Abstract
Eradication or suppression of pathogens is a major goal in periodontal therapy. Due to the increase in antibiotic resistance, the need of new disinfection therapies is raising. Photodynamic therapy (PDT) has demonstrated anti-infective potential. No data are available on the use of light-emitting diode (LED) lights as the light source in PDT. The aim of this study was to investigate the microbiological and clinical adjunctive outcome of a new photodynamic LED device, compared to scaling and root planing in periodontitis patients in maintenance [supportive periodontal therapy (SPT)]. In this masked, split-mouth design study, 30 treated chronic periodontitis subjects (mean age, 46.2 years; 13 males) in SPT were included. Two residual interdental sites with probing pocket depth (PPD) ≥ 5 mm in two opposite quadrants, with positive bleeding on probing (BOP) and comparable periodontal breakdown, were selected. PPD, BOP and subgingival microbiological samples for real-time PCR analysis (Carpegen® PerioDiagnostics, Carpegen GmbH, Münster, Germany) were recorded at baseline and 1 week after treatment. Scaling and root planing was performed under local anesthesia. Randomly one of the sites was selected to receive adjunctive photodynamic therapy by inserting a photosensitizer (toluidine blue O solution) and exposing it to a LED light in the red spectrum (Fotosan®, CMS Dental, Copenhagen, Denmark), according to the manufacturer’s instructions. After 1 week, 73 % of the control sites and 27 % of the test sites were still BOP+. These differences compared to baseline values and in-between groups were statistically significantly different (p < 0.001). Mean PPD decreased from 5.47 mm (±0.68) to 4.73 mm (±0.74, p < 0.001) in control sites and from 5.63 mm (±0.85) to 4.43 mm (±1.25, p < 0.001, test vs control p = 0.01) in the test group. Microbiologically, higher reductions of relative proportions of red complex bacteria were observed in test sites (68.1 vs. 4.1 %; p = 0.01). This study showed that adjunctive photodynamic treatment by LED light may enhance short-term clinical and microbiological outcome in periodontitis subjects in SPT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that for the occurrence of periodontitis, the presence of a pathogenic microbiota in the dental plaque biofilm is essential [1]. Therefore, one of the major goals in periodontal therapy is the disruption of the dental biofilm and the eradication or suppression of periodontal pathogens [2], together with the restoration of the homeostatic relationship between the host immune system and its polymicrobial dental plaque community.
At present, the most widely used treatment to achieve this goal is the instrumentation of the root surface with hand or power-driven instrumentation [3]. Scaling and root planing (SRP) consists of mechanical removal of biofilm and calculus and is often performed along with the adjunctive use of different types of antimicrobials. Despite clinical improvements that result after SRP, complete eradication of periodontal pathogens is very difficult or impossible to achieve [4], mainly because debridement procedures alone may not be always efficient due to the presence of deep pockets or lesions within hard to reach areas such as furcations [5], or due to the fact that some pathogens are able to invade the surrounding soft tissues of the periodontal pocket, or because recolonization of treated sites may occur if other intra-oral niches remain untreated [6].
Clinical and microbiological results of non-surgical periodontal treatment can be implemented by the one-stage full-mouth disinfection approach, especially in severe cases [7, 8]. Furthermore, adjunctive disinfection is also the goal when SRP is combined with systemic or local antibiotics [9, 10].
Although improvement of clinical and microbiological results have been demonstrated when non-surgical periodontal therapy is followed by systemic or local antibiotics [11], there is increasing concern in the worldwide rise in antibiotic resistance. Furthermore, clinical side effects and patient’s compliance limit the use of such adjunctive therapies [12]. Therefore, efforts are made to find alternative strategies to challenge microbial infections.
Light-activated disinfection (LAD) or photodynamic therapy (PDT) was first applied more than a century ago when Oscar Raab [13] reported that acridine hydrochloride and visible light were lethal on Paramecium caudatum. PDT was abandoned for several decades due to the discovery of antibiotics and the difficulties of finding an appropriate photosensitizing agent. Recently, it has been approved for the treatment of a variety of solid-state tumors, and there has been increasing interest on the use of PDT in infective diseases.
