article. Extraction involves the separation of active ingredients of the raw plant [52], which depends on several factors, including temperature, particle size, extraction time, solvent type, solvent to solid ratio, and extraction methods [62,63,64]. Various extraction techniques, such as maceration, percolation, decoction, soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction, ultrasound-assisted extraction, microwave-assisted extraction, pulsed electric field extraction, and enzyme-assisted extraction, hydro distillation, and steam distillation, have been utilized [52]. Different solvents have also been employed for the extraction of bioactive ingredients from plants [52], with each bioactive compound requiring a specific solvent.
GC/MS analysis
Gas chromatography-mass spectroscopy (GC–MS) was employed to analyze the chemical constituents of plant extracts H. sabdariffa and Opuntia, which exhibited potent antibacterial activity and had a greatly desired absorption peak. The results showed the presence of a range of phenolic and non-phenolic compounds with significant peak areas, as indicated in Table 4 and 5. These compounds were identified based on their retention time and area percentage, and are believed to be responsible for the observed antimicrobial effects against human pathogenic microorganisms. Overall, our findings highlight the potential of these plant extracts as natural sources of antimicrobial agents, and pave the way for further investigations into their therapeutic properties. Tables 4 and 5 provide detailed information on the chemical composition of extracts H. sabdariffa, and Opuntia, respectively. Gas chromatography-mass spectroscopy (GC–MS) analysis revealed the presence of various classes of bioactive compounds in both extracts, as evidenced by the 16 and 8 peaks detected in the GC–MS chromatograms of H. sabdariffa and Opuntia, respectively (Figs. 2a, b). Further studies are needed to explore the potential applications of these extracts and their bioactive components in the development of new therapeutic agents. In order to identify specific and non-specific compounds, we compared the identified compounds in our extracts with known compounds reported in the literature. This comparison involved analyzing absorption peaks and comparing them with fingerprints from previous valid references. Specific compounds H.sabdariffa, with antibacterial properties or can combination with that (as components of antibacterial formulations) are Anthocyanins, betacyanins, citric acid, malic acid, propanoic acid, Flavonoids, Hydroxycinnamic acids, 2(3H)-Furanone (γ-butyrolactone), 2-Furancarboxaldehyde (furfuraldehyde), 4-Benzoquinone, Tetradecanoic acid (myristic acid), n-Hexadecanoic acid (palmitic acid), Hexanoic acid (caproic acid), 9,12-Octadecadienoic acid (linoleic acid), 9-Octadecenoic acid (oleic acid). And about non-specific compounds of it, 9,9 Dimethyltetracycloundecan-2-one, Oceanic acid, Bis(2-ethylhexyl) phthalate. Specific compounds Opuntia, with antibacterial properties or can combination with that (as components of antibacterial formulations) are Anthocyanins, betacyanins, Tetradecanoic acid. And about non-specific compounds of it, silane, maleic anhydride, furfural, 2,5-furandicarboxaldehyde, n-Hexadecanoic acid. Some non-specific compounds do not possess antibacterial properties themselves but can enhance and combine as components of antibacterial formulations as silane, maleic anhydride, Furfural, n-Hexadecanoic acid. Specific compounds with both antibacterial properties and being a good photosensitiser in desired wavelength (500–550 nm), anthocyanins, and betacyanins.
Antibacterial activity
One of the alternative approaches to antibiotics for killing or inhibiting the growth of bacteria is the usage of the antimicrobial properties of plant extracts, Since, antibiotics have lost their effectiveness in treating bacterial infections due to antibiotic resistance and side effects. One promising approach is the use of plant extracts with antimicrobial properties. To assess the antibacterial activity of the extracts, disk diffusion and dilution methods were used, both in the absence and presence of the light source. The extracts with better therapeutic performance against both Gram-positive and Gram-negative bacteria were selected for further experiments.
The results of the inhibition zone assay, presented in Table 6, demonstrated that the ethanolic extract of H. sabdariffa exhibited the highest antimicrobial activity against the tested bacteria. This activity can be attributed to the bioactive compounds identified in Table 3, which have been shown to inhibit cell wall synthesis, as well as protein and nucleic acid synthesis [52, 65]. Further analysis using the Spss package showed that all tested extracts, regardless of the extraction method, exhibited a significant reduction in the bacterial population due to their antimicrobial properties. Two extracts with both high zones of inhibition and good absorption peaks were chosen for further testing. Overall, these findings demonstrate the potential of plant extracts as natural sources of antimicrobial agents, and provide a basis for further investigations into their therapeutic properties.
