Abstract
Warm ischemia (WI) and bleeding during laparoscopic partial nephrectomy (LPN) place technical constraints on surgeons. Our aim was to develop a safe and effective laser-assisted LPN-technique without the need for WI. In this study, a diode laser-emitting light at a wavelength of 1,318 nm at output powers between 45 and 70 W in continuous-wave mode was used. Light was coupled into a flexible 600-μm bare fiber to be transported to the tissues. After dry lab experience, 13 patients (six males, seven females) underwent five open and eight laparoscopic/retroperitoneoscopic partial nephrectomies. Postoperative renal function and serum C-reactive protein (CRP) were monitored and coagulation depth and effects on resection margins (RR) were evaluated. Demographic, clinical, and follow-up data are presented. Mean operative time was 116.5 min (range 60–175 min) with mean blood loss of 238 ml (range 50–600 ml). Laser light application took a maximum of 17 min. All patients had a favorable outcome. The locations of the treated tumors (eight left and five right) were central (two), upper pole (two), lower pole (three) and middle kidney parenchyma (six anterior, two posterior, and five peripheral). Mean tumor size was 3.3 cm (range 1.8–5 cm). Two WI (19 and 24 min) were needed. Immediate postoperative serum creatinine and CRP were elevated within 0.1 to 0.6 mg/dl (mean 0.18) and 2.1–10 mg/dl (mean 6.24), respectively. Coagulation depth ranged from <1 to 2 mm without an effect on histopathological evaluation of tumors or RR. One patient had positive RR. During follow-up (2–6 months), one patient developed an A-V fistula that needed embolization. This prospective in-vivo feasibility study showed that the diode laser is a safe and promising device for LPN. Its advantages are minimal gas formation, good hemostasis, and minimal parenchymal damage. Oncological safety appears to be warranted by the use of a diode laser.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent clinical surveys have revealed increasing incidental detection of renal cell carcinoma (RCC) partly due to the widespread use of imaging procedures such as ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) [1, 2]. This has led to a shift in the surgical management of RCC toward widening the indications for nephron sparing surgery (NSS).
Laparoscopic techniques for partial nephrectomy (LPN) are well described [3]. Hemostasis is an important challenge during this procedure and clamping of the renal vasculature is often necessary to allow precise tumor removal in a bloodless field. The consequent warm ischemia (WIT) places significant time constraints on the surgeon during tumor excision and parenchymal reconstruction, adding further technical challenges to the procedure [3].
Several types of lasers are available for surgical intervention. As the laparoscopic approach needs fiber assistance, lasers in the wavelength range up to 2 μm are preferred. Lasers emitting in VIS as well as in the 2-μm spectral range are still under investigation for this procedure. Renal parenchyma is a highly blood-perfused tissue that needs an energy source for good cutting as well as coagulation qualities. Lasers of the VIS wavelength are mainly absorbed by the tissue hemoglobin, while wavelengths in the 2-μm spectrum are mainly absorbed by the tissue water. Therefore, a wavelength is needed that combines both of these features.
The 1,318-nm wavelength shows a good compromise of scattering and absorption in tissues. While high absorption of the radiation by the tissue water is necessary for cutting, the coagulation process needs a suitable penetration. The scattering coefficient from the VIS up to the 2-μm-spectral region differs by a factor of less than five while the absorption coefficient with respect to water differs enormously. Water absorption with respect to the 1,318-nm wavelength is increased by a factor of about 600 of the 2-μm wavelength and about 20 in the case of the 1,470-nm wavelength, whereas a decrease could be estimated to shorter wavelength, such as 0.7 for the 1,064-nm and 0.001 for the 532-nm wavelength [7]. Further, there is no measured absorption to hemoglobin in the spectral range longer than 1,064 nm while hemoglobin is one of the main tissue absorbers for the 532-nm wavelength [8]. The wavelength 1,318 nm appears to be promising because of the sufficient water absorption accompanied by suitable optical penetration into the tissue with reduced hemoglobin absorption. These facts seem responsible for the simultaneous cutting, coagulation, and sealing properties of the 1,318-nm laser during extirpation of lung metastasis, as an example of a parenchymal tissue [4–6]. This experience has not yet been reported for partial kidney resections.
