Abstract
The objective of this study was to investigate the effects of low-level laser therapy (LLLT) treatment alone (λ = 660 nm and λ = 830 nm) or associated with platelet-rich plasma (PRP). We used 54 male rats divided into six groups, with nine animals each: group 1, partial tenotomy; group 2 (GII), PRP; group 3 (GIII): λ660 nm; group 4 (GIV), λ830 nm; group 5 (GV), PRP + λ660 nm; and group 6 (GVI), PRP + λ830 nm. The protocol used was power density 0.35 W/cm2, energy 0.2 J, energy density 7.0 J/cm2, time 20 s per irradiated point, and number of points 3. Animals in groups GII, GV, and GVI received treatment with PRP, consisting of a single dose of 0.2 mL directly into the surgical site, on top of the tenotomy. Animals were killed on the 13th day post-tenotomy and their tendons were surgically removed for a quantitative analysis using polarization microscopy. The percentages of collagen fibers of types I and III were expressed as mean ± SD. Higher values of collagen fibers type I were obtained for groups GV and GVI when compared with all other groups (p < 0.05), whereas groups GIII and GIV showed no significant difference between them (p > 0.05). For collagen type III, a significant difference was observed between GII and all other groups (p < 0.5), but no significant difference was found between GIII and GIV and between GV and GVI. Results showed that the deposition of collagen type I was higher when treatment with PRP and LLLT was combined, suggesting a faster regeneration of the tendon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lesions of the calcaneal tendon (CT) are a common cause of disability and are clinically characterized by pain and swelling in and around the tendon, mainly arising from overuse [1]. These lesions are associated with disruption of collagen fibers, increase in noncollagenous matrix, haphazard proliferation of tenocytes, and subsequent decrease on biomechanical properties of tendon [2].
Tendon matrix is rich in collagens, such as types I and III collagens. Type I collagen is considered to be responsible for the mechanical strength of the tendon tissue and type III collagen has an important role in the healing process [3]. Type I collagen (thick fibers) is the primary collagen incorporated in the tendon structure, and increasing the production of type I collagen may enhance tendon healing [4].
Currently, a variety of treatments for lesions of the CT are used or have been trialed. However, there is little evidence that any conventional therapies are effective. In the last years, low-level laser therapy (LLLT) [4–12] and platelet-rich plasma (PRP) [13–21] have been used in orthopedics, traumatology, and sports medicine showing interesting results in modulation of calcaneal tendon repair. However, optimal parameters and mechanisms behind these effects are not fully understood.
It has been demonstrated that LLLT reduces inflammatory processes [5, 6] and promotes calcaneal tendon healing interfering with the production and realignment of collagen fibers [4, 7, 9–11], as well as enhancing biochemical and biomechanical parameters of the tendon [12]. On the other hand, the properties of high interest in PRP and calcaneal tendon repair are justified by a reduction in total time of recovery of tissue injury [16–18]. Besides, PRP is contributing to the tissue repair through stimulation promoted by the presence of chemotactic cytokines, chemokines, blood proteins, and growth factors present in the plasma [15]. Platelets act in homeostasis, wound healing, and reepithelialization, releasing several growth factors, which in turn stimulates angiogenesis, promoting fibroblast proliferation thereby increasing collagen synthesis [21]. Up to now, we found no study that associates the two therapies (LLLT and PRP) in an attempt to improve tissue repair.
Inserted in this perspective, the objective of this study was to investigate the effects of LLLT treatment (λ = 660 nm and λ = 830 nm) alone or associated with PRP in tenotomies partial of the CT in Wistar rats. The qualitative and quantitative assessment will be carried out aiming to analyze the presence of fibers of collagen type I and type III in the histological slides.
Materials and methods
This work was developed in compliance with the Standards of Animal Experimentation and in accordance with the ethical principles of handling and care of laboratory animals recommended by the Brazilian College of Animal Experimentation. Standards for educational and scientific practice of vivisection of animals were observed at all stages of the study (Law 6638 of 08/05/1979). The approval of the research protocol is registered under the number CEP/UNIPAC 2011/6.