Comparison of antimicrobial activity of extracts with standard antibiotics
The antimicrobial activity of the plant extracts was compared with the relevant antibiotics against the gram-positive and gram-negative bacterium. The antibiotics sensitivity of cyclophosphamide CTX(B), tetracycline TE(B), vancomycin V(B), penicillin P(A), erythromycin E(A), ciprofloxacin CFO(A), clindamycin CC(A) on some gram-positive and negative bacteria were confirmed according to CLSI 2020. The plant extracts with the best zone of inhibition were chosen for comparison. Figure 3 presents the results of comparing the zone of inhibition of antibiotics with the best extracts and its numerical results were shown in Table 7 and 8. For the gram-negative bacterium, A. baumannii, two antibiotics CTX(B) and TE(O) were more effective than the other plant extracts, but the extract of H. sabdariffa had the same therapeutic effect as the vancomycin (V(B)) antibiotic. For the gram-positive bacterium, S. aureus, some antibiotics p(A), E(A), CFO(A), and CC(A) exhibited higher antibacterial activity than the plant extracts, while others had similar zone of inhibition, approximately equal to that of the CC(A), CP(C), and T(B)antibiotic. The calculated P-values for A. baumannii and S. aureus were found to be less than 0.02 and 0.01, respectively, indicating statistically significant differences the two groups.
Comparison of the inhibition zone of antibiotics with the best plant extracts against bacteria. In Fig. 3a, the statistical analysis showed a significant difference with a P value < 0.02, and in Fig. 3b, the difference was even more significant with a P value < 0.01, indicating the strong statistical significant of observed finding
The mechanism of action of antibiotics may vary for each bacterium. Beta-lactam antibiotics, including penicillin, cephalosporins (cephems), monobactams, and carbapenems, act as irreversible inhibitor of the enzyme transpeptidase, which bacteria use to make their cell walls [66]. Aminoglycosides inhibit protein production and affect cell membrane permeability by binding to the 30S subunit of rRNA, causing genetic code misread. Fluoroquinolone interrupts DNA breakage-reunion by binding to DNA-gyrase or topoisomerase II and topoisomerase IV, while tetracycline interferes with amino acid transfer by binding to the 30S subunit of rRNA, preventing protein production [67]. In conclusion, the tested plant extracts with antibacterial properties were found to be appropriate alternatives to antibiotics with fewer side effects for killing bacteria.
MIC and MBC assay
Based on the findings of the previous section, H. sabdariffa and Opuntia extracts exhibited a higher antibacterial activity than the antibiotics, as demonstrated in Fig. 3. Additionally, these extracts displayed strong absorption peaks at specific wavelengths, as illustrated in Table 3, indicating their potential as effective photosensitizers. Specifically, two extracts exhibited exceptional photosensitizing properties, with absorption at approximately 532 nm. As such, the present study aimed to evaluate the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of these two extracts under laser irradiation and non-irradiation conditions.
In the absence of laser radiation
As can be seen in Fig. 3a and 3b, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of H. sabdariffa and Opuntia extracts were evaluated against Gram-positive and Gram-negative bacteria. Interestingly, the ethanolic extract of H. sabdariffa exhibited a higher suppressive effect on Gram-positive bacteria than Gram-negative bacteria. This observation may be attributed to the dissimilarities in the cell wall structure between the two bacterial types. The complex and multilayered cell wall structure of Gram-negative bacteria provides them with higher resistance compared to Gram-positive bacteria. Specifically, the cell wall of Gram-positive bacteria is composed of two layers, namely the cytoplasmic membrane and a thick peptidoglycan layer containing teichoic acid. Conversely, Gram-negative bacteria possess an additional membrane layer in their cell wall architecture, situated outside the peptidoglycan layer, that exhibits an asymmetric lipid structure comprising of strongly negatively charged lipopolysaccharides (LPS), lipoproteins, and proteins with porin function [68].
Moreover, the MIC and MBC results of H. sabdariffa and Opuntia extracts were found to be effective. It is evident that higher concentrations of the extracts led to greater antibacterial activity against the tested bacteria.
In the presence of laser irradiation
In this section, we investigated the therapeutic properties of two plant extracts against gram-positive and negative bacteria. Specifically, we determined the minimum inhibitory concentration (MIC) of the extracts in the presence of laser irradiation, using a photodynamic inactivation (PDI) method. Photosensitizers or photo acceptors are molecules that absorb specific wavelengths of light and can be used in PDI for antimicrobial activity. The combination of photosensitizers and light leads to a promising technique with antimicrobial activity that is called photodynamic inactivation of bacteria (PDI). While various pigments have been reported as good photosensitizers [14], plant extracts can also play this role due to their sharp absorption peak at specific wavelengths, making them not only antibacterial agents but also excellent photosensitizers.
Two selected plant extracts (H. sabdariffa and Opuntia extracts) were found to have an absorption peak at around 532 nm, based on the spectra shown in Table 3. To increase the efficiency of their antibacterial action as photosensitizers, we determined the laser wavelength by their absorption spectra. We used a green (532 nm) low-level laser due to its safety, cost-effectiveness, and availability.