Different types of lasers have been proven experimentally as well as in vivo as surgical options for LPN. The aim of the current prospective study was to evaluate and establish an in vivo laser laparoscopic partial nephrectomy technique (LLPN) without renal hilar clamping.
Patients and methods
Laser equipment
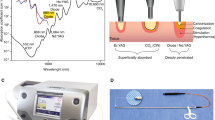
A semiconductor diode laser (Eraser, Rolle & Rolle, Salzburg, Austria) emitting light at wavelength of 1,318 nm in a continuous wave (cw) mode was used. The laser output power could be set between 1 and 100 W. The light is coupled into a flexible bare-ended laser fiber (core diameter 600 μm). This fiber was introduced into a specially designed application hand piece (distal 30° bent) for open surgery or through a commercially available guidance instrument [9] (Karl Storz, Tuttlingen, Germany) during laparoscopic interventions (Fig. 1). This fiber guidance instrument allows for controlled bending of the distal fiber tip between –5 and 50°. Furthermore, an irrigation system was attached (Fig. 1b) to enable rinsing of the incision site to improve the direct vision and cutting efficacy. The whole system fits properly for the introduction in laparoscopic trocars.
Application of 45–70 W output power to the tissue resulted in contact incision mode in an irradiation on the tissue surface of 16–25 kW/cm2, respectively. For coagulation, the fiber tip was slightly retracted to non-contact mode, achieving an irradiation spot size about 4 mm in diameter, thus reducing the irradiation to less than the estimated tissue ablation threshold of 0.5 kW/cm2.
Preclinical investigations
Experimental laser surgery treatment was conducted on ten non-perfused porcine renal units using different cut positions and laser parameters. Clinically relevant technical issues like handling of the laser fiber, effects of cutting angel and cutting velocity, laser effects on instruments, smoke development, and preparation of intra-renal vessels or pelvicalyceal system were evaluated. Moreover, the surgeon's ability to estimate the laser-induced coagulation depth was established by correlating the micro-caliber measured depth with the suggested one from the surgeon.
In a separate set of experiments, the optical transmission through kidney tissue (slices of 200 μm of thickness) of different laser wavelengths (λ = 940, 1,318, 1,470, 1,908, and 2,010 nm) were measured. Collimated light was directed onto the tissue and the transmitted light of only forward-scattered photons were detected as the distance between the tissue and detector was large. Background light as well as scattered photons were inhibited for detection by placing apertures into the optical axe. Evaluation was performed using the Beer–Lambert law to get the total transmission attenuation coefficient (μt), which is the sum of the scattering coefficient (μs) and the absorption coefficient (μa) of this particular tissue. The measurements were performed on five specimens for each wavelength. Mean and standard deviations of μt were listed and compared with the absorption coefficient μa of water.
Clinical study
Thirteen patients (seven men, six women; age range 32–88 years) were included in a prospective feasibility study of diode-laser partial tumor nephrectomy. All of the patients suffered from a suspected malignant renal mass of unknown histology that had been incidentally found in routine US and subsequent CT and were candidates for partial nephrectomy. All patients gave an informed consent after being given detailed information about the planned procedure. The study was approved by the ethics committee of our faculty, (Approval no. 285–09).
As a first step, the technique was established during open surgery (OPN) (n = 5) to standardize the maneuvers and manage/define perioperative complications. Thereafter, retroperitoneoscopic (RPN) (n = 3) and LLPN (n = 5) were performed.
Preoperative co-morbidities of the patients include the following: metastatic mammary carcinoma under chemotherapy and radiation (n = 4), metastatic renal cancer with previous nephrectomy, abdominal metastasectomy and hysterectomy (n = 1), adrenal tumor, pulmonary metastasis and cystic renal and liver disease (n = 1), paraplegia due to previous spinal cord fracture and intracranial bleeding (n = 1), AIDS with high viral load, neutropenia and previous opportunistic pneumonitis (n = 1), a long history of metastatic Hodgkin’s lymphoma, spinal cord fixation, chemotherapy, radiotherapy and pulmonary embolism (n = 1), cerebral infarction, cardiac infarction, hypertension, and continuous anticoagulant therapy (n = 1). The remaining patients (n = 3) showed previous different abdominal operations without malignancy.