The photodynamic reaction involves the use of a photosensitizer (PS) and a light source of a specific wavelength in the presence of oxygen [14]. Briefly, due to the absorption of energy from a specific light source, PS is excited and converted from the ground state to the triplet state. In this state, the interaction with the surrounding molecules determines the formation of cytotoxic products, mainly singlet oxygen. This highly reactive oxygen state has a very short lifetime (<0.04 μs) and a limited radius of action (100 nm) [15, 16].
Some PS have shown the property to bind only with bacteria, therefore to be effective only against target microorganisms without inducing damage to the host tissues [17]. Antimicrobial PDT (aPDT) can be directed also to Gram negative bacteria if specific cationic photosensitizers are used [15]. Toluidine blue (TBO) has been shown to be an effective PS against many microorganisms, including periodontal pathogens in vitro [18, 19], and in animal models [20].
Recently, an increasing number of clinical studies have been published [16], dealing with the adjunctive effects of aPDT in periodontal treatment, using low-level power diode lasers and phenothiazine photosensitizers (mainly TBO and methylene blue). There is conflicting evidence arising from these clinical trials which reported conflicting clinical and microbiological evidence, some showing no adjunctive effects, while other researchers reported better clinical and/or microbiological results after an adjunctive single or multiple course of aPDT towards SRP alone.
Low-level diode lasers were mostly used as the light-emitting source to excite the photosensitizer, although, in principle, all type of lamps can be used if set on the specific excitement wavelength of the dye. Non-coherent light sources, as light-emitting diode lamps (LED), have some advantages in comparison to lasers: longer irradiation times are possible and lower costs and simpler to use [14, 21]. The aim of this clinical trial is to investigate the microbiological and clinical photodynamic adjunctive effect of a new LED lamp emitting in the red spectrum, compared to scaling and root planing in periodontitis patients in maintenance.
Materials and methods
The present study was a single-blinded, split-mouth design, randomized parallel clinical trial.
Subjects
Thirty adult patients (mean age of 46.2 years, 13 males) treated for chronic periodontitis and participating in a supportive periodontal therapy program at the Department of Periodontology of the Dental Clinic of the University of Rome “Sapienza” were included in this study. Recruitment of participants started October 2010 and ended February 2011. Only four were smokers at the time of enrolment. The study met the criteria of the Helsinki Declaration of 1975, revised in 2008. The study design was reviewed and approved by the ethical committee of the University of Rome (Italy). All subjects received oral and written explanation of the purpose of the study and signed an informed consent.
Exclusion criteria were any systemic disease or intake of any pharmaceutical that could influence the outcome of the study or influence inflammatory clinical indices and use of systemic antibiotics or any local antimicrobials in the previous 3 months before the start of the trial. Pregnancy or lactation, for female subjects, was considered an exclusion criterion.
To be included in the study, subjects should meet the following criteria:
-
1.
Diagnosed suffering from moderate to severe chronic periodontitis;
-
2.
Being compliant to the supportive periodontal program;
-
3.
Had the last supportive treatment appointment 3 months before the start of the trial;
-
4.
Full-mouth plaque score and full-mouth bleeding score ≤ 20 %;
-
5.
Presence of at least two residual interdental sites with a probing pocket depth (PPD) ≥ 5 mm in two opposite quadrants, which showed bleeding upon probing, radiographically comparable amount of periodontal breakdown and good matching in tooth type.
Clinical parameters
In each subject, only the two experimental sites selected for the study were included in the analysis. At baseline and 1 week after the treatment, one single experienced periodontist, blinded towards treatment, recorded the following clinical parameters:
-
1.
Probing pocket depth, measured to the nearest millimeter from the gingival margin to the base of the clinical pocket, using a manual periodontal probe (PCP-UNC 15, Hu-Friedy, Chicago, IL, USA) with a gentle probing force (approximately 25 g);
-
2.