MIC testing was performed by the standard broth dilution method according to CLSI, and MBC testing was done in the same manner as before irradiation. Interestingly, a significant increase in the antibacterial effects of the photosensitizers (H. sabdariffa and Opuntia) against Gram-positive and Gram-negative bacteria was achieved in the presence of the green low-level laser beam. The laser was irradiated to each well of the microplate in 4-time steps of 6, 12, 18, and 20 min. It is worth noting that, each experiment was repeated three times. The results of laser irradiation were presented in Fig. 4, its numerical results in Table 9. The P value of MIC/MBC for H. sabdariffa and Opuntia on both A. baumannii and S. aureus was statistically significant (P value < 0.037), indicating the efficacy of the PDI method.
The results of MIC and MBC testing for the absence of laser irradiation in 0 min and also, after 6 to 20 min of laser irradiation against a) gram-positive and b) gram-negative. MIC and MBC testing had the same results. The calculated p values of MIC/MBC of H. sabdariffa and Opuntia against both A. baumannii and S. aureus were found to be less than 0.037, indicating significant inhibitory effects
Since two tested extracts had significant antibacterial properties in the absence of laser, the result of increasing the antibacterial properties of these extracts was considered in the presence of the laser irradiation in consecutive periods of about 6, 12, 18, and 20 min. The MIC/MBC values showed that the antibacterial properties of two tested extracts were significantly increased in the presence of laser irradiation as indicated in Figs. 4a and 4b. This enhancement was attributed to the synergy between the laser and the photosensitizers in the PDI process, which produced singlet oxygen and free radicals that increased the antibacterial activities. The mechanism behind this enhancement was explained through the biophysical expression of an increase in antibacterial properties, where the laser radiation on photosensitizers increased and accelerated the number of reactions with oxygen molecules, which ultimately led to an increased antibacterial effect [69]. The study highlighted the potential of using photosensitizers and laser irradiation in the development of new antibacterial therapies.
Following the second, third, and fourth periods of irradiation, which had a mean duration of 12, 18, and 20 min, respectively, the antibacterial properties of the photosynthetic solution remained constant as it shown in Figs. 4a, 4b. However, this phenomenon can be attributed to the process of laser beam irradiation on wells containing photosynthetic solution and bacteria, which resulted in the generation of heat. As a low-level laser was employed in this study, the heat generated was insufficient to cause bacterial death but may have created a conducive environment for bacterial growth during prolonged irradiation periods [67].
As the laser irradiation continued, two competing processes emerged, the bacterial killing process related to the antibacterial activities and the heating process, which promoted bacterial growth. Consequently, with prolonged laser irradiation, the antibacterial effect gradually subsided, and the bacteria continued to grow. Additionally, photosensitizers, including the synthetic pigments of plant extracts, that highly absorb laser light can potentially decompose and lose their antibacterial properties. Overall, the findings of this study suggest that the antibacterial efficacy of laser irradiation is dependent on several factors, including the type of laser used, the duration of irradiation, and the presence of photosensitizers. Therefore, future studies should consider these factors when investigating the antibacterial properties of laser irradiation on photosynthetic solutions.
The biophysical process can be described as follows, when the photosensitizers absorb the light, a higher number of molecules are excited compared to the absence of a laser. These molecules are excited to a singlet excited state, followed by rapid vibrational relaxation. The energy of the excited state decays through two pathways, fluorescence radiative decay, where the excited molecule returns to the ground state by emitting quantum, and non-radiative decay, such as intersystem crossing from the excited molecule transfers from the singlet excited state to the triplet excited state. Intersystem crossing occurs due to the different couplings between the nucleus and the electron in each molecule [69,70,71].
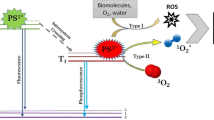
The absorption of laser light by photosensitizers increases the probability of their transition to the triplet excited state from the singlet excited state through intersystem crossing, resulting in more molecules being in the triplet excited state. The triplet excited state is more stable in terms of energy compared to the singlet excited state, as illustrated in Fig. 5. The energy level of the triplet excited state is lower than that of the singlet excited state, and the energy difference between the triplet excited and the ground state is smaller than in the case of the singlet excited state. However, the energy in the triplet excited state is also unstable and can decay through various mechanisms, including radiation decay, which releases energy similar to phosphorescence radiation. This leads to the occurrence of two types of photosensitized oxidation (PDI), referred to as type I and type II PDIs. The elevated absorption of energy during photosynthesis stems from the extended excitation phase of the photosensitizer. A prolonged singlet excitation phase results in heightened electronic energy conversion from the ground level to an excited triplet state. The surplus energy at the excited triplet state allows for the transfer of energy to neighboring oxygen molecules, leading to the generation of reactive oxygen species (ROS) [72].
Photophysical process of the photosensitizer excitations and production of the reactive species, SES: Singlet excited state, GS: Ground state, TES: Triplet excited state, PS: Photosensitizer, 1PS*: Excited-state (singlet), 3PS*: Excited-state (triplet), ISC: Inter-system crossing, IC: Internal conversion (vibrational decay), ROS: Reactive oxygen species
Then, type I of PDI involved direct interaction between the photosensitizers in the triplet excited state and the biological substances, resulting in the transfer of an electron or a proton to them. This transfer can generate radical activated species, as well as react with oxygen molecules to produce reactive oxygen species (ROS) such as hydroxyl radicals (HO●) and superoxide anion radicals (O2•).