This prospective pilot study was constructed to evaluate the method in patients with different general conditions, co-morbidities, and ASA scores having different tumor characteristics within T1 (as shown in Table 1) in a single surgeon (WYK) series omitting the effect of the surgeon's level of experience. Furthermore, all specimens were examined by a single genitourinary pathologist (SS)
Data (OPN, RPN, LPN) were brought together and merged in the datasets without any selection as this study aimed to evaluate the feasibility and efficiency of this new laser technique seeking no proof of superiority for any approach.
Surgical procedure
In the flank lateral patient position, the kidney was approached through a 10-cm supra-costal incision (OPN) or through three trocars (RPN/LPN). Trocars were inserted after establishment of the retroperitoneal space at the tip of last rib, in anterior axillary line (2–3 cm above and medial to anterior superior iliac spine) and at the middle point of a line connecting both ports for RPN while at 3 cm above and cranial to the umbilicus, in the midclavicular line (2 and 12 cm from thorax cage) for LPN. The laser apparatus was assembled nearby at the tableside. The sterile laser fiber was fixed at the sister table and prepared through the fiber-guidance instrument shown in Fig. 1.
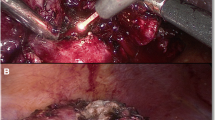
After incision of Gerota's fascia and preparation of renal hilum, the perirenal fat was removed from either the upper, lower, or middle parts of the kidney for adequate tumor exposition. The fiber-guidance system was introduced through a 10-mm trocar positioned as close as possible to the kidney to function as a protection sheath. A laparoscopic suction device was simultaneously held in left hand. The renal tumor was excised by parenchymal fiber contact using laser energy output of 45–70 W with adequate free resection edges (RR) and placed in laparoscopic sac to be removed later. The renal cut surface was then biopsied and coagulated using the laser without tissue contact. Opened calyx (n = 1) or large vessels were sutured with 3–0 Vicryl sutures. The renal parenchyma was covered with a cellulose mesh and closed using 0–0 Vicryl sutures (Fig. 2). After removal of the specimen, a drain was placed through the 5-mm port site with closure of the other sites.
In case of failure of laser coagulation of large parenchymal vessels resulting in obscured visibility (6th and 9th cases), the parenchymal surface was compressed with gauze until selective clamping of the pre-dissected renal artery. None of patients became clinically unstable.
The drainage tubes and urethral catheters were removed on the second and third postoperative days, respectively.
All specimens were measured and fixed in formalin for histological examination to evaluate the depth and effects of the laser on RR.
Postoperative renal function, acute phase proteins, and blood analysis as well as surgical outcomes and required analgesia during convalescence were monitored. After discharge, the patients were reviewed after 1 month. US is used routinely on our postoperative wards and at all follow-up appointments as a non-invasive assessment of the urinary tract. CT was performed at 3 months.
Results
The basic experimental part revealed that laser adjustment between 45 and 70 W in a moderate cut velocity (1–3 mm/s) is sufficient for adequate manipulation (cutting as well as preparation). Logically, with a slow cut velocity, the coagulation zone becomes deeper (up to 4 mm) rather than with fast cutting where the coagulation effect is almost lost. Dissection of vessels and pelvicalyceal system was feasible. Furthermore, heavy smoke began to form when the laser application continued more than 1 min per application.
As shown in Table 2, the light transmission measurement (μt) follows the absorption coefficient of water (μa). As μt could be assumed to be the sum of μs and μa of a water-and-tissue-chromophore mixture, the difference between μt and μa (water) reflects the μs of the tissue plus an unknown μa of the remaining chromophores except water. This value showed that the scattering coefficient decreases with increasing wavelengths.
