Abstract
The purpose of this randomized, parallel, triple-blinded clinical trial was to compare efficacy and tooth sensitivity (TS) after use of an in-office bleaching agent of 6 % hydrogen peroxide containing nanoparticles of nitrogen-doped titanium oxide (HP6) vs. 35 % hydrogen peroxide (HP35). Forty-eight volunteers were randomly divided either a HP6 or HP35. Two clinical sessions were performed with an interval of 7 days between them for each group. In each session, two consecutive applications of each bleaching agent were performed and activated by a hybrid LED/laser light. Efficacy was determined by color alteration (ΔE), recorded with reflectance spectroscopy. It was assessed at baseline and after the first and second bleaching session. TS was characterized according to occurrence, intensity, duration, and type. Efficacy was analyzed by repeated measures analysis of variance (ANOVA) and post hoc Bonferroni test, and TS was analyzed by means of chi2 test (α = 0.05). For HP35, highest and significant values of ΔE were found after bleaching when compared to HP6 (p = 0.002). However, HP35 showed a significantly higher occurrence of TS than HP6 (p = 0.008). Also, intensity and duration were higher in HP35. The majority of volunteers classified the type experienced in their sensitivity in the form of a “shock.” The use of HP6 despite reducing efficacy when compared to an in-office bleaching in higher concentration (35 %) produced less tooth sensitivity. Clinical relevance: In terms of tooth sensitivity, the use of lower concentrations of in-office bleaching should be the first choice, suggesting greater biocompatibility and safety compared to a conventional HP35.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although dental bleaching is considered a simple and very safe technique, some studies have emphasized that in-office dental bleaching can cause different side effects to dental tissues, mainly when higher concentration of hydrogen peroxide was used [1, 2]. The major side effect is tooth sensitivity trans- and post-treatment [3, 4]. It is supposed that transdentinoenamel fast diffusion of hydrogen peroxide and/or its free radicals towards the pulp and the consequent chemical irritation produced may be responsible for this sensitivity [5], and therefore the occurrence of tooth sensitivity may represent the degree of biological aggression of this cosmetic procedure [1, 6].
In fact, de Souza Costa et al. [6] alerted that in-office bleaching using higher concentration of hydrogen peroxide caused irreversible pulp damages in the human lower incisors, and it was recently confirmed by Roderjan et al. [7]. This damage may have been caused by direct oxidation of the pulp tissues when in contact of hydrogen peroxide or as a result of intense inflammatory response of the pulp. Several studies have shown that the indirect cytotoxicity of the free radicals is proportional to the concentration of the bleaching agent and the contact time with the enamel [8–10]. Keeping this concept in mind, some manufacturers release low concentrated in-office bleaching gels in the market (15–20 %), but unfortunately, despite minimizing the tooth sensitivity, they do not reach the same bleaching efficacy [11].
More recently, new in-office bleaching agents with lower hydrogen peroxide (3.5–15 %) containing a semiconductor agent (nanoparticles of titanium dioxide doped with nitrogen, TiO_N) have been introduced aiming at increased safety and maintaining efficacy over conventional formulations. However, only hydrogen peroxide at a concentration of 15 % has been recently evaluated. In these studies, a lower sensitivity rate and same bleaching pattern were shown [4, 12, 13] when compared with 35 % of hydrogen peroxide, but unfortunately, around 30 % of the patients submitted to in-office bleaching with 15 % of hydrogen peroxide still have shown tooth sensitivity with different intensity rates [13].
In this context, it is worth mentioning that, according to the Guide of the European Community, only concentrations of >0.1–≤6 % of hydrogen peroxide present or released in bleaching products can only be sold to dental practitioners, because this range is considered safe to the patients (CUE 2012). However, to the extent of our knowledge, there are no clinical studies evaluating in-office tooth bleaching when concentration of hydrogen peroxide lower than 15 % was used. This randomized triple-blind clinical study investigated the efficacy (as primary outcome) and the tooth sensitivity (as secondary outcome) resultant of a low concentration bleaching agent of 6 % hydrogen peroxide containing nanoparticles TiO_N, testing the null hypotheses that (HP6) containing nanoparticles of TiO_N present similar efficacy and tooth sensitivity than 35 % hydrogen peroxide (HP35).
Materials and methods
This clinical study was approved by the Ethics Committee of the Araraquara Dental School, UNESP, São Paulo, Brazil (protocol number 01095812.4.0000.5416) and took place at this location, between July 2013 and June 2014. It is registered in the Brazilian Clinical Trials Registry (registration number U1111-1150-4466) and was conducted according to the Consolidated Standards of Reporting Trials statement [14].
