Abstract
Low-level laser therapy has been shown to decrease ischemia–reperfusion injuries in the skeletal muscle by induction of synthesis of antioxidants and other cytoprotective proteins. Recently, the light-emitting diode (LED) has been used instead of laser for the treatment of various diseases because of its low operational cost compared to the use of a laser. The objective of this work was to analyze the effects of LED therapy at 904 nm on skeletal muscle ischemia–reperfusion injury in rats. Thirty healthy male Wistar rats were allocated into three groups of ten rats each as follows: normal (N), ischemia–reperfusion (IR), and ischemia–reperfusion + LED (IR + LED) therapy. Ischemia was induced by right femoral artery clipping for 2 h followed by 2 h of reperfusion. The IR + LED group received LED irradiation on the right gastrocnemius muscle (4 J/cm2) immediately and 1 h following blood supply occlusion for 10 min. At the end of trial, the animals were euthanized and the right gastrocnemius muscles were submitted to histological and histochemical analysis. The extent of muscle damage in the IR + LED group was significantly lower than that in the IR group (P < 0.05). In comparison with other groups, tissue malondialdehyde (MDA) levels in the IR group were significantly increased (P < 0.05). The muscle tissue glutathione (GSH), superoxide dismutases (SOD), and catalase (CAT) levels in the IR group were significantly lower than those in the subjects in other groups. From the histological and histochemical perspective, the LED therapy has alleviated the metabolic injuries in the skeletal muscle ischemia reperfusion in this experimental model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prolonged ischemia and reperfusion frequently occur in a variety of conditions such as reconstructive plastic surgery, orthopedic surgery, thrombolytic therapy, transplantation, and limb trauma such as crush injury and fractures. The events during ischemia reperfusion are basically similar in different organs and tissues but the time scale of the event varies [1–8]. Ischemia leads to muscle cell energy failure, inflammatory reaction, and biochemical alterations. These are worsened by reperfusion, which triggers high free radical production and neutrophil activation, making local and systemic lesions more severe [1–9]. The effect of ischemia reperfusion on skeletal muscle has been well described in several studies [10–15]. Therefore, it is important to find preservation methods for the skeletal muscle that decrease injuries caused by ischemia reperfusion.
Recently, phototherapy with light-emitting diode (LED) has attracted attention for inducing tissue repair through several mechanisms [16–18]. LED radiation has many biological effects similar to those of lasers, such as increases the adenosine triphosphate (ATP) synthesis, angiogenesis, collagen and protein synthesis, fibroblast proliferation; inhibits pain and oxidative stress; and decreases inflammatory reactions [16–25]. Phototherapy in wavelengths ranging from <600 to 1000 nm leads to activation of cytochrome c oxidase and improvement of ATP synthesis in cell cultures, which favors the expression of genes related to tissue repair [19–21, 26, 27]. No information is available about the applicability of LED therapy on muscle reperfusion injury. Since LED therapy can counteract many of the deleterious effects induced by high intensity exercise on the skeletal muscle [28, 29], this treatment may be a suitable method for preventing symptoms of skeletal muscle damage after ischemia reperfusion. This approach may be useful for decreasing injuries during ischemia reperfusion but this possibility has yet to be tested. The aim of this study, therefore, was to investigate the effects of 940-nm wavelength LED therapy on a skeletal muscle injury in a rat model following acute ischemia reperfusion. The study hypothesis was that LED therapy could decrease muscle damage induced by ischemia reperfusion.
Material and methods
Thirty healthy male Wistar rats, aged between 3 and 4 months with body weight between 300 and 320 g, were purchased from the Pasteur Institute of Iran. This study was conducted according to the guidelines of the animal care review board of the Islamic Azad University Faculty of Specialized Veterinary Sciences and adhering to the guide for care and use of laboratory animals, and the study is approved by the ethics committee. They were kept in a controlled temperature and humidity, 12 h/12 h light/dark cycle, with ad libitum access to commercial food and filtered tap water.
Experimental groups
The rats were divided randomly into three groups of ten rats each (of these ten, five were used for histochemical assays and five for histological analysis), normal (N), ischemia–reperfusion (IR), and ischemia–reperfusion + LED (IR + LED) therapy.