Patients included in this prospective study were candidates for NSS. As listed in Table 3, the mean operative time (skin to skin) was 116.5 min (ranging from 60 to 90 min for open surgery and 110–175 min for the laparoscopic approach) with mean estimated blood loss of 240 ml (50–600 ml) with no real differences between open compared to laparoscopic approach. The laser activation time for tissue removal was 7–13 min during open surgery and 9–17 min during the laparoscopic intervention.
There was no conversion from LPN/RPN to OPN. Two patients needed vascular clamping with an average WIT of 21.5 min (19 and 24). The individual operative data including approach, operative time, intraoperative blood loss, and pathological stage are shown in Table 1. One OPN was performed in a single kidney. Tumors were at the left (n = 8) and right (n = 5) kidney. Maximal tumor diameter was 1.8–5 cm (mean 3.3 cm). Three patients had central tumors without the renal parenchyma separating the tumor from renal sinus as measured by preoperative-CT. The remaining patients had peripheral tumors of the upper pole (n = 2), lower pole (n = 3) and middle kidney parenchyma (n = 4). Furthermore, the localization on the kidney surface was on the anterior (6/13), posterior (2/13), and lateral (5/13) border.
There were no perioperative complications. Two patients developed post-operative fever that was not surgery-related. There was no increased need for postoperative analgesics compared to postoperative standard pain managements in these cases. All patients resumed a normal diet without any delay.
Histologically, the coagulation depth ranged from <1 to 2 mm without limiting the histological evaluation of tumors or RR (Fig. 3). This was comparable with the given coagulation depth from the surgeon. Positive RR was found in one (6th) patient with central tumor and bleeding necessitated arterial clamping. There was no normal renal tissue between tumor and major vessels resulting in unclear margin status. Three-month follow-up CT showed normal kidney size and function without any tumor recurrence.
Histological findings of the resection margins (H&E, 200-fold) in two different specimens. The coagulation zone (long arrow) is visible. Tubular structures and stroma are partially destroyed. The tubular epithelium shows marked hypereosinophilia. The blue/black ink (short arrows) marks the surgical resection margin
In-vivo experience confirmed the experimental results that smoke (which obscured laparoscopic vision) developed if the laser was activated for >1 min per application. In case of bleeding, suction of blood in combination with water irrigation is mandatory for adequate laser coagulation. Beginning with parenchyma around the bleeding vessel shrinks tissues and controls parenchymal bleeding, which helps to intensify direct laser coagulation on this vessel afterwards. Parenchymal contact is necessary for cutting and non-contact for coagulation as already mentioned. Parameters such as cutting velocity, changing laser adjustments, and objective appearance of RR remain dependant on the surgeon’s experience, operative situation, and learning curve. The surgeon intended to re-resect the tumor bed in two cases because of unclear RR status, however, histological examination revealed normal parenchyma, indicating that the laser had not altered the surgeon-dependant oncological results.
The postoperative creatinine (0.6–2.4 mg/dl, median 1.0) was expectantly elevated within 0.1–0.6 mg/dl (mean 0.18 mg/dl) compared to the pre-operative values (0.6–2.2 mg/dl, median 0.9 mg/dl) (p = 0.034). This was found to be significant and returned to preoperative values within the third to eighth postoperative day. None of the patients needed hemodialysis. Serum C-reactive protein was significantly increased postoperatively with a median of 6.24 mg/dl (0.5–17.8 mg/dl, p = 0.003).
Follow-up ranged from 3 to 6 months. One (the 9th) patient developed A-V fistula after 1 month, which was successfully embolized. Otherwise, all of the patients showed uneventful follow-up examinations.
Discussion
While there is no doubt about the oncologic and functional efficacy of OPN, the morbidity of the flank incision remains considerable. Indications and contraindications of LPN/RPN are essentially the same as OPN, however, they should be performed by surgeons with advanced laparoscopic skills [3, 10].