Bleeding on probing (BOP) measured by recording the presence or the absence of bleeding up to 30 s after gentle probing.
Examiner calibration and reproducibility were ensured by duplicating measurements of five periodontitis patients, not included in the study, with a 2-day interval, prior to the start of the study. Duplicate measurements where within 1 mm difference in 92.5 % of the recordings.
Microbiological evaluation
At baseline and 1 week later, two different subgingival samples from each subject were collected, one for the control and one for the test site, by the same blinded examiner, using a commercially available real-time PCR test (Carpegen® Perio Diagnostics, Carpegen GmbH, Münster, Germany).
Briefly, each experimental site was isolated with cotton rolls, supragingival plaque was carefully removed with a sterile curette and the site was air-dried. Subsequently, subgingival plaque was collected by inserting one #40 sterile paper point, present in the commercial kit, both from the buccal and the palatal aspect of the site. The paper points were left in place for 10 s. After removal, they were inserted in the sterile transport vial and sent for real-time PCR analysis.
This commercially available test identifies the presence and the number of bacterial cells of six putative periodontopathogens, together with the total bacterial counts in the sample and relative proportions of each tested pathogen. The bacteria analysed by the test are the following: Aggregatibacter actinomycetemcomitans (previously Actinobacillus actinomycetemcomitans), Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia (previously Tannerella forsythensis), Fusobacterium nucleatum spp. and Prevotella intermedia. The level of detection of this test is set at 102 bacterial cells/sample.
Since all three pathogens of the “red complex species” (P. gignivalis, T . denticola and T. forsythia) are detected in this microbiological analysis, the relative proportion (towards the total bacterial count) and the total number of the “red complex bacteria” (RCB) can be calculated.
Treatment procedures
The same experienced periodontist performed all treatment procedures. At baseline, both sites in each patients were treated with SRP under local anaesthesia if needed, using periodontal Gracey curettes (Hu-Friedy, Chicago, IL, USA). The debridement was terminated when the operator felt that the instrumentation provided a hard and smooth root surface. Furthermore, in each subject, sites were randomly allocated by a toss of a coin to receive adjunctive treatment (test) or to serve as a control site.
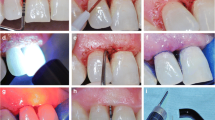
Additionally, test sites received a single episode of adjunctive LAD procedure. LAD was performed using a LED lamp emitting in the red spectrum with a peak frequency at 628 nm (Fotosan®, CMS Dental ApS, Copenhagen, Denmark). According to the manufacturer, the lamp used in the study has an output power of 1,000 mW, an intensity of 2,000 mW/cm2, providing an energy of 20 J/cm2 for every 10 s of exposition time. Two different tips of the LAD system were used: a blunt short tip to be used from the outside of the periodontal pocket, touching the gingiva, and a periodontal tip to be inserted in the depth of the pocket. The PS provided consisted in a toluidine blue O solution (Fotosan Agent®, CMS Dental ApS, Copenhagen, Denmark) at a concentration of 0.1 mg/ml dissolved in a 1 % xanthan gel. The photosensitizer is available in several viscosities: low, medium and high viscosity, respectively. All solutions have the same concentration of active ingredients. For the purpose of this study, only the high-viscosity PS was used. The experimental sites in all subjects were additionally treated as follows:
The periodontal pocket of the experimental site was isolated with cotton rolls, air-dried and filled with the PS by introducing the blunt end tip of the pre-filled syringe to the bottom of the pocket till the complete filling was achieved. After 1 min in place, the pocket was rinsed with water to remove excess PS. LAD was performed by applying the lamp for 10 s with the short blunt tip, from the outside of the pocket, touching the gingiva and afterwards by inserting the periodontal tip inside the pocket till the bottom was reached and irradiating for additional 10 s. The same procedure was repeated also on the palatal aspect. Total exposition was 40 s, giving an energy of 80 J/ cm2.