Surgery
Surgery was performed at the Laboratory of Experimental Surgery, Science and Research Faculty of Specialized Veterinary Sciences. The rats were weighed and anesthetized using an intramuscular injection of ketamine hydrochloride 10 % and xylazine hydrochloride 2 % (50 mg/kg and 10 mg/kg, respectively). The animals were placed on a table, in dorsal recumbency, with their fore- and rear limbs immobilized with adhesive tape. After clipping, disinfecting with antiseptic povidone-iodine solution, and draping, a skin incision was made on the medial surface of the right hind limb. After the isolation of the femoral artery and vein from the surrounding tissues, the femoral artery was exposed. Ischemia was induced by 2 h of femoral artery occlusion with a mini vascular clamp and followed by 2 h of reperfusion. Rats were maintained in dorsal recumbency and kept anesthetized (additional doses were given in case of necessity) throughout the ischemic period. Body temperature was maintained with a heating pad and monitored using a rectal thermometer. Following the ischemic period, the vascular forceps were removed and the surgical site was routinely closed with 3/0 nylon sutures.
LED therapy
LED equipment was developed for this study according to parameters that were suggested by Costa Santos et al. [28]. The optical output power of the light source was verified prior to experimental procedures. The animals in IR + LED group were submitted to irradiation at a wavelength of 940 nm, delivering an energy intensity of 4 J/cm2 and a power density of 9.5 mW/cm2 to 1 cm2 of area immediately and 1 h following blood supply occlusion for 10 min. The radiation source was attached to a support and kept perpendicular 1 cm above the skin surface, on the middle region of the right gastrocnemius muscle belly.
Specimen collection
At the end of the trial, euthanasia was performed by using an overdose of pentobarbital (300 mg/kg) intraperitoneally. The right gastrocnemius muscle was harvested immediately, accomplished by incision in the posterior face of the leg then it was submitted for histological and biochemical assays.
Histological analysis
The muscle samples were paraffin-embedded. Using standard techniques, paraffin sections were obtained at 5 μm, stained with hematoxylin and eosin, and studied under optical microscopy by two pathologists who were blinded to the experiment and data. Histological changes were scored on a scale from 0 to 3 where 0 = absence (<5 % of myocytes injury), 1 = mild (<10 %), 2 = moderate (15–20 %), and 3 = severe (20–25 %) [30]. A total of ten fields of view from each muscle sample were randomly screened, and the mean was accepted as the representative value of the sample.
Biochemical assays
Glutathione (GSH), superoxide dismutases (SOD), catalase (CAT), and malondialdehyde (MDA) were detected in skeletal muscle tissue cuts. Each tissue was stocked in a separate bowl at −80 °C until analysis. Then, tissues were homogenized with cold Tris–HCl buffer (pH 7.4) to make a 10 % homogenate. GSH measurements were taken using a modification of the Ellman procedure [31]. The results were expressed as nanomoles/100 mg protein in tissue samples. The total SOD activity was measured kinetically by a method described by Sun et al. [32]. SOD activity was expressed as units/milligram of tissue protein. Muscle tissue CAT activity was measured according to Aebi’s method [33]. Results were expressed as k (rate constant per gram protein; k/g protein). The MDA levels were assayed for products of lipid peroxidation by monitoring thiobarbituric acid-reactive substance formation as described previously [34]. The results are expressed as nanomole MDA/gram tissue.
Statistical analysis
Data were analyzed by using SPSS statistical software package (version 18). Distribution of the groups was analyzed with one sample Kolmogorov–Smirnov test. The results were analyzed by using analysis of variance (ANOVA) for the comparison of multiple means with post hoc test analysis. Results of the study were expressed as the mean ± standard deviation. Values of P < 0.05 were considered as statistically significant.
Results
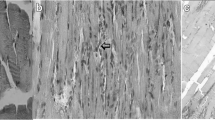
The experimental procedure was well tolerated, and no animal died during the experiment. The muscles in the IR group exhibited destruction of muscle fibers with edema and inflammatory cell infiltration 2 h after reperfusion. Shrinkage was observed in muscle structure, and cell gaps were obviously widened. The degree of injuries was largely uniform in most subjects, and all subjects had a high histologic damage score after ischemia–reperfusion injury (Fig. 1). In contrast to the muscle tissues observed after reperfusion injury in the IR group, damage to muscle fibers was rarely observed in the IR + LED therapy group (Fig. 2). Degeneration was not obvious and cell gap, slightly wide; however, there were some cases of inflammatory cell infiltration and edema between muscle fibers (Fig. 3). Histological findings indicate that the scavenging of free radicals by LED therapy may inhibit inflammatory cascades.