The LPN technique has evolved mainly in developing hemostatic tools to control bleeding, efficient suturing techniques to shorten WIT, and achieving cold ischemia. Clamping of renal vessels allows NSS similar to the well-established OPN principles [10], However, the time factor by LPN is still of concern. To avoid irreversible kidney damage, WIT should not exceed 30 min [11, 12], hence warm ischemia is not recommended for tumors in unfavorable locations necessitating difficult reconstruction after excision. In addition, unexpected problems can be encountered during LPN/RPN that may prolong WIT and thus endangering renal function [13]. When working without ischemia, it is important to control parenchymal bleeding which often obscures vision, adding more technical and oncological difficulties.
Many alternative treatment methods are used for small RCC such as cryotherapy, thermoablation, radio frequency ablation, high-intensity focused ultrasonography, ethanol ablation, microwave coagulation, and others [14–18], hoping to decrease the ischemic damage to renal remnant. These methods have an essential drawback: they leave the tumor without excision, which makes the radiological follow-up evaluations very difficult, limiting its clinical use.
Recently, reports about laser-supported partial nephrectomy using different laser types both experimentally [19, 20] and in vivo [21, 22] have been published. Our experimental results and preclinical handling confirmed the published results about laser partial nephrectomy on a porcine kidney model revealing feasibility, safety, and establishing the approach. The presented clinical results are comparable to the published literature of OPN [23–25] as well as LPN [26, 27].
There are reports on the feasibility of the Tm laser OPN in five cases of small peripheral tumors (1.2–3.8 cm) [21]. The reported technique was feasible and showed similar clinical results to the current series. Our shorter operative time may be related to surgical experience. Mattioli et al. [22] reported their experience with a thulium laser under cold ischemia in nine patients (OPN (n = 8) and LPN (n = 1)) with a median tumor diameter of 3.8 cm. The operative time and blood loss were higher than the current series. Furthermore, follow-up in our series is the longest of both studies.
Generally, there are definite advantages of renal vessel clamping during LPN that allows safe renal reconstruction, which widens the indications to more complex tumors. The challenge of the current series was to excise the tumors without clamping as far as it was possible. This led, in two cases of central/deep tumors, to increased blood loss, obscuring vision, which was within the allowed limits for a surgical patient. Clamping was necessary in these cases (25% of LPN/RPN) until reconstruction was finished. WIT was relatively shorter as it would be required for the whole procedure. Therefore, central/deep tumors with large vessels remain the limits of this method. From our point of view, whether the surgeon could accept more blood loss to avoid clamping still needs to be discussed.
This prospective feasibility study of laser tumor excision for tumors in different positions in patients with different general conditions by one surgeon demonstrated many advantages. The laser system used is being tested in vivo for this indication for the first time. While other wavelengths such as the Revolix laser (2,013 nm), KTP (532 nm) and thulium laser (1,900–2,000 nm) were used for this kind of intervention [19, 21, 22], the 1,318-nm wavelength was chosen with respect to its good combination of tissue water absorption and optical penetration depth [28]. Comparing these characteristics to other wavelengths by means of the water absorption curve [7] and the hemoglobin absorption curve [8], the KTP 532-nm shows minor absorption by the tissue water but good absorption by tissue hemoglobin, therefore, having a low tissue optical penetration depth and a good cutting/vaporization effect mainly based on the hemoglobin absorption. On the other hand, the 2,000-nm wavelength shows a very high absorption by water with a decreased optical penetration depth, thus an efficient cutting with sufficient tissue coagulation is only possible with less blood perfusion. Here the wavelength of 1,318 nm showed a good compromise of sufficient cutting and efficient coagulation.
As mentioned, measurement of the optical attenuation coefficient for kidney tissue revealed a mean optical attenuation coefficient of μt = 6.5 mm−1, which indicates a good combination of water absorption μa = 0.2 mm−1 and other attenuation properties of (μt–μa) = 6.3 mm−1. In contrast, the values of the 940 nm showed extremely reduced water absorption and a high amount of the other attenuation components such as scattering and absorption by other chromophores, while for 2,010 nm, both parts are of a similar amount, as shown in Table 3.