The 48 volunteers selected received a dental prophylaxis and oral hygiene instructions 1 week prior to the beginning of this study in order to create similar oral conditions. They also signed a term of free and informed consent.
Study design
This was a randomized, triple-blinded (patients, evaluator, and statistician), and parallel study following nonprobability sampling. The patients were invited to participate in the study through posters around the city, as well as those who had previously participated in other studies in the same department were contacted by email and/or phone.
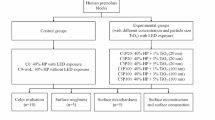
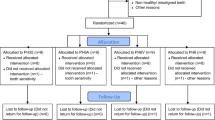
A total of 106 patients were examined in a dental chair to check if they meet the inclusion and exclusion criteria (Fig. 1). Patients included in this study were between 18 and 25 years old and selected under the following inclusion criteria: anterior teeth without restorations, previously submitted to bleaching procedure, cervical lesions or dental pain, and with improperly aligned teeth. Patients who are pregnant or lactating, have moderate and severe fluorosis, have tetracycline stains, undergoing orthodontic treatment, have periodontal disease, have orofacial tumors, have trauma, have tooth malformation or analgesic, are anti-inflammatory, or are taking antibiotic drug were excluded. Two trained operators performed the bleaching treatments. A third participant that does not have contact with the patients was responsible for conducting the randomization. The allocation of patients in the groups was performed by random drawing, using software Microsoft Excel 2010 (Microsoft, Redmond, Washington, USA) from coding assigned to each participant. The distribution of the operators was made by block randomization. To ensure triple blinding, the following procedures were adopted: (1) the operators were instructed not to reveal details about the products applied; (2) label, logos, packaging, and any other aspect that could identify the products used were removed and procedures and instruments used were standardized; (3) bleaching protocol was performed in a different room from where the evaluator examined the patients; (4) the randomization was alpha-numerically coded to ensure blinding of the research team; (5) operators received the opaque and sealed envelope containing the bleaching protocol that would be applied in each patient immediately before the clinical procedure; (6) evaluator received envelope containing just the code of the patient without containing any information about bleaching protocol; and (7) statistician received data tabulated in code that did not allow identifying the treatment applied to each group.
Sample size calculation
The primary outcome of this study was efficacy determined by color alteration (ΔE). Previously, studies showed that use of the in-office bleaching agent of 35 % hydrogen peroxide HP35 associated or not to LED/laser light would lead to a (ΔE) value of 7.0 ± 2.0 after two bleaching sessions [13, 15–17]. In order to have an 80 % chance of detecting significance at the level of 5 %, considering an increase in the primary outcome measure from “7” in the control group to “5” in the experimental group, a minimum of 16 participants would be required in each group. Due to higher dropout rate in the last two clinical studies of our research group, we decided to add more 50 % of patients, which led to 24 patients in each group.
Bleaching agents and LED/laser light
Two bleaching agents were used: a low concentration hydrogen peroxide product (Lase Peroxide Lite 6 %, DMC, São Carlos, SP, Brazil) and a traditional agent 35 % hydrogen peroxide (Total Blanc, Nova DFL, Rio de Janeiro, RJ, Brazil) proportioned and handled in accordance with the recommendations of each manufacturer. Both bleaching agents have yellowish-red coloring, in order to enable a better visualization, and blue absorption wavelength in the case of treatment with photocatalization.
The device (Whitening Lase Light Plus, DMC, São Carlos, SP, Brazil) was used for photocatalization. It is a specific equipment for in-office dental bleaching. It presents six LEDs (470 ± 15 nm, 300 mW each), generators of 1800 mW of power, and also three infra-red laser diodes (810 nm, 200 mW each) and generators of 600 mW of power, irradiating a total area of 8.5 cm2, with an intensity of 300 mW/cm2.
Bleaching protocol
In each session, volunteers received prophylaxis with pumice powder and water. Then, gingival tissue was protected using a light-cured resin gum barrier applied according to the manufacturer instructions (Lase Protect, DMC, São Carlos, SP, Brazil).