Statistical analysis
Mean values and standard deviations were used for descriptive purposes. Primary outcome variables where changes in BOP and changes in relative proportion of the RCB. Secondary outcome measures were reduction of PPD, changes in numbers of single pathogenic species tested and changes in total bacterial cell.
To detect differences among groups and taking account of the dependence, intra-group ANOVA for repeated measures was used as well as logistic regression adjusted for repeated measures for dichotomic variables. Level of significance was set at 95 %, and all analyses were performed with a commercially available software (STATA, StataCorp LP, College Station, TX, USA).
Results
All subjects completed the study. Compared to control sites, none of the patients reported any complications associated with LAD adjunctive therapy such as burning sensations, pain or any other discomfort.
The descriptive characteristics of the study sample are presented in Table 1. The mean age (±standard deviations) of the subjects was 46.2 (±10.8), with 17 being female and 4 subjects were current smokers.
Clinical outcomes
Since positivity of BOP was an inclusion criterion to enter the site in the experimental groups, all sites showed BOP at baseline. One week after treatment, mean values for BOP decreased to 73 % with still positive BOP in the control sites and to 27 % at sites with adjunctive LAD (Table 2). Compared to baseline readings, these changes were statistically significant in both groups (p < 0.001). The difference between groups at 1 week was statistically significant (p < 0.001).
Mean probing pocket depths at baseline were 5.47 mm (±0.68) and 5.63 mm (±0.85) in the control and in the test sites, respectively. Differences between test and control sites were not significantly different at baseline. After treatment, these values decreased to 4.73 mm (±0.74) in the control group and to 4.43 mm (±1.25) in the test group. This decrease in probing pocket depth mean values after treatment was statistically significant (p < 0.001) in both groups compared to baseline values (Table 2). Intergroup difference at 1 week was significantly different (p = 0.01) with a higher number of LAD-treated sites showing a PPD reduction of ≥2 mm. Figure 1 shows the frequency distribution of changes in PPD for test and control sites.
Microbiological outcomes
At baseline, total bacterial counts (TBC; ±SD) were on average 5.16 × 107 (±5.26 × 107) in control samples and 5.01 × 107 (±4.98 × 107) in test samples. After treatment, both control and test group showed a reduction in TBC (3.42 × 107 ±5.26 × 107 and 3.94 × 107 ±5.2 × 107, respectively). For both groups, these reductions did not reach statistical significance (Table 3; even if the overall analysis showed a significant decrease at p = 0.0412). When groups were compared, nonsignificant differences were observed at all intervals.
The detection frequency of single pathogenic bacteria was different for the six species tested. Only three subjects were positive for A. actinomycetemcomitans; therefore, data for this pathogen were excluded from any statistical comparison. Table 3 shows detection frequency of the other five pathogens tested and the treatment effect, for both control and test sites, on mean numbers of bacteria and mean relative proportions. No statistical differences were noticed between test and control sites at baseline.
Both treatments reduced the number of pathogens and the relative proportion towards total bacterial counts. The adjunctive LAD treatment for test sites resulted, in general, in higher reduction in bacterial cell numbers and in relative proportion.
At baseline, high numbers and high relative proportions of red complex bacteria (RCB) were detected, with no significant difference between test and control group (test sites: 5.2 × 106 ±7.7 × 106; 11.6 % ± 16.2; control sites: 4.11 × 106 ±5.23 × 106; 9.7 % ± 11.4). Both treatments resulted in a statistical significant reduction of absolute cell numbers and relative proportion of RCB, compared to baseline values. Furthermore, when compared to sites treated with mechanical instrumentation only, sites treated with SRP + LAD achieved a significantly greater mean reduction both in total cell numbers of RCB (3.2 × 106 versus 1.69 × 106; p < 0.05; Fig. 2). When the relative proportion of RCB towards total bacterial counts was considered, test sites showed a reduction from 11.6 to 3.7 % vs. 9.7 to 9.3 % in control sites at 1 week (respectively 68.1 vs. 4.1 % reduction; p = 0.01; Fig. 3). In fact, almost double as much sites treated with LAD did reach a complete reduction of RCBs under the detection capacity of the microbiological test used, compared to sites treated with SRP only (nine versus five, respectively; data not shown).