Animals and groups
This study was conducted with 54 male Wistar rats (Rattus norvegicus albinos), with 8 weeks of age, body mass of 200 ± 12.3 g, and maintained at temperature range of 20–22 °C, from the Central Animal Facility at the University of São Paulo. The animals remained in the vivarium of the Laboratory of Physiology and Pathology, President Antonio Carlos University (Itajubá, Minas Gerais, Brazil) in seven standard polypropylene cages, kept in a controlled environment with 12-h light–dark cycle, and received water and food “ad libitum.”
The animals were randomly divided into six groups with nine animals each: Group I (GI), partial tenotomy of the CT but received no treatment; group II (GII), partial tenotomy of the CT + PRP treatment; group III (GIII), partial tenotomy of the CT + LLLT λ660 nm; group IV (GIV), partial tenotomy of the CT + LLLT λ830 nm; group V (GV), partial tenotomy of the CT + PRP + LLLT λ660 nm; and group VI (GVI), partial tenotomy of the CT + PRP + LLLT λ830 nm treatment.
Procedure for preparation of PRP
Animals from groups GII, GV, and GVI, after being anesthetized, were subjected to a puncture of the caudal vein, and then 0.3 mL of blood was removed from each animal for PRP preparation. It is suggested in the literature that the amount of blood withdrawn should be not more than 6.4 % of the animal body weight [22]. The quantity of PRP obtained was approximately 10 to 15 % of the total volume of blood.
Most of the protocols for PRP production used a small fraction of blood (0.3–0.5 mL). This blood is first subjected to a 10-min centrifugation at 800 rpm, followed by another 20 min at 1,600 rpm [23, 24]. A 10 % calcium chloride activator was added in a ratio of 1:20 for obtaining the total volume of PRP. Platelet counts were performed to calculate the PRP concentrate, which should be around 400 % of the peripheral blood platelet count [25]. The platelet concentrate was stored at 20 °C until the exact time for use at the surgical site [22].
Procedure for the partial lesion of the CT
Rats were previously medicated with acepromazine (0.2 % Acepram, Univest SA) and butorphanol (Fort Dodge Lab Ltd.) at doses of 0.02 and 0.1 mL/kg, respectively, injecting intramuscularly in the region of the right quadriceps muscle. After 15 min, anesthetic Zoletil 50® (Virbac) at a dose of 0.1 mL/kg was applied. Trichotomy was performed in the entire right thigh, and then a longitudinal incision was made 3 cm on the skin just above the origin and insertion of the CT. The partial tenotomy was performed with a scalpel blade number 11, with a cut of 2 mm in the middle third of the tendon, the medial to lateral. Then, the skin was sutured with nonabsorbable monofilament polyamide 4.0 (Ethicon, Johnson & Johnson) and subjected to local asepsis [26]. These procedures occurred with all animals.
PRP treatment
The animal in groups GII, GV, and GVI received treatment with PRP. Each animal received a single dose of 0.2 mL directly into the surgical site, on top of the tenotomy. The application of PRP was performed immediately after injury but before suturing the lesion [27].
Laser treatment
For laser therapy, the low-intensity laser device Laser Flash® DMC III (DMC Equipments Ltda, São Carlos, SP) was used which can be operated in two wavelengths: λ = 660 nm (red laser, mid-activity: InGaAlP) that was used for groups GIII and GV and λ = 830 nm (infrared laser, mid-activity: GaAlAs) applied to groups IV and VI [28]. Laser irradiations were made at the same day time (10:00 a.m.) leaving an interval of 1 day between applications. The animals were immobilized manually, exposing the right side of the thigh and leg. Animals from groups GIII, GIV, GV, and GVI were irradiated by the laser according to the protocol described in Table 1.