In type II PDI, the reaction depends on the availability of oxygen molecules. If the energy difference between the excited state of the photosensitizer molecules and oxygen molecule’s excitation energy is matched, the oxygen molecule becomes activated by accepting energy from the triplet excited state of photosensitizers, leading to the formation of singlet oxygen (3O2 to 1O2). Single oxygen possesses an unoccupied π*2p orbital, which readily react with unsaturated lipids, alkenes (fatty acids), nitrogen and sulfur groups (amino acids), or nucleobases. The harmful effects of reactive oxygen species (ROS) include damaging cell membranes, disrupting cell division, and breaking DNA strands. Once cell membranes are compromised, photosensitizers can enter cells and harm organelles like lysosomes, mitochondria, and nuclei. ROS compounds interact with biomolecules within cells, leading to the breakdown of cellular structures and loss of function. Additionally, when ROS compounds react with phospholipids in cell membranes, they trigger lipid peroxidation, generating malondialdehyde and further damaging and rupturing cells. [73]. These reactions in bacteria cells lipids result in the formation of lipid hydroperoxides through photooxidation of unsaturated lipids, leading to cell death. Additionally, the acidification of the cell due to an oxidative burst of the endosomes and/or lysosomes can also contribute to cell death. Furthermore, photooxidation of DNA can result in genomic mutation or cell death [14, 69].
Time-killing assay
For more accuracy, time-killing assay was performed on both plant extracts that exhibited the significant antibacterial properties against tested bacteria in the MIC and the MBC assay in the absence and the presence of laser irradiation. Time-killing assay was performed using the colony count method [74]. As depicted in Fig. 6, the colony counts of all samples at 3.125 ppm in the seventh dilution tube were compared under different laser irradiation durations (the absence of laser irradiation (t = 0), the presence of laser irradiation (t = 6, 12, 18, and 20 min)). The results of colony counting demonstrated the effectiveness of our study in inhibiting bacterial growth and revealed the bactericidal properties of the herbal samples. Specifically, the graphs in Fig. 6 showed that all samples at 3.125 ppm exhibited excellent reduction in bacterial colony count, with a decrease from log7 to complete elimination as the irradiation time increased. The colony count of all bacteria was zero in the presence of laser irradiation, indicating the potent antibacterial effect of the photosensitizers at this concentration. As it shown in Fig. 7, the absence of bacterial colony images in comparison with the colony study groups is evident.
Conclusion
One of the significant threats to human life is antibiotic resistance which leads to serious illnesses. A safe alternative approach to antibiotics is the PDI method. To optimize the efficiency of this method, we have proposed using medicinal plant extracts as natural photosensitizers. The antibacterial effect of the proposed medicinal plant’s extracts was tested and compared with antibiotics. To enhance the antibacterial effect, the absorption peak of the natural photosensitizers should be matched with the wavelength of laser light. So, the spectroscopy of the plant extracts was utilized to find excellent photosensitizers.
Our results demonstrated that two plant extracts, Hibiscus sabdariffa, and Opuntia, had inhibitory effects against both Gram-positive and Gram-negative bacteria at low concentration. The ethanolic extracts had excellent absorption peaks at 532 nm, and gas chromatography-mass spectrometry (GC/MS) analysis presented some excellent details of the chemical composition of the selected great natural photosensitizers include of H. sabdariffa and Opuntia.
The MIC and MBC tests were conducted in the absence and presence of laser irradiation, and the results showed that a 12 min exposure of the natural photosensitizers to low-level laser led to maximum inhibition of bacteria. The MIC/MBC values of H. sabdariffa and Opuntia against A. baumannii were 1562 and 6250 ppm, respectively, and 1562 and 3125 ppm against S. aureus. Moreover, colony counting revealed a significant reduction in the number of colonies in the presence of herbal samples and laser irradiation, with a decrease in bacterial count from log107.
The experiments showed a significant increase in the antibacterial effect of medicinal natural photosensitizers against both bacteria after laser exposure, which was attributed to increased production of reactive oxygen species through biophysical phenomena. Our findings suggest that the simultaneous use of plant extracts and laser irradiation can provide a safe and cost-effective alternative to antibiotics, particularly for the treatment of bacterial skin infections.
In conclusion, our study highlights the potential of medicinal plant extracts as natural photosensitizers to enhance the antibacterial effect of PDI. Further investigations are warranted to fully understand the underlying mechanisms and optimize the conditions for bacterial inhibition. Nonetheless, our findings provide promising evidence for the use of medicinal plants as low-cost antibacterial agents.