The histological coagulation depth in the removed specimens was found to be up to 2 mm. The coagulation depth in the parenchymal side is not known. Laser energy was applied with a reduced irradiance in a non-contact application to perform a proper visual sealing of the parenchymal bleeding. There is no mentioned coagulation depth for control of renal parenchymal bleeding, but this should be as minimal as possible to minimize the loss of kidney tissue. Sealing of the vessels depends on the lumen diameter and the intraluminal blood pressure. Laser-induced blood vessel obstruction with a larger diameter (more than 2 mm) seems to be problematic.
Increased smoke development could be a problem during laser light application. There is no available data about the relation of smoke development and the wavelength. Theoretically, potential differences could be due to whether the wavelengths were predominantly absorbed by hemoglobin or tissue water. From a clinical point of view, a first optimization step could be the development of a special fiber guidance instrument serving for sufficient smoke suction without affecting laparoscopic insufflation of the peritoneum.
This study aimed to evaluate the clinical challenges during laser partial nephrectomy. The instrumentation, surgeon's experience, method of handling, as well as technique may be different (e.g., in OPN cases one can manipulate the renal unit differently than in LPN). So it was intended to establish a standard maneuver.
In addition to omission of hilar clamping, there was less smoke, adequate laser cutting ability under complete surgeon control, as well as acceptable coagulation depth (<1 mm), which is important for NSS. Lastly, the operative oncological results were not compromised through the technique. The pathological results documented the objectively reported free-RR by the surgeon. Even in case of suspicion (two cases) the tumor bed was found free, which indicates the reproducibility of the technique. Furthermore, the operative time was not prolonged with the technique.
In spite of the discussed advantages, some study limitations should be mentioned such as the necessity of special training, the limited number of patients, heterogeneous patient group, single-center/surgeon experience and the limited follow-up. However, the main intention of this study was to show the technical feasibility and reproducibility using quality-control parameters such as complication rate and perioperative morbidity and to propose a standardized approach that is both suitable and safe, from the clinical point of view, for further in vivo studies evaluating laser renal surgery. The next step is directed towards improvement of the equipment and fiber-guidance devices with sufficient blood and smoke suction.
Conclusions
The described preliminary results of the 1,318 diode laser partial nephrectomy showed that the technique is feasible, is adherent to surgical standards, and adds benefits to both laparoscopic as well as open techniques without compromising oncological results. Developments are needed to optimize the laser-fiber guidance instrumentation.
References
Pantuck AJ, Zisman A, Belldegrun AS (2001) The changing natural history of renal cell carcinoma. J Urol 52:447–450
Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr (1999) Rising incidence of renal cell cancer in the United States. JAMA 281:1628–1631
Lane BR, Gill IS (2007) 5-year outcomes of laparoscopic partial nephrectomy. J Urol 177:70–74
Federico VF, Rolle A, Anile M, Martucci N, Bis B, Rocco G (2010) Techniques used in lung metastasectomy. J Thorac Oncol 5(6) Suppl 2:S145–S150
Rolle A, Pereszlenyi A, Koch R, Bis B, Baier B (2006) Laser resection technique and results of multiple lung metastasectomies using a new 1,318-nm Nd:YAG laser system. Lasers Surg Med 38:26–32
Rolle A, Pereszlenyi A, Koch R, Richard M, Baier B (2006) Is surgery for multiple lung metastases reasonable? A total of 328 consecutive patients with multiple-laser metastasectomies with a new 1,318-nm Nd:YAG laser. J Thorac Cardiovasc Surg 131:1236–1242