Discussion
The main goal of supportive periodontal therapy (SPT) is to maintain the clinical situation achieved by the active phase of the treatment of periodontitis subjects. On the microbiological aspect, SPT recall visits aim to maintain a microflora compatible with periodontal health. Deeper periodontal probing pocket depth and bleeding on probing may represent an increased risk of periodontal disease recurrence [22] and the loss of the tooth in a long-term perspective [23].
To enhance the clinical and microbiological outcome in aggressive forms of periodontitis, mechanical instrumentation is often implemented by the adjunctive use of systemic or local antimicrobial drugs [7, 24]. Recently, however, there has been an increasing number of reports of bacterial strains becoming multidrug-resistant due to the frequent use of antibiotics [25]. Therefore, big efforts are done in the development of alternative antimicrobial strategies. LAD and aPDT have been demonstrated to have a potential antimicrobial action by the combination of a certain wavelength light source and a photosensitizer, without development of drug-resistant strains [15, 16].
In the present study, we demonstrated that in subjects in SPT, the adjunctive LAD treatment is able to improve clinical and microbiological outcomes of sites at risk of disease recurrence. However, these results were very difficult to compare since there are very few in vivo studies dealing with aPDT in periodontitis patients in supportive periodontal therapy, and, to our knowledge, none using a LED light as the source for the photodynamic effect. This makes direct comparison very difficult.
Although most studies dealing with aPDT used a low-power laser as the light source, in this study a LED system in the red spectrum was chosen because it represents an easier to use technology at a lower cost, compared to laser devices [21].
In the present study, significantly more sites treated with SRP and LAD showed reduction in bleeding on probing compared to sites treated only with SRP. Our results are consistent with a number of recent clinical investigations that reported significantly better clinical results by the adjunctive use of aPDT.
Chondros et al. [26] demonstrated that the combination of a single episode of aPDT to SRP in subjects in SPT results in significantly higher reduction in bleeding on probing compared to SRP only, but failed to significantly improve the reduction in probing pocket depth. Rühling et al. [27] in a randomized clinical trial, also on chronic periodontitis subjects in SPT, but using a different photosensitizer and wavelength diode laser, could not demonstrate any difference in BOP reduction and other clinical parameters, when the PDT was compared to ultrasonic treatment.
Clinical trials on the adjunctive effect of PDT in non-surgical periodontal therapy mostly observed greater reduction in BOP scores when PDT was associated with mechanical instrumentation compared to debridement only. Christodoulides et al. [28] in 24 chronic periodontitis patients treated with either scaling and root planing followed by a single episode of PDT or scaling and root planing only reported a statistically significant higher improvement of full-mouth bleeding scores at 3 and 6 months for subjects in the test group. In patients with aggressive periodontitis, enrolled in a split-mouth design clinical trial, de Olivera et al. [29] observed a significant reduction in BOP values at sites treated by aPDT.
On the other hand, in terms of PPD reduction, the results of this study showed a significant adjunctive effect when LAD was applied. This observation is not in agreement with results in the Chondros et al. [26] where authors could not demonstrate any additional effect on PPD reduction in sites treated by mechanical debridement and PDT compared to mechanical debridement alone, and with the results of Rühling et al. [27]. These differences may be explained partly by the different design of the studies and partly by the differences in the light sources used.
Clinical studies evaluating the adjunctive effect of PDT to mechanical non-surgical periodontal therapy in periodontitis patients show conflicting evidence [16, 30]. More recently, Liu et al. [31] observed a significant greater probing depth reduction when chronic periodontitis patients treated with scaling and root planing and one course of adjunctive PDT and low-level laser therapy, compared to mechanical debridement only. In a split-mouth design randomized clinical trial, Braun et al. [32], in 20 untreated chronic periodontitis subjects, reported significantly higher impact of adjunctive photodynamic therapy in terms of reduction in BOP and PPD if compared to quadrants treated only with scaling and root planning, after 3 months.