Tissue samples
All animals were killed at the 13th day after surgery, receiving an intracardiac application of anesthetic sodium thiopental (crystal) at a dose of 0.05 mL per 100 g body weight, followed by 19.1 % potassium chloride via intracardiac, with a single dose of 0.4 mL per 100 g body weight. After confirmation of euthanasia by verification of vital data and absence of reflexes, the entire triceps surae muscle was dissected and extirpation occurred at the calcaneal insertion and myotendinous junction.
Morphometrical analysis
The CT was fixed in 10 % neutral buffered formalin for 48 to 72 h and processed in routine histological processing order: dehydration, bleaching, paraffin inclusion, and dyeing [29]. Semi-serials cuts were obtained with 5 μm thick and stained with Picrosirius Red, which allows visualization of collagen fibers. The material was examined with a polarized microscope Olympus CX31 trinocular, YS100 model, equipped with digital camera Olympus SC20 and coupled to a microcomputer. Morphometric analysis of collagen fibers was performed according to Silva [29]: Collagen area = (Σ regions with fibers of the same pixel / area on the tendon) × 100.
Statistical analysis
One-way analysis of variance was used for comparison between groups. The calculations were performed using the GraphPad Prism®. All statistical tests were performed at a significance level of p < 0.05.
Results
Figure 1 shows histological analysis using the Picrosirius sections of calcaneal tendons with partial rupture showing the presence of type I collagen fibers, which are thick and yellow or red, and type III collagen fibers, which are thin and greenish.
Histological analysis using the Picrosirius sections of calcaneal tendons with partial rupture showing the presence of type I collagen fibers, which are thick and yellow or red, and type III collagen fibers, which are thin and greenish. Bar scale 1/4 100 μm. a GI, tenotomy; b GII, PRP; c GV, PRP + λ660 nm; and d GVI, PRP + λ830 nm. Representative histological sections (5 μm, Picrosirius Red under polarized light, ×10 objective) of the longitudinal axis of the central region of the tendon. The control group collagen fibers increased as evidenced by greenish areas in the image (a)
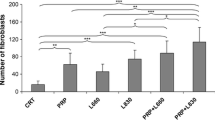
Figure 2 shows the percentage of the type I collagen fibers. Type I collagen fibers were more frequent in all treated groups than in the untreated (group I). Both groups that associated LLLT and PRP treatments (V and VI groups) presented higher values (p < 0.05) when compared with all other groups (I, II, III, and IV). Additionally, LLLT groups (III and IV) showed higher values (p < 0.05) than PRP alone (group II). However, no difference (p > 0.05) was found between LLLT groups (III versus IV).
Percentage of the type I collagen fibers. Results are expressed as mean ± SD. Statistically significant differences (p < 0.05) when compared groups: one asterisk GI with GII thru GVI; two asterisks, GII with GIII thru GVI; three asterisks, GII–GIV with GV and GVI; number sign, GV with GVI; n.s. indicates no statistically significant difference (p > 0.05)
Figure 3 displays the percentage of the type III collagen fibers. Type III collagen fibers were more frequent in untreated group (group I) than all treated groups. Both groups that associated LLLT and PRP (V and VI groups) presented lower values (p < 0.05) when compared with the all other groups (I, II, III, and IV). Additionally, LLLT groups (III and IV) showed higher values (p < 0.05) than PRP alone (group II). However, no difference (p > 0.05) was found between LLLT groups (III versus IV) and between groups treated with LLLT plus PRP.
Percentage of the type III collagen fibers. Results are expressed as mean ± SD. Statistically significant differences (p < 0.05) when compared groups: one asterisk, GI with GII thru GVI; two asterisks, GII with GIII thru GVI, three asterisks, GII–GIV with GV and GVI; n.s. indicates no statistically significant difference (p > 0.05)
A predominance of fibers type III was observed for animals in groups GI (untreated), GIII, and GIV, a result that can be justified by the absence of treatment in GI and the low efficacy of only LLLT on groups GIII (λ660 nm) and GIV (λ830 nm).