Wieliczka DM, Weng S, Querry MR (1989) Wedge-shaped cell for highly absorbent liquids: infrared optical constants of water. Appl Opt 28:1714–1719
Takatani S, Graham MD (1987) Theoretical analysis of diffuse reflectance from a two-layer tissue model. IEEE Trans Biomed Eng BME-26:656–664
Sroka R, Rösler P, Janda P, Grevers G, Leunig A (2000) Endonasal laser surgery with a new laser fibre guidance instrument. Laryngoscope 110:332–334
Campbell SC, Novick AC (2006) Expanding the indications for elective partial nephrectomy: is this advisable? Eur Urol 49:952–954
Porpiglia F, Volpe A, Billia M, Scarpa RM (2008) Laparoscopic versus open partial nephrectomy: analysis of the current literature. Eur Urol 53:732–743
Godoy G, Ramanathan V, Kanofsky JAO, Malley RL, Tareen BU, Taneja SS, Stifelman MD (2009) Effect of warm ischemia time during laparoscopic partial nephrectomy on early postoperative glomerular filtration rate. J Urol 181(6):2438–2443
Abukora F, Nambirajan T, Albqami N, Leeb K, Jeschke S, Gschwendtner M, Janetschek G (2005) Laparoscopic nephron sparing surgery: evolution in a decade. Eur Urol 47:488–493
Gill I, Novick A, Soble J et al (1998) Laparoscopic renal cryoablation: initial clinical series. Urology 52:543–551
Hsu T, Fidler M, Gill I (2000) Radiofrequency ablation of the kidney: acute and chronic histology in porcine model. Urology 56:872–875
Rendon R, Kachura J, Sweet J et al (2002) The uncertainty of radiofrequency treatment of renal cell carcinoma: findings at immediate and delayed nephrectomy. J Urol 167:1587–1592
Desai M, Gill I (2002) Current status of cryoablation and radiofrequency ablation in the management of renal tumors. Curr Opin Urol 12:387–393
Sewell PE, Howard JC, Shingleton WB et al (2003) Interventional magnetic resonance image-guided percutaneous cryoablation of renal tumors. South Med J 96:708–710
Eret V, Hora M, Sykora R, Hes O, Urge T, Klecka J, Matejovic M (2009) GreenLight (532 nm) laser partial nephrectomy followed by suturing of collecting system without renal hilar clamping in porcine model. Urology 73:1115–1118
Bui MH, Breda A, Gui D, Said J, Schulam P (2007) Less and minimal tissue carbonization using a thulium laser for laparoscopic partial nephrectomy without hilar clamping in a porcine model. J Endourol 21:1107–1111
Gruschwitz T, Stein R, Schubert J, Wunderlich H (2008) Laser-supported partial nephrectomy for renal cell carcinoma. Urology 71:334–336
Mattioli S, Muñoz R, Recasens R, Berbegal C, Teichmann H (2008) What does Revolix laser contribute to partial nephrectomy. Arch Esp Urol 61:1126–1129
Gill IS, Matin SF, Desai MM et al (2003) Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol 170:64–68
Gill IS, Kavoussi LR, Lane BR et al (2007) Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 178:41–46
Porpiglia F, Volpe A, Billia M, Scarpa RM (2008) Laparoscopic versus open nephrectomy: analysis of the current literature. Eur Urol 53:732–742
Breda A, Stepanian SV, Liao J et al (2007) Positive margins in laparoscopic partial nephrectomy in 855 cases: a multiinstitutional survey from the United States and Europe. J Urol 178:47–50
Frank I, Colombo JR Jr, Rubinstein M, Desai M, Kaouk J, Gill IS (2006) Laparoscopic partial nephrectomy for centrally located renal tumors. J Urol 175:849–852
Seitz M, Reich O, Gratzke C, Schlenker B, Karl A, Bader M, Khoder W, Fischer F, Stief C, Sroka R (2009) High-power diode laser at 980 nm for the treatment of benign prostatic hyperplasia: ex vivo investigations on porcine kidneys and human cadaver prostates. Lasers Med Sci 24:172–178
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wael Y. Khoder and Ronald Sroka contributed equally to this investigation and manuscript.
Rights and permissions
About this article
Cite this article
Khoder, W.Y., Sroka, R., Hennig, G. et al. The 1,318-nm diode laser supported partial nephrectomy in laparoscopic and open surgery: preliminary results of a prospective feasibility study. Lasers Med Sci 26, 689–697 (2011). https://doi.org/10.1007/s10103-011-0897-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-011-0897-y