In IR + LED therapy group, treatment of phototherapy significantly decreased (P < 0.05) skeletal muscle MDA level compared to the IR group (Fig. 4). SOD, CAT, and GSH levels were measured in the skeletal muscle. Results of oxidative stress markers in each group were shown in Figs. 5, 6, and 7. The muscle tissue GSH levels in the IR group were significantly lower (P < 0.05) than those in the LED treatment group. Ischemia–reperfusion injury to the skeletal muscle significantly decreased (P < 0.05) SOD activity muscle tissues. CAT activity in muscle tissue decreased (P < 0.05) markedly after reperfusion. A significant decrease in the activity of CAT was observed in the IR group compared with the IR + LED group.
Discussion
At the onset of ischemia, the biochemical alterations in the basic cellular functions can be reversed; however, when the ischemia time is longer, another sequence of reactions is triggered as a consequence of cellular energetic failure [35, 36]. The anaerobic metabolism prevails up to 2 h of ischemia, increasing the amount of lactate and inorganic phosphate and reducing pH, ATP, and creatine [37–39].
The ATP synthesized by the anaerobic metabolism maintains the ion pumps, the membrane potential, and the contractile function, although the production of lactic acid infuses into the interstitial space, causing edema and academia [40]. When this energetic source is over, the pumps fail and the ionic gradient of the cells is altered with potassium and magnesium release and sodium and calcium input into the intracellular medium, causing edema in the cell, matrix, or mitochondrial crests [37, 40, 41]. Increased intracellular calcium concentration activates the cytoplasmic proteases. In combination with the high concentration of hypoxanthine resultant from the uninterrupted degradation of ATP, the proteases convert the enzyme xanthine dehydrogenase into the oxidase enzyme. This enzyme has an important role in reperfusion injuries. Calcium also activates the lysosomal enzymes that impair organelles and the phospholipase enzyme A2, which degradates the arachidonic acid, originating inflammation mediators like leukotrienes, prostaglandins, prostacyclins, and thromboxanes [37, 39, 40]. Neutrophils are activated by the leukotriene B4 [37].
Paradoxically, although reperfusion is critical for reverting ischemia, it worsens injuries that occurred during the ischemia period. The production of exceeding free radicals and the intense participation of neutrophils increase the inflammatory reaction thereby promoting muscular edema formation, tissue necrosis, impairment of systemic clinical conditions and lead to limb loss and even death.
The photobiomodulation has been ascribed to activation of the mitochondrial respiratory chain, which leads to activation of several intracellular signaling cascades and cell proliferation [16, 18, 21]. Cytochrome c oxidase is the photon acceptor of LED irradiation, and increased ATP synthesis may be one of the beneficial effects of phototherapy. Other benefits include increased tissue repair and the maintenance of tissue homeostasis [19, 20, 24]. LED irradiation also acts as a radical scavenger, reducing reactive oxygen species production and inhibiting activation of phospholipase A2. This mechanism avoids oxidative damage to muscle and reduces inflammation caused by the release of prostaglandins [24]. Another mechanism involved in muscle protection that is affected by LED therapy is the expression of several genes related to angiogenesis, synthesis of basement membrane components, and muscle differentiation [21]. Other biological effects of phototherapy include the alleviation of pain and nociception [24, 42, 43]. Effects on tissue repair and inflammation can be reached in wavelengths ranging from 600 to 1000 nm [18, 21, 23, 44].
Some studies have suggested that 940-nm LED irradiation could decrease serum markers of muscle injury and inflammation after exercise [28, 29]. On the basis of these studies, the LED equipment was developed for this study according to parameters that were suggested [28, 29]. In the present study, we observed that LED irradiation at 940 nm has the potential to prevent skeletal muscle injury from ischemia reperfusion and local inflammatory cell infiltration. LED irradiation decreased the damaged muscle fibers, decreased areas of necrosis, and reduced leukocyte infiltration.
Conclusion
The results of this study indicate that 940-nm LED phototherapy is effective for reducing muscle inflammation and muscle fiber injury induced by ischemia reperfusion. Its effect may be related to anti-inflammatory action and the preservation of muscle fiber cell membrane integrity.