Discussion
Defragmentation of the PRP promotes the release of various substances: platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor beta-1, fibroblast growth factor, connective tissue growth factor, transforming growth factor such as insulin or stimulatory (IGF-1), epidermal growth factor, platelet thromboplastin, calcium, serotonin, and fibrinogen hydrolytic enzymes [30, 31].
The results show the percentage difference of the types of fibers of collagen type I and type III. The type I collagen presents closely packed, thick non-argyrophilic, strongly birefringent, yellow or red fibers and it is responsible for the tensile strength, whereas type III collagen (1 %) presents loose argyrophilic network of thin, weakly birefringent, greenish, reticular fibers and its main function is the structural maintenance in expansible organs [32, 33].
The fibers of type I collagen are responsible for tensile strength; this collagen type constitutes the major portion of the vertebrate body and they are the most abundant component of the tendons. The type I collagen in normal adult tissues appears in the form of thick (2–10 mm) fibers under the optical microscope. When these fibers are observed under polarized light, the enhancement of collagen birefringence promoted by Picrosirius staining is specific for collagen and discloses its distinct patterns of physical aggregation: type I collagen (thick fibers) displays a strong birefringence and are yellow or red [34]. It is suggested that the predominance of this type of fiber in the GV group (PRP + LLLT λ660 nm) and GVI (PRP + LLLT λ830 nm) is due to combination of LLLT and PRP.
On the other hand, fibers of collagen type III are responsible for maintaining the structure and this collagen type is usually found intermixed with type I collagen. It is present in many organs and is mainly, but not exclusively, related to smooth muscle cells [35]. Histochemical evidence has been presented suggesting that type III collagen appears by optical microscopy under the form of thin (0.5–2 mm) argyrophilic, weakly birefringent, greenish fibers. Polarized light microscopy of Picrosirius-stained sections has been widely used to quantify types I and III collagen [36]. A predominance of fibers type III was observed in GI (untreated) and GIII and GIV, being justified by the absence of treatment in GI and low efficacy of the proposed protocol for GIII (LLLT λ660 nm) and GIV groups (LLLT λ830 nm).
The study by Neves et al. [7], which aimed to evaluate the effect of the GaAlAs laser with λ = 830 nm; a power of 40, 60, 80, and 100 mW; and an energy density of 20 J/cm2 on the repair of partial lesions tendons of rats, showed that the fibers of type I collagen responded better for a laser power of 80 mW, whereas a better response was obtained for 60 mW for the fibers of type III collagen. The wavelengths of 660 and 830 nm were chosen because laser radiation at wavelengths between 660 and 840 nm is less absorbed by superficial chromospheres, resulting in better tissue penetration [36].
LLLT and PRP treatments separately showed positive results in the stimulation of healing of the Achilles tendon [37]. Studies involving treatment with LLLT and ultrasound combined showed positive results in the regeneration of the calcaneal tendon, with respect to the increase of type I collagen [38]. It is believed by some authors that the cellular response of any tissue, particularly the calcaneal tendon tissue, depends on the physical agent, a combination of parameters, and associations [37].
In the present study, the results showed that the treatment of animals with PRP or LLLT alone, groups GII (PRP), GIII (λ660 nm), and GIV (λ830 nm), has significant advantages over untreated animals (p < 0.05), as the percentage of type I collagen fibers is concerned. Furthermore, it was found that the combined treatment with PRP and LLLT is even more efficient than when each of the two treatments is used alone. However, the treatments combining PRP and LLLT showed significant results between groups GV (PRP λ660 nm) and GVI (λ830 nm) (p < 0.05). These encouraging results suggest a decrease in the time of tendon regeneration using the two therapies combined, accelerating the healing process. The inflammatory signals also showed rapid transition but were not measured in this study.
It is also interesting to observe that no significant difference is found (p > 0.05) when animals are treated with either one of the two laser wavelengths λ = 660 nm or λ = 830 nm (groups III and IV).