The 6 % hydrogen peroxide containing TiO_N (HP6) bleaching agent was prepared by mixing the “peroxide” and “thickening” compounds, according to the manufacturer’s instructions. For each application, 24 “peroxide” drops and eight “thickening” drops for each patient were used. The (HP35) bleaching agent was also prepared according to the manufacturer’s instructions. The two syringes containing the “peroxide” and “thickening” were connected, and the pistons were pushed up alternately. For each application, one “peroxide” syringe and one “thickening” syringe for each patient were used. The resultant gels were distributed uniformly on the buccal surfaces of the upper and lower teeth. A total of 16 teeth, between the first premolars, were bleached for each patient. In each bleaching session, the bleaching gels were applied twice, 12 min each one. In each application of 12 min, the surface of gel was light activated six times for each arcade, with alternating irradiance every 1 min, using LED/laser light. The application was performed perpendicular to upper and lower jaw, with 1 cm away from the tooth structure. The interval of sessions was 7 days for both groups.
Efficacy evaluation (E)
A calibrated evaluator (ICC = 0.74) measured the tooth color for the baseline (T0) and 7 days after first and second sessions (T1 and T2). The color evaluation was obtained from an area of 6 mm located in the middle third of the labial surface of the right central incisor. To standardize this evaluation, an impression of the maxillary arch using high-putty silicone (Zetaplus, Zhermack, Badia Polesine, Rovigo, Italy) was realized in order to make a guide. A window was created on the labial surface in the middle third of the central incisor, using a device with well-formed borders, 3 mm in radius corresponding to the reflectance spectrophotometer Vita EasyShade Advance tip (Wilcos, Petrópolis, Rio de Janeiro, Brazil). The shade was determined using the parameters of L*, a*, and b* obtained, and the color alteration after each session was given by the differences between the values obtained at the session and the baseline (∆E). It was calculated using the formula ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2 [18].
Tooth sensitivity evaluation (S)
Tooth sensitivity was characterized by variables occurrence, intensity, duration, and type. These data were obtained by a self-completed form. The occurrence was analyzed according to the report or not of sensitivity. The intensity was measured at four levels, according to a verbal scale: none, mild, moderate, or severe [19]. The duration was classified into five levels: 1 s, 1 min, 5 min, 1 h, and more than 1 h. For type, the volunteers should describe in one word the sensitivity noticed. The volunteers were instructed to fill out a form for each bleaching session, in case of sensitivity in any of bleached teeth and any time.
Statistical analysis
After verifying the normality of the data distribution (Shapiro-Wilk; p > 0.05 for HP35 at T1 and p ≥ 0.114 for other groups and times) and the homogeneity of variances-covariances (Box’s M test; p = 0.071), efficacy of the treatments was evaluated over color alteration (ΔE) and analyzed by a mixed repeated measures analysis of variance (ANOVA) test associated to Bonferroni correction. The statistical analyzes were performed using PASW Statistics 21.0 (SPSS Inc, Chicago, IL, USA) considering an α = 0.05.
Occurrence, intensity, duration, and type were evaluated taking in consideration the concentration of hydrogen peroxide (HP6 and HP35). For occurrence, we used the chi-square test with α = 0.05 (PASW Statistics 21.0, SPSS Inc, Chicago, IL, USA). For a description of intensity and duration, respectively, the highest intensity and greatest duration for each patient during all treatment were selected. The intensity, duration, and type were only qualitatively evaluated. The absolute risk reduction sensitivity (ARR) and the number needed to treat (NNT) were calculated by the following equations:
where CSR = control sensitivity rate and ESR = experimental sensitivity rate.
Results
The characteristics of the participants included in this clinical trial are described in Table 1. Figure 1 shows the participant flow diagram in different phases of the study design.
Efficacy
The ANOVA test demonstrated significant differences for time (p < 0.001), treatment (p = 0.008), and for the interaction time and treatment (p = 0.002). Considering the effect size, it can be concluded that the evaluation time has greater effect on the values of ΔE (ɳ p 2 = 0.309) compared to the effect of treatment (ɳ p 2 = 0.144) and the interaction between factors (ɳ p 2 = 0.183). The means and 95 % confidence interval (CI) for treatments and times are described in Fig. 2, which shows that only for HP35 the ΔE values were significantly higher after second bleaching session (p < 0.001).