References
Takhtfooladi MA, Jahanshahi A, Sotoudeh A, Jahanshahi G, Aslani K, Khansari M, Adibi S, Takhtfooladi HA (2012) Effects of N-acetylcysteine upon the rat serum enzyme changes in skeletal muscle ischemia reperfusion. Ann Biol Res 3(8):4099–4102
Takhtfooladi MA, Jahanshahi A, Sotoudeh A et al (2012) Synergistic protective effect of ischemic preconditioning technique in combination of N-acetylcysteine on skeletal muscle ischemia reperfusion injury in rat; Histopathological study. Eur J Exp Biol 2(5):1766–1770
Takhtfooladi MA, Jahanshahi G, Sotoudeh A, Jahanshahi A (2013) Protective effects of N-acetylcysteine on myocardial injury induced by hindlimb ischaemia–reperfusion: a histological study in rat model. Comp Clin Pathol. doi:10.1007/s00580-013-1768-7
Takhtfooladi MA, Jahanshahi A, Sotoudeh A et al (2013) Effect of tramadol on lung injury induced by skeletal muscle ischemia-reperfusion: an experimental study. J Bras Pneumol 39(4):434–439
Takhtfooladi MA, Jahanshahi A, Sotoudeh A et al (2013) Neuroprotective effects of tramadol on cerebral injuries caused by hind limb ischaemia/reperfusion in rats. Comp Clin Pathol. doi:10.1007/s00580-013-1753-1
Takhtfooladi MA, Jahanshahi A, Jahanshahi GH, Sotoudeh A, Takhtfooladi HA, Khansari M (2012) Protective effect of N-acetylcysteine on kidney as a remote organ after skeletal muscle ischemia-reperfusion. Acta Cir Bras 27(9):611–615
Sotoudeh A, Takhtfooladi MA, Jahanshahi A, Khiabanian Asl AH, Takhtfooladi HA, Khansari M (2012) Effect of N-acetylcysteine on lung injury induced by skeletal muscle ischemia-reperfusion. Histopathological study in rat model. Acta Cir Bras 27(2):168–171
Takhtfooladi MA, Jahanshahi A, Sotoudeh A, Daneshi MH, Khansari M, Takhtfooladi HA (2013) The antioxidant role of N-acetylcysteine on the testicular remote injury after skeletal muscle ischemia and reperfusion in rats. Pol J Pathol 64(3):204–209. doi:10.5114/PJP.2013.38140
da Silveira M, Yoshida WB (2004) Ischemia and reperfusion in skeletal muscle injury mechanisms and treatment perspectives. J Vasc Br 3(4):367–378
Brunelli GA, Brunelli GR (1995) Tissue changes at different periods of ischemia. Int Angiol 14(3):253–263
Gordon L, Buncke HJ, Townsend JJ (1978) Histological changes in skeletal muscle after temporary independent occlusion of arterial and venous supply. Plast Reconstr Surg 61(4):576–579
Lagerwall K, Daneryd P, Schersten T, Soussi B (1995) In vivo 31P nuclear magnetic resonance evidence of the salvage effect of ascorbate on the postischemic reperfused rat skeletal muscle. Life Sci 56(6):389–397
Skjeldal S, Grogaard B, Reikeras O, Muller C, Torvik A, Svindland A (1991) Model for skeletal muscle ischemia in rat hindlimb: evaluation of reperfusion and necrosis. Eur Surg Res 23(5–6):355–365
Lindsay TF, Liauw S, Romaschin AD, Walker PM (1990) The effect of ischemia/reperfusion on adenine nucleotide metabolism and xanthine oxidase production in skeletal muscle. J Vasc Surg 12(1):8–15
Labbe R, Lindsay T, Walker PM (1987) The extent and distribution of skeletal muscle necrosis after graded periods of complete ischemia. J Vasc Surg 6(2):152–157
Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT (2004) Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 4(5–6):559–567. doi:10.1016/j.mito.2004.07.033
Douris P, Southard V, Ferrigi R, Grauer J, Katz D, Nascimento C, Podbielski P (2006) Effect of phototherapy on delayed onset muscle soreness. Photomed Laser Surg 24(3):377–382. doi:10.1089/pho.2006.24.377
Komine N, Ikeda K, Tada K, Hashimoto N, Sugimoto N, Tomita K (2010) Activation of the extracellular signal-regulated kinase signal pathway by light emitting diode irradiation. Lasers Med Sci 25(4):531–537. doi:10.1007/s10103-009-0743-7
Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT (2005) Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 280(6):4761–4771. doi:10.1074/jbc.M409650200
Liang HL, Whelan HT, Eells JT, Wong-Riley MT (2008) Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience 153(4):963–974. doi:10.1016/j. neuroscience.2008.03.042
Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MT, Eells JT, Gould LJ, Hammamieh R, Das R, Jett M (2003) Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg 21(2):67–74. doi:10.1089/104454703765035484
Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC (2003) Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci 18(2):95–99. doi:10.1007/s10103-003-0262-x
Xavier M, David DR, de Souza RA, Arrieiro AN, Miranda H, Santana ET, Silva JA Jr, Salgado MA, Aimbire F, Albertini R (2010) Anti-inflammatory effects of low-level light emitting diode therapy on Achilles tendinitis in rats. Lasers Surg Med 42(6):553–558. doi:10.1002/lsm.20896
Lim W, Lee S, Kim I, Chung M, Kim M, Lim H, Park J, Kim O, Choi H (2007) The anti-inflammatory mechanism of 635-nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med 39(7):614–621. doi:10.1002/lsm.20533
Casalechi HL, Nicolau RA, Casalechi VL, Silveira L Jr, De Paula AM, Pacheco MT (2009) The effects of low-level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci 24(4):659–665. doi:10.1007/s10103-008-0607-6
Karu TI, Pyatibrat LV, Kalendo GS (2001) Cell attachment modulation by radiation from a pulsed light diode (lambda0820 nm) and various chemicals. Lasers Surg Med 28(3):227–236. doi:10.1002/lsm.1043
Sussai DA, Carvalho Pde T, Dourado DM, Belchior AC, dos Reis FA, Pereira DM (2010) Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci 25(1):115–120. doi:10.1007/s10103-009-0697-9
da Costa Santos VB, de Paula Ramos S, Milanez VF, Corrêa JC, de Andrade Alves RI, Dias IF, Nakamura FY (2014) LED therapy or cryotherapy between exercise intervals in Wistar rats: anti-inflammatory and ergogenic effects. Lasers Med Sci 29(2):599–605. doi:10.1007/s10103-013-1371-9
Camargo MZ, Siqueira CP, Preti MC, Nakamura FY, de Lima FM, Dias IF, Toginho Filho Dde O, Ramos Sde P (2012) Effects of light emitting diode (LED) therapy and cold water immersion therapy on exercise-induced muscle damage in rats. Lasers Med Sci 27(5):1051–1058. doi:10.1007/s10103-011-1039-2
Ulrich R, Roeder G, Lorber C et al (2001) Continuous venovenous hemofiltration improves arterial oxygenation in endotoxin-induced lung injury in pigs. Anesthesiology 95(2):428
Boyne AF, Ellman GL (1972) A methodology for analysis of tissue sulfhydryl components. Anal Biochem 46:639
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497
Aebi H (1974) Catalase. In: Bergmeyer U (ed) Methods of enzymatic analysis. Academic, New York, pp 673–677
Beuge JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302
Perry MO (1988) Compartment syndromes and reperfusion injury. Surg Clin N Am 68:853–864
Quinones BWJ, Saleh S (1991) Acute arterial occlusion. In: Moore WS (ed) Vascular surgery. A comprehensive review, vol 33. Saunders, Philadelphia, pp 578–597
Morin D, Hauet T, Spedding M, Tillement J (2001) Mitochondria as target for antiischemic drugs. Adv Drug Deliv Rev 49:151–174
Barie PS, Mullins RJ (1988) Experimental methods in the pathogenesis of limb ischemia. J Surg Res 44:284–307
Swartz WM, Cha CJ, Clowes GH Jr, Randall HT (1978) The effect prolonged ischemia on high energy phosphate metabolism in skeletal muscle. Surg Ginecol Obstet 147:872–876
Yoshida WB (1996) Radicais livres na síndrome da isquemia e reperfusão. Cir Vasc e Angiol 12:82–95
Brooks B (1922) Pathologic changes in muscle as a result of disturbances of circulation: an experimental study of Volkmann’s ischemic paralysis. Arch Surg 5:188–216
Jimbo K, Noda K, Suzuki K, Yoda K (1998) Suppressive effects of low-power laser irradiation on bradykinin evoked action potentials in cultured murine dorsal root ganglion cells. Neurosci Lett 240(2):93–96
Simunovic Z, Trobonjaca T, Trobonjaca Z (1998) Treatment of medial and lateral epicondylitis—tennis and golfer’s elbow—with low level laser therapy: a multicenter double blind, placebo-controlled clinical study on 324 patients. J Clin Laser Med Surg 16(3):145–151
Serafim KG, Ramos SD, de Lima FM, Carandina M, Ferrari O, Dias IF, Toginho Filho DD, Siqueira CP (2011) Effects of 940 nm light-emitting diode (led) on sciatic nerve regeneration in rats. Lasers Med Sci. doi:10.1007/s10103-011-0923-0
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takhtfooladi, M.A., Shahzamani, M., Takhtfooladi, H.A. et al. Effects of light-emitting diode (LED) therapy on skeletal muscle ischemia reperfusion in rats. Lasers Med Sci 30, 311–316 (2015). https://doi.org/10.1007/s10103-014-1670-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1670-9