In supportive periodontal therapy, professional supra- and sub-gingival plaque control aim to maintain a microflora compatible with periodontal health, in order to prevent the recurrence of periodontal disease. Socransky et al. [33] showed that in periodontal lesions, bacteria are frequently found in microbial complexes. The red complex, which appears later in the biofilm development, is represented by three different microorganisms: P. gingivalis, T. forsythia and T. denticola, and is considered the most pathogenic microbial complex [34].
The microbiologic response is one of the main goals of periodontal therapy. Mechanical debridement and adjunctive antimicrobial therapy aim to reduce or eliminate periodontopathogens. The shift of the microbial ecology from a pathogenic state to a condition of relative health is often due to a reduction of the proportion of the red complex bacteria [34].
The two treatment protocols tested in this study resulted in a significant decrease of both cell numbers and relative proportions of the putative periodontopathogens detected by the commercially available real-time PCR test used. This is in agreement with other studies that showed that mechanical instrumentation in deep inflamed pockets is able to decrease the levels of pathogenic bacterial species in periodontitis patients [35]. Results from our study showed that, by implementing mechanical debridement with LAD treatment, we were able to reduce significantly (by 16 times more) the proportions of RCB in the bacterial counts of the sites enrolled in the study, compared to sites treated only by mechanical instrumentation.
Data from different in vitro studies showed that it is possible to kill bacteria, sensitised with an appropriate photosensitizer and irradiated with a low-power laser light in a correlated spectrum [36]. Zanin et al. [37] carried out an in vitro study of the use of anti-bacterial photodynamic therapy on in vivo formed natural oral plaque biofilms using toluidine blue combined with a red light-emitting diode (620–660 nm). An up to 99 % killing efficacy was observed after photosensitization of biofilms containing Streptococcus mutans, Streptococcus sobrinus and Streptococcus sanguinis. Lethal photosensitization was found also for P. gingivalis in vitro [18] and in vivo in an animal model [38], and for A. actinomycetemcomitans biofilms [39].
Clinical trials involving adjunctive PDT treatment in periodontitis patients reported contrasting microbiological results. Polansky et al. [40] could not find any statistical significant difference on clinical and microbiological parameters, except in bleeding on probing, when non-surgical periodontal treatment of chronic periodontitis patients was implemented by a single cycle of PDT. In the Chondros et al. [26] study, differences in the microbiological profile were only found for Fusobacterium nucleatum and Eubacterium nodatum at 3 months and for Eikenella corrodens and Capnocytophaga species at 6 months in favour of the adjunctive PDT treatment. In localized chronic periodontitis patients infected by F. nucleatum and treated by scaling and root planing, a single full-mouth course of antimicrobial PDT was able to reduce significantly more the DNA concentration of this single pathogen, compared to baseline values [41]. Using the same commercially available test as in our study, Rühling et al. [27] showed immediate reduction of the pathogens analysed, but the decrease was not maintained after 3 months, both in PDT and in ultrasonic treatment of residual pockets in maintenance subjects.
The photosensitizer used consisted in a toluidine blue O solution at a concentration of 0.1 mg/ml dissolved in a 1 % xanthan gel. Kömerick et al. [38], using different concentrations of this photosensitizer and different light doses, demonstrated, in an animal model, that a concentration of 0.1 mg/ml at any light exposure dose is able to reduce P. gingivalis viable bacterial cells of about 2 log10.These findings are consistent with the in vivo results reported in our study, although other factors seem to be important, since not all sites treated with LAD showed the same reduction in cell counts of this periodontopathogen. A possible explanation could be the presence of serum proteins in the sites treated by LAD, which can compete in the uptake of the dye [42].