Tooth sensitivity
In regard to the occurrence of tooth sensitivity, significant difference was observed between groups as seen in Table 2 (X 2 = 7.1; p = 0.008). The sensitivity rate in the HP6 and HP35 was significantly different with an average of 8.3 and 41.7 %, respectively. The absolute risk reduction and the number needed to treat calculated were 33.4 and 2.99, respectively (Table 2). Treatment HP35 presented highest intensity and longer duration of sensitivity than HP6 (Table 3). The most cited word by the volunteers to describe the type of sensitivity was “shock” (50 % HP6 and 60 % HP35). Besides that, other terms used by the volunteers were “shiver” (10 % HP35), “twinge” (50 % HP6 and 10 % HP35), “pain” (10 % HP35), and “tingle” (HP35).
Discussion
The null hypothesis of this study was rejected, given that the use of the HP6 showed lower efficacy and lower sensitivity when compared to HP35, rejected both null hypotheses.
Although a color change compared to baseline was shown in both groups, 35 % hydrogen peroxide obtained statistically significant greater color change (ΔE) than 6 % hydrogen peroxide after second bleaching sessions (Fig. 2), led us to reject the first null hypothesis. This result is probably due to the lower concentration of hydrogen peroxide in HP6.
Actually, we expected that, despite the use of lower concentration of hydrogen peroxide, the use of HP6 associated with nanoparticles of TiO_N would improve the efficacy of bleaching, mainly because these particles can be activated by visible light from the violet to blue range [20–22]. Recently, an optimization of light-curing units has occurred, with the development of equipment with wavelengths corresponding to the maximum absorbance peaks of semiconductor nanoparticles. It can maximize the heterogeneous oxidative reactions [23] and consequently the efficacy of dental bleaching with lower concentration bleaching agents [13], allowing the use of in-office bleaching agents with concentrations similar to those used for at-home bleaching, which potentially reduces the risk of sensitivity to dental tissues [4, 12, 13].
However, the lowest results in terms of efficacy for HP6 may have occurred due to the bleaching protocol used in this clinical trial. To avoid source of variation between groups and compare the effect of hydrogen peroxide concentration on the efficacy of tooth bleaching, the same protocol of light application was used for both bleaching agents. As 35 % hydrogen peroxide is considered the control group, we use the protocol recommended by the manufacturer of 35 % hydrogen peroxide, which unfortunately reduced the contact time of 6 % hydrogen peroxide for half of the recommended time for the manufacturer. Also, different studies indicate that, when lower concentration of hydrogen peroxide is used, it is necessary to have a higher number of application to optimize dental bleaching result [24]. Thus, we believe that HP6 can achieve the same result than HP35 if more sessions and/or more applications are performed in the same session. Future studies need to be done to evaluate these hypotheses.
Regarding tooth sensitivity, significant differences in occurrence of tooth sensitivity can be seen as HP6 showed fewer occurrences compared to HP35 (Table 2), which led us to reject the second null hypothesis. Also, while HP35 had reports of moderate and severe sensitivity lasting more than an hour, HP6 provided only mild sensitivity with shorter duration, only 1 min (Table 3). These results are in agreement with the literature that shows less report of tooth sensitivity and lower intensities when lower concentrations of bleaching agents are used [4, 11–13]. It is worth to mention that the most frequently reported type of tooth sensitivity was in the form of “shock,” typical “zinger” responses.
Despite the fact that tooth sensitivity is a common adverse effect, occurring for 30 to 80 % of patients who undergo dental bleaching, the mechanism through which tooth sensitivity occurs during and after dental bleaching is not completely established. Theories that explain the dentin hypersensitivity, such as Brännström hydrodynamic theory [25], have been proposed as possible explanations. However, there are major differences between the pain caused by dental bleaching and dentin hypersensitivity [26]. It has been suggested that the bleaching-induced tooth sensitivity occurs as a result of a reversible inflammatory process due to the presence of hydrogen peroxide and its degradation products in the pulp chamber [6, 26–28]. Although a hard tissue, enamel has pores that allow the penetration of hydrogen peroxide [29], which causes the release of inflammatory mediators that can sensitize or depolarize nociceptors that innervate the pulp tissue [30]. Recently, it has been shown that this inflammatory reaction also reduces the pulp blood flow [31]. According to Markowitz [26], the reason that most patients experience a pain as a “twinge” or a “shock” with no stimulus is because oxidant agents, as higher concentration of hydrogen peroxide, stimulate nociceptive afferents leading to sensitivity and inflammation.