References
Petrovska BB (2012) Historical review of medicinal plants’ usage. Pharmacogn Rev 6(11):1–5. https://doi.org/10.4103/0973-7847.95849
Yang Y, Liu Q, Shi X, Zheng Q, Chen L, Sun Y (2021) Advances in plant-derived natural products for antitumor immunotherapy. Arch Pharm Res 44(11):987–1011. https://doi.org/10.1007/s12272-021-01355-1
Gurib-Fakim A (2006) Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects of Med 27:1–93. https://doi.org/10.1016/j.mam.2005.07.008
Kolar M, Urbanek K, Latal T (2001) Antibiotic selective pressure and development of bacterial resistance. Int J Antimicrob Agents 17:357–363
Mainous AG, Diaz VA, Matheson EM, Gregorie SH, Hueston WJ (2011) Trends in hospitalizations with antibiotic-resistant infections. Public Health Rep 126:354–361
Zamani S, Nasiri MJ, Khoshgnab BN, Ashrafi A, Abdollahi A (2014) Evaluation of antimicrobial resistance pattern of nosocomial and community bacterial pathogens at a teaching hospital in tehran. Iran Acta Medica Iranica 52:182–186
Taylor PW, Stapleton PD, Luzio JP (2002) New ways to treat bacterial infections. Drug Discovery Today 7:1086–1091
Hamblin MR, Hasan T (2004) Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3:436–450
Quishida CCC, Mima EGDO, Jorge JH, Vergani CE, Bagnato VS, Pavarina AC (2016) Photodynamic inactivation of a multispecies biofilm using curcumin and LED light. Lasers Med Sci 31:997–1009
Mantareva V, Kussovski V, Durmuş M, Borisova E, Angelov I (2016) Photodynamic inactivation of pathogenic species Pseudomonas aeruginosa and Candida albicans with lutetium (III) acetate phthalocyanines and specific light irradiation. Lasers Med Sci 31:1591–1598
Makdoumi K, Hedin M, Bäckman A (2019) Different photodynamic effects of blue light with and without riboflavin on methicillin-resistant Staphylococcus aureus (MRSA) and human keratinocytes in vitro. Lasers Med Sci 34:1799–1805
Barroso RA, Navarro R, Tim CR, de Paula L, Ramos LD, de Oliveira Â, Araki T, Fernandes KGC, Macedo D, Assis L (2021) Antimicrobial photodynamic therapy against Propionibacterium acnes biofilms using hypericin (Hypericum perforatum) photosensitizer: in vitro study. Lasers Med Sci 36:1235–1240
Ghorbani J, Rahban D, Sh Aghamiri A, Teymouri AB (2018) Photosensitizers in antibacterial photodynamic therapy: an overview. Laser Therapy 27:293–302
Polat E, Kang K (2021) Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 9:9060584
Huang L, El-Hussein A, Xuan W, Hamblin MR (2017) Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J Photochem Photobiol, B 178:277–286
Huang L, Bhayana B, Xuan W, Sanchez RP, McCulloch BJ, Lalwani S, Hamblin MR (2018) Comparison of two functionalized fullerenes for antimicrobial photodynamic inactivation: Potentiation by potassium iodide and photochemical mechanisms. J Photochem Photobiol, B 186:197–206
Grinholc M, Szramka B, Kurlenda J, Graczyk A, Bielawski KP (2008) Bactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependent. J Photochem Photobiol, B 90:57–63
Niea X, Ch Jiangb Sh, Wua WC, Lva P, Wanga Q, Liua J, Ch Narha X, Caod RA, Ghiladia QW (2020) Carbon quantum dots: A bright future as photosensitizers for in vitro antibacterial photodynamic inactivation. J Photochem Photobiol, B 206:111–864
Wang Y, Guo X, Sh Zhou L, Wang YF, Xing L, Zhao Y, Zhang L, Qiu H, Zeng J, Gu Y (2021) Selective photodynamic inactivation of Helicobacter pylori by a cationic benzylidene cyclopentanone photosensitizer - an in vitro and ex vivo study. J Photochem Photobiol, B 223:112287
Regensburger J, Maisch T, Felgentrager A, Santarelli F, Baumler W (2010) A helpful technology- the luminescence detection of singlet oxygen to investigate photodynamic inactivation of bacteria (PDIB). J Biophotonics 3:5–6
Kostelanska M, Freisleben J, Hanusova ZB, Mosko T, Vik R (2019) Optimization of the photodynamic inactivation of prions by aphthalocyanine photosensitizer: the crucial involvement of singlet oxygen. J Biophotonic 12:8
Tinkler JH, Biihm F, Schalch W, Truscott TG (1994) Dietary carotenoids protect human cells from damage. J Photochem Photobiol 26:283–285
Lyu JI, Ryu J, Jin CH, Kim DG, Kim JM, Seo KS, Kim JB, Kim SH, Ahn JW, Kang SY, Kwon SJ (2020) Phenolic compounds in extracts of hibiscus acetosella (Cranberry Hibiscus) and their antioxidant and antibacterial properties. Molecules 25(18):419. https://doi.org/10.3390/molecules25184190
