Abstract
The aim of this study was to evaluate the effects of 820-nm diode laser on osteoclastic and osteoblastic cell proliferation-activity and RANKL/OPG release during orthodontic tooth movement. Thirty-eight albino Wistar rats were used for this experiment. Maxillary incisors of the subjects were moved orthodontically by a helical spring with force of 20 g. An 820-nm Ga-Al-As diode laser with an output power of 100 mW and a fiber probe with spot size of 2 mm in diameter were used for laser treatment and irradiations were performed on 5 points at the distal side of the tooth root on the first, second, and 3rd days of the experiment. Total laser energy of 54 J (100 mW, 3.18 W/cm2, 1717.2 J/cm2) was applied to group II and a total of 15 J (100 mW, 3.18 W/cm2, 477 J/cm2) to group III. The experiment lasted for 8 days. The number of osteoclasts, osteoblasts, inflammatory cells and capillaries, and new bone formation were evaluated histologically. Besides immunohistochemical staining of PCNA, RANKL and OPG were also performed. No statistical difference was found for the amount of tooth movement in between the control and study groups (p > 0.05). The number of osteoclasts, osteoblasts, inflammatory cells, capillary vascularization, and new bone formation were found to be increased significantly in group II (p < 0.05). Immunohistochemical staining findings showed that RANKL immunoreactivity was stronger in group II than in the other groups. As to OPG immunoreactivity, no difference was found between the groups. Immunohistochemical parameters were higher in group III than in group I, while both were lower than group II. On the basis of these findings, low-level laser irradiation accelerates the bone remodeling process by stimulating osteoblastic and osteoclastic cell proliferation and function during orthodontic tooth movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laser irradiation has a variety effects on tissues, ranging from biostimulation to photodisruption. Arising effect in the tissue depends on the irradiation time and the energy density. The effects of laser radiation which are not accompanied by local temperature increase in tissues by more than 1°C are called ‘biostimulating effects’ [1]. Treatments take effect via biostimulation potency of laser radiation are called ‘low-level laser therapy’ (LLLT).
Researchers have been studying the biostimulatory effects of low-level laser use since 1971. Many processes like fibroblast [2] and chondrocyte proliferation [3], collagen synthesis [4], nerve regeneration [5], wound healing [6], and bone regeneration [7] can be stimulated with laser radiation. In dentistry, biostimulation is useful for the treatment of aphthous ulcers, bone repair in some periodontal defects, and acceleration of osteointegration after implantation.
In orthodontics, low-level laser radiation can be used for reduction of post-adjustment pain [8], bone regeneration in midpalatal suture area after rapid maxillary expansion [9], and accelerating tooth movement [10–12].
Osteoclasts are specialized members of the monocyte/macrophage family that differentiate from hematopoietic precursors. As an alternative method to define osteoclasts, it is possible to evaluate RANKL (Receptor Activator for Nuclear Factor κ B Ligand) level, which is an osteoclast differentiation factor. RANKL, also known as TNF-related activation-induced cytokine (TRANCE), is a member of the tumor necrosis factor (TNF) cytokine family which functions as a key factor for osteoclast differentiation and activation. Recently, a novel inhibitor of osteoclastogenesis was identified and named as osteoprotegerin (OPG) by Simonet et al. [13]. This novel cytokine is also a member of the TNF receptor family and is expressed ubiquitously in murine and human tissues. It is a strong inhibitor of osteoclast differentiation in co-cultures of osteoblastic and hematopoietic cells. Current evidence suggests that OPG inhibits osteoclast terminal differentiation from their progenitors, as well as the function of mature osteoclasts. Ogasawara et al. determined RANKL in osteoblasts and periodontal ligament cells during orthodontic tooth movement and emphasized that RANKL-OPG release and their interaction is very important for bone remodeling [14].
PCNA (proliferating cell nuclear antigen) protein is a cell cycle-related nuclear protein that is maximally elevated in the late G1 and S phases of proliferating cells. Immunohistochemical staining for PCNA in paraffin-embedded sections has been reported to be a useful method for evaluation of cell-proliferative activity [10].
As is well known, orthodontic tooth movement arises due to an inflammatory response. Many inflammatory cytokines are released and osteoclastic and osteoblastic activity increases during this process. Because low-level laser therapy (LLLT) is very beneficial in treatment of inflammatory problems, it is logical to think that orthodontic tooth movement can be stimulated by LLLT.
In consideration of the previous articles, the aim of this study was to evaluate the effects of 820-nm diode laser on osteoclastic and osteoblastic cell proliferation-activity during orthodontic tooth movement. For this purpose, in addition to histomorphological assessment, immunohistochemical staining by using the monoclonal antibody of PCNA was performed to elucidate the effects of laser irradiation on osteoblasts, osteoclasts, and fibroblasts and immunohistochemical staining with monoclonal antibodies of RANKL and OPG were performed to define the changes in the immunoreactivities of these factors.