In the same study, Kömerick et al. [38] demonstrated that at this concentration, toluidine blue alone, in the absence of light exposure, has no killing effect on bacterial cells. The same observations were reported also when the light exposure alone was tested, in the absence of the photosensitizer. When histological examination was carried out, no adverse effects on the periodontal structures of this animal model were reported, at any combination of toluidine blue concentration and light dose exposure.
This is in accordance with our findings. No adverse effects of the LAD procedure applied in our study were reported by any of the 30 subjects included.
Conclusions
Within the limits of our study, we were able to demonstrate that a single episode of adjunctive LAD therapy, based on the photoactivation of a toluidine blue solution by a LED light system in the red spectrum, is able to significantly enhance short-term clinical and microbiological outcomes of mechanical instrumentation in moderate-deep residual pockets in chronic periodontitis subjects in maintenance.
References
Socransky SS, Haffajee AD (1992) The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol 63:322–331
Bernimoulin JP (2003) Recent concepts in plaque formation. J Clin Periodontol 30(suppl 5):7–9
Tunkel J, Heinecke A, Flemmig TF (2002) A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol 29(suppl 3):72–81
Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Socransky SS (1997) The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol 24:324–334
Wylam JM, Mealey BL, Mills MP, Waldrop TC, Moskowicz DC (1993) The clinical effectiveness of open versus closed scaling and root planing on multirooted teeth. J Periodontol 64:1023–1028
Teughels W, Dekeyser C, Van Essche M, Quirynen M (2009) One-stage, full-mouth disinfection: fiction or reality? Periodontol 2000(50):39–51. doi:10.1111/j.1600-0757.2008.00292.x
Mongardini C, van Steenberghe D, Dekeyser C, Quirynen M (1999) One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. I. Long-term clinical observations. J Periodontol 70:632–645
Quirynen M, Mongardini C, Pauwels M, Bollen CM, Van Eldere J, van Steenberghe D (1999) One stage full-versus partial mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. II. Long-term impact on microbial load. J Periodontol 70:646–656
Bollen CM, Quirynen M (1996) Microbiological response to mechanical treatment in combination with adjunctive therapy. A review of the literature. J Periodontol 67:1143–1158
Sigusch B, Beler M, Klinger G, Pfister W, Glockmann E (2001) A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J Periodontol 72:275–283
Bonito AJ, Lux L, Lohr KN (2005) Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J Periodontol 76:1227–1236
Walker CB (1996) The acquisition of antibiotic resistance in the periodontal microflora. Periodontol 10:79–88
Raab O (1900) Über die Wirkung fluoriziender Stoffe auf Infusorien. Z Biol 39:524–546 (In German)
Meisel P, Kocher T (2005) Photodynamic therapy for periodontal disease: state of the art. J Photochem Photobiol B 13:159–170
Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G (2006) Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med 38:468–481
Soukos NS, Goodson JM (2011) Photodynamic therapy in the control of oral biofilms. Periodontol 2000 55:143–166
Kömerik N, MacRobert AJ (2006) Photodynamic therapy as an alternative antimicrobial modality for oral infections. J Environ Pathol Toxicol Oncol 25:487–504
Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M (1997) Effect of dosimetric and physiological factors on the lethal photosensitization of Porphyromonas gingivalis in vitro. Photochem Photobiol 65:1026–1031
Wilson M, Dobson J, Sarkar S (1993) Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol Immunol 8:182–187
Qin YL, Luan XL, Bi LJ, Sheng YQ, Zhou CN, Zhang ZG (2008) Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J Periodont Res 43:162–167. doi:10.1111/j.1600-0765.2007.01007.x
Brancaleon L, Moseley H (2002) Laser and non-laser light sources for photodynamic therapy. Lasers Med Sci 17:173–186
Drisko CH (2001) Non surgical periodontal therapy. Periodontol 2000(25):77–88
Matuliene G, Pjetursson BE, Salvi GE, Schmidlin K, Brägger U, Zwahlen M, Lang NP (2008) Influence of residual pocket on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol 35:685–695