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18(2):2328–2375. https://doi.org/10.3390/molecules18022328
Daglia M (2011) Polyphenols as antimicrobial agents. Curr Opin Biotechnol 23:174–181
Kim J-S (2015) Production, separation and applications of phenolic-rich bio-oil–a review. Bioresour Technol 178:90–98. https://doi.org/10.1016/j.biortech.2014.08.121
Meral G, Tasar F, Kocago S, Sener C (2003) Factors affecting the antibacterial effects of Nd:YAG Laser In Vivo. Lasers Surg Med 32:197–202
Trzaska WJ, Wrigley HE, Thwaite JE, May RC (2017) Species-specific antifungal activity of blue light. Sci Rep 7:4605. https://doi.org/10.1038/s41598-017-05000-0
Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M (2005) Low-level laser therapy for wound healing mechanism and efficacy. Am Soc Dermatol Surg 31:334–340
Dadras S, Mohajerani E, Eftekhar F, Hosseini M (2006) Different photoresponses of staphylococcus aureus and pseudomonas aeruginosa to 514, 532, and 633 nm low-level lasers in vitro. Curr Microbiol 53:282–286
Yoshida A, Sasaki H, Toyama T, Araki M, Fujioka J, Tsukiyama K, Hamada N, Yoshino F (2017) Antimicrobial effect of blue light using Porphyromonas gingivalis pigment. Scientific Reports 7:5225. https://doi.org/10.1038/s41598-017-05706-1
Brasel M, Pieranski M, Grinholc M (2020) An extended logistic model of photodynamic inactivation for various levels of irradiance using the example of Streptococcus agalactiae. Sci Rep 10:14168. https://doi.org/10.1038/s41598-020-71033-7
Mamonea L, Di Venosa G, Gándara L, Sáenz D, Vallecorsa P, Schickinger S, Rossetti MV, Batlle A, Buzzola F, Casas A (2014) Photodynamic inactivation of Gram-positive bacteria employing natural Resources. J Photochem Photobiol, B 133:80–89
Alam ST, Hwang H, Son JD, Nguyen UTT, Park J, Ch H, Kwon JK, Kang K (2021) Natural photosensitizers from Tripterygium wilfordii and their antimicrobial photodynamic therapeutic effects in a Caenorhabditis elegans model. J Photochem Photobiol, B 218:112–184
Tim M (2015) Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J Photochem Photobiol, B 150:2–10
Mishra AP, Saklani S, Sharifi-Rad M, Iriti M, Salehi B, Maurya VK, Rauf A, Milella L, Rajabi S, Baghalpour N, Sharifi-Rad (2018) Antibacterial potential of Saussurea obvallata petroleum ether extract A spiritually revered medicinal plant. J Cell Mol Biol (Noisy-le-grand) 64(8):65–70
Nocedo-Mena D, Garza-González E, González-Ferrara M, Del Rayo C-C (2020) Antibacterial activity of cissus incisa extracts against multidrug- resistant bacteria. Curr Top Med Chem 20(4):318–323. https://doi.org/10.2174/1568026619666191121123926
Benramdane E, Chougui N, Ramos PAB, Makhloufi N, Tamendjari A, Silvestre AJD, Santos SAO (2022) Lipophilic compounds and antibacterial activity of opuntia ficus-indica root extracts from algeria. Int J Mol Sci 23(19):11161. https://doi.org/10.3390/ijms231911161
Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E (2021) Towards advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives. Microorganisms 9:2041
Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M (2014) Hibiscus sabdariffa L. - a phytochemical and pharmacological review. Food Chem 165:424–443. https://doi.org/10.1016/j.foodchem.2014.05.002
Riaz G, Chopra R (2018) A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharmacother 102:575–586. https://doi.org/10.1016/j.biopha.2018.03.023
Ojulari OV, Lee SG, Nam JO (2019) Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa on Obesity. Molecules 24(1):210. https://doi.org/10.3390/molecules24010210
Abdel-Shafi S, Al-Mohammadi A, Sitohy M, Mosa B, Ismaiel A, Enan G, Osman A (2019) Antimicrobial activity and chemical constitution of the crude, phenolic-rich extracts of hibiscus sabdari_a. Brassica oleracea and Beta vulgaris, Molecules 24:4280
Ghareaghajlou N, Hallaj-Nezhadi S, Ghasempour Z (2021) Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem 365:130482. https://doi.org/10.1016/j.foodchem.2021.130482
(2007) Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin. Int J Toxicol 26 Suppl 1:3–106. https://doi.org/10.1080/10915810601163939
Anaya-Esparza LM, Mora ZV, Vázquez-Paulino O, Ascencio F, Villarruel-López A (2021) Bell Peppers (Capsicum annum L.) Losses andWastes: Source for Food and Pharmaceutical Applications. Molecules 26:5341
Hosseinpour-Jaghdani F, Shomali T, Gholipour-Shahraki S, Rahimi-Madiseh M, Rafieian-Kopaei M (2017) Cornus mas: a review on traditional uses and pharmacological properties. J Complement Integr Med 14(3). https://doi.org/10.1515/jcim-2016-0137