The amount of peroxide detected in the pulp chamber is related to the concentration of hydrogen peroxide in the gel applied [8, 32] and the contact time of the gel with the dental structure [33]. Therefore, the higher rate of occurrence, intensity, and longer duration of sensitivity to HP35 are probably the result of greater penetration of hydrogen peroxide into the pulp tissue associated to its higher concentration [9, 10, 33, 34]. Also, the reduced concentration of hydrogen peroxide in HP6 and its consequently reduced penetration into the pulp tissue might have given more time for the pulp cells to produce enough peroxidases, catalases [35], and oxygenases [36] to protect the connective pulp tissue from the immediate damage caused by hydrogen peroxide. Soares et al. [9] showed in vitro study the reduction of pulpal cytotoxicity when the concentration of the bleaching agent was reduced, suggesting that the lower intensity and shorter duration of tooth sensitivity to the 6 % hydrogen peroxide bleaching agent are related to lower cytotoxicity of bleaching gel and smaller damages to the pulp and thus safer biologically.
This study was limited to the age range of 18–28 years old, which does not allow us to extrapolate the results to other ages, since the thickness of enamel, dentin, and size of pulp varies according to the age of the subjects [37, 38], which can alter the results of both efficacy and tooth sensitivity. New protocols using the lower hydrogen peroxide concentration bleaching agents may be tested in order to establish the efficacy, cytotoxicity, and tooth sensitivity.
Conclusion
The use of 6 % hydrogen peroxide containing nanoparticles of doped nitrogen titanium oxide produced less tooth sensitivity but reduced the efficacy of in-office bleaching when compared to a protocol in-office bleaching in higher concentration 35 %.
References
Dahl J, Pallesen U (2003) Tooth bleaching—a critical review of the biological aspects. Crit Rev Oral Biol Med 14:292–304
Bruzell EM, Pallesen U, Thoresen NR, Wallman C, Dahl JE (2014) Side effects of external tooth bleaching. Br Dent J 215:E17
Kossatz S, Martins G, Loguercio AD, Reis A (2012) Tooth sensitivity and bleaching effectiveness associated with use of a calcium-containing in-office bleaching gel. J Am Dent Assoc 143:e81–e87
Martin J, Fernandez E, Bahamondes V, Werner A, Elphick K, Oliveira O Jr, Moncada G (2013) Dentin hypersensitivity after teeth bleaching with in-office systems. Randomized clinical trial. Am J Dent 26:10–14
Benetti AR, Valera M, Mancini M, Miranda C, Balducci I (2004) In vitro penetration of bleaching agents into the pulp chamber. Int Endod J 37:120–124
de Souza Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:e59–e64
DA Roderjan SR, Hebling J, de Souza Costa CA, Soares DG, Reis A, Loguercio AD (2014) Histopathological features of dental pulp tissue from bleached mandibular incisors. J Mater Sci Eng B 4:8
Hanks CT, Fat JC, Wataha JC, Corcoran JF (1993) Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, in vitro. J Dent Res 72:931–938
Soares DG, Basso FG, Hebling J, de Souza Costa CA (2014) Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent 42:185–198
Soares DG, Ribeiro APD, da Silveira VF, Hebling J, de Souza Costa CA (2012) Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel. Clin Oral Investig 17:1901–1909
Reis A, Kossatz S, Martins GC, Loguercio AD (2013) Efficacy of and effect on tooth sensitivity of in-office bleaching gel concentrations: a randomized clinical trial. Oper Dent 38:386–393
Moncada G, Sepúlveda D, Elphick K, Contente M, Estay J, Bahamondes V, Fernandez E, Oliveira O, Martin J (2013) Effects of light activation, agent concentration, and tooth thickness on dental sensitivity after bleaching. Oper Dent 38:467–476
Bortolatto JF, Pretel H, Floros MC, Luizzi ACC, Dantas AAR, Fernandez E, Moncada G, de Oliveira OB (2014) Low concentration H2O2/TiO_N in office bleaching: a randomized clinical trial. J Dent Res 93:66S–71S
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 8:18
Bortolatto JF, Pretel H, Neto CS, Andrade MF, Moncada G, Junior OBO (2013) Effects of LED–laser hybrid light on bleaching effectiveness and tooth sensitivity: a randomized clinical study. Laser Phys Lett 10:085601