Page RC (2004) The microbiological case for adjunctive therapy for periodontitis. J Int Acad Periodontol 6(Suppl):143–149
Vergidis PI, Falagas ME (2008) Multidrug-resistant Gram-negative bacterial infections: the emerging torea and potential novel treatment option. Curr Opin Investig Drugs 9:176–183
Chondros P, Nikolidakis D, Christodoulides N, Rössler R, Gutknecht N, Sculean A (2009) Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers Med Sci 24:681–688
Rühling A, Fanghänel J, Houshmand M, Kuhr A, Meisel P, Schwahn C, Kocher T (2010) Photodynamic therapy of persistent pockets in maintenance patients—a clinical study. Clin Oral Investig 14:637–644
Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwar F, Rossler R, Sculean A (2008) Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized, controlled clinical trial. J Periodontol 79:1638–1644
de Olivera RR, Schwartz-Filho HO, Novaes AB, Taba M (2007) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: a preliminary randomized controlled clinical study. J Periodontol 78:965–973
Azarpazhooh A, Shah PS, Tenenbaum HC, Goldberg MB (2010) The effect of photodynamic therapy for periodontitis: a systematic review and meta-analysis. J Periodontol 81:4–14
Liu J, Corbet EF, Jin L (2011) Combined photodynamic and low-level laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. J Periodont Res 46:89–96
Braun A, Dehn C, Krause F, Jepsen S (2008) Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol 35:877–884
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144
Holt SC, Ebersole JL (2005) Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 38:72–122
Rhemrev GE, Timmerman MF, Veldkamp I, Van Winkelhoff AJ, Van der Velden U (2006) Immediate effect of instrumentation on the subgingival microflora in deep inflamed pockets under strict plaque control. J Clin Periodontol 33:42–48
Maisch T (2007) Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci 22:83–91
Zanin IC, Lobo MM, Rodrigues LK, Pimenta LA, Hofling JF, Goncalves RB (2006) Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur J Oral Sci 114:64–69
Kömerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M (2003) In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother 47:932–940
Suci P, Kang S, Gmür R, Douglas T, Young M (2010) Targeted delivery of a photosensitizer to Aggregatibacter actinomycetemcomitans biofilm. Antimicrob Agents Chemother 54:2489–2496
Polansky R, Haas M, Heschl A, Wimmer G (2009) Clinical effectiveness of photodynamic therapy in the treatment of periodontitis. J Clin Periodontol 36:575–580
Sigusch B, Engelbrecht M, Völpel A, Holletschke A, Pfister W, Schütze J (2010) Full-mouth antimicrobial photodynamic therapy in Fusobacterium nucleatum infected periodontitis patients. J Periodontol 81:975–981
Lambrechts SAG, Aalders MCG, Verbraak FD, Lagerberg JWM, Dankert J, Schuitmaker JJ (2005) Effect of albumin on the photoinactivation of microorganisms by a cationic porphyrin. J Photochem Photobiol B 79:51–57
Conflict of interest
All authors declare that they have no financial relationships related to any products involved in this study and that there are no conflicts of interest in this study.
Funding source
This study was partially supported by CMS Dental (Copenhagen, Denmark) by a grant and by supplying materials used in this study for free.
Author information
Authors and Affiliations
Corresponding author
Additional information
Key findings
Although further studies are needed, photodynamic therapy, applying a LED light as the light source, is an easy to use anti-infective therapy for the daily practice in the periodontal clinic, without the problems raised by antibiotic treatments and with less initial costs compared to laser lights.
Rights and permissions
About this article
Cite this article
Mongardini, C., Di Tanna, G.L. & Pilloni, A. Light-activated disinfection using a light-emitting diode lamp in the red spectrum: clinical and microbiological short-term findings on periodontitis patients in maintenance. A randomized controlled split-mouth clinical trial. Lasers Med Sci 29, 1–8 (2014). https://doi.org/10.1007/s10103-012-1225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1225-x