Efenberger-Szmechtyk M, Nowak A, Nowak A (2020) Cytotoxic and DNA-damaging e_ects of aronia melanocarpa, cornus mas, and chaenomeles superba leaf extracts on the human colon adenocarcinoma cell line Caco-2. Antioxidants 9:1030
Silva MA, Albuquerque TG, Pereira P, Ramalho R, Vicente F, Oliveira MBPP, Costa HS (2021) Opuntia ficus-indica (L.) Mill: a multi-benefit potential to be exploited. Molecules 26(4):951. https://doi.org/10.3390/molecules26040951
Aragona M, Lauriano ER, Pergolizzi S, Faggio C (2018) Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat Prod Res 32(17):2037–2049. https://doi.org/10.1080/14786419.2017.1365073
Thamkaew G, Sjöholm I, Galindo F (2021) A review of drying methods for improving the quality of dried herbs. Crit Rev Food Sci Nutr 61:11
Zhang Q-W, Lin L-G, Ye W-C (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medicine 20:13–20
Bhatta S, Janezic TS, Ratti C (2020) Review of Freeze-Drying of Plant-Based Foods. Foods 9:87
Gong D, Chen J, Li X, Sun G, Sun W (2021) A smart spectral analysis strategy-based UV and FT-IR spectroscopy fingerprint: Application to quality evaluation of compound liquorice tablets. J Pharm Biomed Anal 5(202):114172. https://doi.org/10.1016/j.jpba.2021.114172
Khuanekkaphan M, Noysang CH, Khobjai W (2020) Anti-aging potential and phytochemicals of Centella asiatica, Nelumbo nucifera, and Hibiscus sabdariffa extracts. J Adv Pharm Technol Res 11:174–178
Alsaad AJA, Mohammed LS (2021) Study of the Antioxidant Activity of Cactus (Opuntia dellienii) Fruits (Pulp and Peels) and Characterisation of their Bioactive Compounds by GC-MS. Basrah J Agric Sci 34:204–219
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Subramaniam P, Reddy KRM, Eswara U (2012) Reddy Effect of different types of tea on Streptococcus mutans: an in vitro study. Indian J Dent Res 23(1):43–48
Denis TGS, Dai T, Izikson L, Astrakas CH, Anderson RR, Hamblin MR, Tegos GP (2011) All you need is light Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 6:509–520
Williamson G (2017) The role of polyphenols in modern nutrition. Nutr Bull 42:226–235
Riaz G, Chopra R (2018) A review on phytochemistry and therapeutic uses of Hibiscus sabdari_a L. Biomed Pharmacother 102:575–586
Li P, Xu G, Li SP, Wang YT, Fan TP, Zhao QS, Zhang QW (2008) Optimizing ultra performance liquid chromatographic analysis of 10 diterpenoid compounds in Salvia miltiorrhiza using central composite design. J Agric Food Chem 56:1164–1171
Yi Y, Zhang QW, Li SL, Wang Y, Ye WC, Zhao J, Wang YT (2012) Simultaneous quantification of major flavonoids in “Bawanghua”, the edible flower of Hylocereus undatus using pressurised liquid extraction and high performance liquid chromatography. Food Chem 135:528–533
Du G, Zhao HY, Song YL, Zhang QW, Wang YT (2011) Rapid simultaneous determination of isoflavones in Radix puerariae using high-performance liquid chromatography-triple quadrupole mass spectrometry with novel shell-type column. J Sep Sci 34:2576–2585
Zeng D, Debabov D, Hartsell TL, Cano RJ, Adams S, Schuyler JA, McMillan R, Pace JL (2016) Approved glycopeptide antibacterial drugs: mechanism of action and resistance. Cold Spring Harb Perspect Med 6(12):026989. https://doi.org/10.1101/cshperspect.a026989
Holten KB, Onusko EM (2000) Appropriate prescribing of oral beta-lactam antibiotics. Am Fam Physician 62:611–620
Khardori N, Stevaux C, Ripley K (2020) Antibiotics: From the Beginning to the Future: Part 2. Indian J Pediatr 87(1):43–47. https://doi.org/10.1007/s12098-019-03113-0
Fekrazad R, Khoei F, Bahador A, Hakimiha N (2020) Comparison of different modes of photo-activated disinfection against Porphyromonas gingivalis: An in vitro study. Photodiagnosis Photodyn Ther 32:101951
Siewert B, Stuppner H (2019) The photoactivity of natural products – An overlooked potential of phytomedicines? Phytomedicine 60:152985
Wanarska E, Mielko KA, Maliszewska I, Młynarz P (2022) The oxidative stress and metabolic response of Acinetobacter baumannii for aPDT multiple photosensitization. Sci Rep 12:1913. https://doi.org/10.1038/s41598-022-05650-9
Crocker LB, Lee JH, Mital S, Mills GC, Schack S, Bistrović-Popov A, Franck ChO, Mela I, Kaminski CF, Christie G, Fruk L (2022) Tuning riboflavin derivatives for photodynamic inactivation of pathogens. Sci Rep 12:6580. https://doi.org/10.1038/s41598-022-10394-7