Conclusion
The results showed the predominance of type I collagen fibers in groups treated with the combination of PRP with LLLT (λ = 660 nm, λ = 830 nm); nevertheless, further studies are necessary to identify which are the mechanisms by which this rapid regeneration occurs and the influence of LLLT on growth factors in PRP.
References
Longo UG, Ronga M, Maffulli N (2009) Achilles tendinopathy. Sports Med Arthrosc Rev 17(2):112–126
Sharma P, Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 87:187–202
Liu SH, Yang RS, Al-Shaikh R, Lane JM (1995) Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop 318:265–278
Chen CH, Tsai JL, Wang YH, Lee CL, Chen JK, Huang MH (2009) Low-level laser irradiation promotes cell proliferation and mRNA expression of type I collagen and decorin in porcine Achilles tendon fibroblasts in vitro. J Orthop Res 27:646–650
Casalechi HL, Leal-Junior EC, Xavier M, Silva JA Jr, de Carvalho PD, Aimbire F, Albertini R (2012) Low-level laser therapy in experimental model of collagenase-induced tendinitis in rats: effects in acute and chronic inflammatory phases. Lasers Med Sci. doi:10.1007/s10103-012-1189-x
Pires D, Xavier M, Araújo T, Silva JA Jr, Aimbire F, Albertini R (2011) Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 26:85–94
Neves MA, Pinfildi CE, Wood VT, Gobbato RC, da Silva FM, Parizotto NA, Hochman B, Ferreira LM (2011) Different power settings of LLLT on the repair of the calcaneal tendon. Photomed Laser Surg 29(10):663–668
Joensen J, Gjerdet NR, Hummelsund S, Iversen V, Lopes-Martins RA, Bjordal JM (2012) An experimental study of low-level laser therapy in rat Achilles tendon injury. Lasers Med Sci 27(1):103–111
Carrinho PM, Renno AC, Koeke P, Salate AC, Parizotto NA, Vidal BC (2006) Comparative study using 685-nm and 830-nm lasers in the tissue repair of tenotomized tendons in the mouse. Photomed Laser Surg 24(6):754–758
Oliveira FS, Pinfildi CE, Parizoto NA, Liebano RE, Bossini PS, Garcia EB, Ferreira LM (2009) Effect of low level laser therapy (830 nm) with different therapy regimes on the process of tissue repair in partial lesion calcaneous tendon. Lasers Surg Med 41(4):271–276
Elwakil TF (2007) An in-vivo experimental evaluation of He-Ne laser photostimulation in healing Achilles tendons. Lasers Med Sci 22(1):53–59
Demir H, Menku P, Kirnap M, Calis M, Ikizceli I (2004) Comparison of the effects of laser, ultrasound, and combined laser + ultrasound treatments in experimental tendon healing. Lasers Surg Med 35(1):84–89
Wang X, Qiu Y, Triffitt J, Carr A, Xia Z, Sabokbar A (2012) Proliferation and differentiation of human tenocytes in response to platelet rich plasma: an in vitro and in vivo study. J Orthop Res 30(6):982–990
de Mos M, van der Windt AE, Jahr H et al (2008) Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med 36:1171–1178
Monto RR (2012) Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int 33(5):379–385
Xiong X, Wu L, Xiang D, Ni G, Zhao P, Yu B (2012) Effect of platelet-rich plasma injection on early healing of Achilles tendon rupture in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 26(4):466–471
Del Buono A, Papalia R, Denaro V, Maccauro G, Maffulli N (2011) Platelet rich plasma and tendinopathy: state of the art. Int J Immunopathol Pharmacol 24(1 Suppl 2):79–83
Taylor DW, Petrera M, Hendry M, Theodoropoulos JS (2011) A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med 21(4):344–352
Geng Z, Wang C, Zhou H (2011) Effect of platelet-rich plasma on tendon healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 25(3):344–348
Paoloni J, De Vos RJ, Hamilton B, Murrell GA, Orchard J (2011) Platelet-rich plasma treatment for ligament and tendon injuries. Clin J Sport Med 21(1):37–45