Kossatz S, Dalanhol A, Cunha T, Loguercio A, Reis A (2011) Effect of light activation on tooth sensitivity after in-office bleaching. Oper Dent 36:251–257
Reis A, Dalanhol AP, Cunha TS, Kossatz S, Loguercio AD (2011) Assessment of tooth sensitivity using a desensitizer before light-activated bleaching. Oper Dent 36:12–17
Hoffmann G (2000) Cie color space. online http://www.fho-emdende/hoffmann/-ciexyz29082000pdf
Jorgensen MG, Carroll WB (2002) Incidence of tooth sensitivity after home whitening treatment. J Am Dent Assoc 133:1076–1082
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Suyama Y, Otsuki M, Ogisu S, Kishikawa R, Tagami J, Ikeda M, Kurata H, Cho T (2009) Effects of light sources and visible light-activated titanium dioxide photocatalyst on bleaching. Dent Mater J 28:693–699
Tano E, Otsuki M, Kato J, Sadr A, Ikeda M, Tagami J (2012) Effects of 405 nm diode laser on titanium oxide bleaching activation. Photomed Laser Surg 30:648–654
Kishi A, Otsuki M, Sadr A, Ikeda M, Tagami J (2011) Effect of light units on tooth bleaching with visible-light activating titanium dioxide photocatalyst. Dent Mater J 30:723–729
Sulieman M, Addy M, MacDonald E, Rees J (2004) The effect of hydrogen peroxide concentration on the outcome of tooth whitening: an in vitro study. J Dent 32:295–299
Brännström M, Aström A (1972) The hydrodynamics of the dentine: its possible relationship to dentinal pain. Int Dent J 22:219–227
Markowitz K (2010) Pretty painful: why does tooth bleaching hurt? Med Hypotheses 74:835–840
Trindade FZ, Ribeiro APD, Sacono NT, Oliveira CF, Lessa FCR, Hebling J, Costa CAS (2009) Trans‐enamel and trans‐dentinal cytotoxic effects of a 35% H2O2 bleaching gel on cultured odontoblast cell lines after consecutive applications. Int Endod J 42:516–524
Li Y (1997) Toxicological considerations of tooth bleaching using peroxide-containing agents. J Am Dent Assoc 128:31S–36S
Pinto CF, Oliveira RD, Cavalli V, Giannini M (2004) Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz Oral Res 18:306–311
Charakorn P, Cabanilla LL, Wagner WC, Foong WC, Shaheen J, Pregitzer R, Schneider D (2009) The effect of preoperative ibuprofen on tooth sensitivity caused by in-office bleaching. Oper Dent 34:131–135
Cartagena AF, Parreiras SO, Loguercio AD, Reis A, Campanha NH (2015) In-office bleaching effects on the pulp flow and tooth sensitivity–case series. Braz Oral Res 29:1–6
Thitinanthapan W, Satamanont P, Vongsavan N (1999) In vitro penetration of the pulp chamber by three brands of carbamide peroxide. J Esthet Dent 11:259–264
Marson FC, Gonçalves RS, Silva CO, Cintra L, Pascotto RC, Santos PH, Briso ALF (2014) Penetration of hydrogen peroxide and degradation rate of different bleaching products. Oper Dent 40:72–79
de Almeida LC, Costa CA, Riehl H, dos Santos PH, Sundfeld RH, Briso AL, Brito-Júnior M, Nobre SA, Freitas JC, Camilo CC (2012) Occurrence of sensitivity during at-home and in-office tooth bleaching therapies with or without use of light sources. Acta Odontol Latinoam 25:3–8
Bowles WH, Burns H (1992) Catalase/peroxidase activity in dental pulp. J Endod 18:527–534
Anderson DG, Chiego DJ, Glickman GN, McCauley LK (1999) A clinical assessment of the effects of 10% carbamide peroxide gel on human pulp tissue. J Endod 25:247–250
Morse DR (1991) Age-related changes of the dental pulp complex and their relationship to systemic aging. Oral Surg Oral Med Oral Pathol 72:721–745
Bernick S, Nedelman C (1975) Effect of aging on the human pulp. J Endod 1:88–94
Acknowledgments
This study was supported by CAPES.
We thank NUPEN and the undergraduate students Rafaela Nanami Handa Inada, Camila Yumi Morita, and Priscila Petrucelli Freire de Carvalho for the technical and scientific support.
We gratefully acknowledge the contribution of Michael Floros (Trent University, ON, Canada) for his English language and grammar corrections.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Trial registration: U1111-1150-4466
Rights and permissions
About this article
Cite this article
Bortolatto, J.F., Trevisan, T.C., Bernardi, P.S.I. et al. A novel approach for in-office tooth bleaching with 6 % H2O2/TiO_N and LED/laser system—a controlled, triple-blinded, randomized clinical trial. Lasers Med Sci 31, 437–444 (2016). https://doi.org/10.1007/s10103-016-1866-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-1866-2