Astuti SD, Arifianto D, Drantantiyas NDG, Nasution AMT (2016) Efficacy of CNC-diode laser combine with chlorophylls to eliminate Staphylococcus aureus biofilm. Publishing in International Seminar on Sensors, Instrumentation, Measurement, and Metrology (ISSIMM)
Astuti SD, Suhariningsih AB, Astuti SD (2019) The efficacy of photodynamic inactivation of the diode laser in inactivation of the candida albicans biofilms with exogenous photosensitizer of papaya leaf chlorophyll. J Lasers Med Sci 10(3):215–224
Putman M, Burton R, Nahm MH (2005) Simplified method to automatically count bacterial colony forming unit. J Immunol Methods 302(1–2):99–102. https://doi.org/10.1016/j.jim.2005.05.003
Zhen J, Villani TS, Guo Y, Qi Y, Chin K, Pan M, Ch Ho JE, Simon QWu (2016) Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem 190:673–680
Deli M, Nguimbou R, Baudelaire E, Yanou N, Scher J, Mbofung C (2020) Effect of controlled differential sieving processing on micronutrient contents and in vivo antioxidant activities of Hibiscus sabdariffa L. calyxes powder. Food Sci Biotechnol 29:1741–1753
Chauhan ES, Tiwari A (2016) A Singh Phytochemical screening of red cabbage (Brassica oleracea) powder and juice - A comparative study. Journal of Medicinal Plants Studies 4:196–199
Rybak K, Wiktor A, Witrowa-Rajchert D, Parniakov O, Nowacka M (2021) The Quality of Red Bell Pepper Subjected to Freeze-Drying Preceded by Traditional and Novel Pretreatment. Foods 10:226
Klymenko S, Kucharska AZ, Sokól-Letowska A, Piórecki N, Przybylska D, Grygorieva O (2021) Iridoids flavonoids, and antioxidant capacity of cornus mas, c officinalis, and c mas _ c officinalis fruits. Biomolecules 11:776
Silva MA, Albuquerque TG, Pereira P, Ramalho R, Vicente F, Oliveira MBPP, Costa HS (2021) Opuntia ficus-indica (L.) Mill.: A multi-benefit potential to be exploited. Molecules 26(4):951. https://doi.org/10.3390/molecules26040951
Tsao R (2010) Chemistry and biochemistry of dietary polyphenols. Nutrients 2(12):1231–1246. https://doi.org/10.3390/nu2121231
Kh Khorsandi Z, Kianmehr EG (2022) Combination effect of red light irradiation and Traychspermum ammi essential oil on colorectal cancer cells (SW480). Lasers Med Sci 37:1031–1040
Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B (2022) Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem 30(383):132531. https://doi.org/10.1016/j.foodchem.2022.132531
Barbasz A, Oćwieja M, Barbasz J (2015) Cytotoxic activity of highly purified silver nanoparticles sol against cells of human immune system. Appl Biochem Biotechnol 176:817–834
Andersen M, Fossen T, Torskangerpoll K, Fossen A, Hauge U (2004) Anthocyanin from strawberry (Fragaria ananassa) with the novel aglycone, 5- carboxypyranopelargonidin. Phytochemistry 65:405–410
Kasal A, Budesinsky M, Griffiths WJ (2010) Spectroscopic Methods of Steroid Analysis. Steroid Analysis 27:161
Vinokurova NG, Vinokurova NG, Zelenkova NF, Baskunov BP (2001) Determination of diketopiperazine alkaloids of the roqefortine group by UV spectroscopy, thin-layer chromatography, and high-performance liquid chromatography. J Anal Chem 56:258–262
Maoka T (2019) Carotenoids as natural functional pigments. J Nat Med. https://doi.org/10.1007/s11418-019-01364-x
Toth M, Kukor Z, Valent S (2002) Chemical stabilization of tetrahydrobiopterin by L-ascorbic acid: contribution to placental endothelial nitric oxide synthase activity. Mol Hum Reprod 8:271–280
Galili G, Amir R, Hoefgen R, Hesse H (2005) Improving the levels of essential amino acids and sulfur metabolites in plants. Biol Chem 386(9):817–831. https://doi.org/10.1515/BC.2005.097
Author information
Authors and Affiliations
Contributions
Z. Aghaebrahimi: methodology, data collection and analysis, design, and writing the first draft of the manuscript.
M. Ranjbaran: helping to write the final manuscript, and assisting in setting up the laser setup.
J. Sabaghzadeh: methodology, design, Supervision.
S. Soudi, M. Tanhayi Ahary, S. H. Nabavi, and all authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aghaebrahimi, Z., Sabaghzadeh, J., Soudi, S. et al. Simultaneous effect of medicinal plants as natural photosensitizers and low-level laser on photodynamic inactivation. Lasers Med Sci 39, 95 (2024). https://doi.org/10.1007/s10103-024-04037-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-024-04037-8