Jo CH, Kim JE, Yoon KS, Shin S (2012) Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med 40(5):1035–1045
Tohidnezhad M, Varoga D, Wruck CJ, Brandenburg LO, Seekamp A, Shakibaei M, Sönmez TT, Pufe T, Lippross S. Platelet-released growth factors can accelerate tenocyte proliferation and activate the anti-oxidant response element. 2011; 135(5):453-460
Froum SJ, Wallace SS, Tarnow DP, Chao SC (2002) Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restor Dent 22(1):45–53
Carmona JU (2006) Use of autologous platelet concentrates for the treatment of musculoskeletal injuries in the horse. 91 f. Tesis (Doctorado en Medicina y Sanidad Animales) - Universitat Autònoma de Barcelona
Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, Fortier LA (2007) Platelet rich plasma (PRP) enhanced anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res 25(2):230–240
Silva RD, Glazebrook MA, Campos VC (2011) Vasconcelos AC Achilles tendinosis: a morphometrical study in a rat model. Int J Clin Exp Pathol 4(7):683–691
Aspenberg P, Virchenko O (2004) Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand 75(1):93–99
Barbosa D, de Souza RA, Xavier M, da Silva FF, Arisawa EA, Villaverde AG (2012) Effects of low-level laser therapy (LLLT) on bone repair in rats: optical densitometry analysis. Lasers Med Sci. doi:10.1007/s10103-012-1125-0
Silva RD (2008) Modelo experimental de indução à tendinose de Aquiles: Um estudo morfométrico [Dissertação] Belo Horizonte: Faculdade de Medicina de Minas Gerais (MG): Universidade Federal de Minas Gerais - UFMG
Debus ES, Schmidt K, Ziegler UE, Thiede A (2000) The role of growth factors in wound healing. Zentralbl Chir 125(Suppl 1):49–55
Shanaman R, Filtein MR, Danesh MMJ (2001) Localized ridge augmentation using GBR and platelet-rich plasma: case reports. Int J Periodontics Restor Dent 21(4):345–355
Bihari J, Mester AR (1997) The biostimulative effect of low level laser therapy on long standing crural ulcers using helium-neon laser, helium-neon laser plus infrared lasers, and noncoherent light: preliminary report of a randomized double blind comparative study. Laser Ther 11(2):11–18
Dugrillon A, Kluter H (2002) Current use of platelet concentrates for topical application in tissue repair. Ther Transfus Med 29(23):67–70
Junqueira LCU, Cossermerlli R (1978) Brentani. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn 41(3):267–274
Hock JM, Canalis E (1994) Platelet-derived growth factor enhances bone cell replication, but not differentiated function of osteoblasts. Endocrinology 134(3):1423–1428
Karu T (1998) The science of low power laser therapy. Gordon and Breach Science Publishers, Australia, pp 23–29
Reddy G, Gum S, Stehno B, Enwemeka C (1998) Biochemistry and biomechanics of healing tendon: part II. Effects of combined laser therapy and electrical stimulation. Med Sci Sports Exerc 30(6):794–800
Wood VT, Pinfildi CE, Neves MA, Parizoto NA, Hochman B, Ferreira LM (2010) Collagen changes and realignment induced by low-level laser therapy and low-intensity ultrasound in the calcaneal tendon. Lasers Surg Med 42(6):559–565
Acknowledgments
We thank the Instituto de Ciências Biomédicas, Universidade de São Paulo, for the technical and laboratory assistance related to histological analysis.
Conflict of interest
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbosa, D., de Souza, R.A., de Carvalho, W.R.G. et al. Low-level laser therapy combined with platelet-rich plasma on the healing calcaneal tendon: a histological study in a rat model. Lasers Med Sci 28, 1489–1494 (2013). https://doi.org/10.1007/s10103-012-1241-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1241-x