Materials and methods
Animals and appliance used
In the study we used 38 male albino Wistar rats 10 weeks old (weighing 175 ± 10 g), which were obtained from the animal laboratory of Cumhuriyet University Faculty of Medicine. The Animal Ethics Committee of Cumhuriyet University approved our study protocol. Guidelines for using laboratory animals were strictly followed throughout the study. All animals were housed in a 12-h light/dark environment at a constant temperature of 23°C and fed a standard pellet diet with tap water ad libitum. The animals were randomly divided into four groups. Groups I, II, and III served as experimental groups containing 11 rats each and group IV was the control group and contained five rats. In the first group, an orthodontic spring was placed for orthodontic tooth movement. In the second and the third groups, the spring was placed and right maxillary incisor was irradiated with different doses.
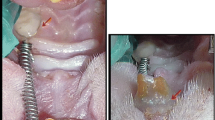
A helical spring fabricated from 0.015” stainless-steel wire was prepared for orthodontic tooth movement. Springs were placed on a grid and activated with pliers. The force of the spring was calibrated with a gauge to 20 g in order to prevent expansion of the suture and obtain tooth movement solely. Appliances were attached to maxillary incisors of all animals under anesthesia with xylazine (Rompun, Bayer, Leverkusen, Germany, 3 mg/kg) and ketamine (Ketalar, Pfizer, USA, 90 mg/kg) combination. A groove at the level of the gingival papilla was prepared on the distal sides of the incisor teeth using a stainless-steel disc for retention of the spring. Then, the spring was fixed with 0.012” stainless-steel ligature wires (Figs. 1, 2).
The rats were monitored during the experiment, and all the animals were weighed each day of the experiment. A sharp decrease in their body weights were observed in the first 2 days; then they recovered.
It has been reported that the changes in RANKL and OPG levels after loading orthodontic forces become significant in 24 h while they had lost their significance 168 h later. Consequently, for immunohistochemical assessment of RANKL and OPG, experimental groups were divided into subgroups (A groups) consisting of four rats which were killed at the end of the third day of the experiment. The other seven rats in the experimental groups (B groups) and the five rats in the control group were killed on the eighth day of the experiment with 200 mg/kg sodium pentobarbital (Petothal, Abbot, ABD). Their pre-maxillae were dissected and placed in bottles contained 10% formalin solution. The appliances were removed after fixation in order to prevent relapse of teeth.
Laser irradiation
A Ga-Al-As diode laser (Doris, CTL-1106MX) with a wavelength of 820 nm and an output power of 100 mW was used in this study. The irradiation was performed with continuous waves by a fiber probe 2 mm in diameter (CTL-2214) on the first, second, and third days of the experiment. The tip was held perpendicular and in contact with the mucosa during irradiation (Fig. 3).
In group II, the root of the right maxillary incisor was irradiated from five points (two points at distobuccal, one at distal approximal, and two at distopalatinal side) for 108 s each (10.8 J/point or 343.9 J/cm2). Total energy dose corresponding to a 9-min exposure was 54.0 J (540 s, 100 mW, 3.18 W/cm2, 1717.2 J/cm2).
In group III, the root of the right maxillary incisor was irradiated from five points (two points at distobuccal, one at distal approximal, and two at distopalatinal side) for 30 s each (3 J/point or 95.5 J/cm2). Total energy dose corresponding to a 2.5-min exposure was 15.0 J (150 s, 100 mW, 3.18 W/cm2, 477 J/cm2).
Measurement of tooth movement
The amount of tooth movement was measured each day of the experiment with a digital caliper compass at the level of gingival papilla between incisors.
Tissue preparation
After killing, the pre-maxillae were fixed in 10% formalin solution at room temperature for 24–48 h and then a routine paraffin procedure was performed. Briefly, tissue samples were dehydrated through graded alcohol series and cleared in xylene and embedded in paraffin. Sections (5 μm thick) were cut and prepared for both histochemical and indirect immunohistochemical stainings. Hematoxylin and eosin (H&E) stain was applied for histochemical evaluation. For immunohistochemical evaluation, the avidin–biotin peroxidase system was used. Anti-receptor activator of nuclear factor kappa ligand (anti-RANKL) (1:100 dilution, Santa Cruz, sc-7628, CA, USA), anti-osteoprotegerin (anti-OPG) (1:100 dilution, Santa Cruz, sc-8468, CA, USA), anti-PCNA (1:100 dilution, Santa Cruz, sc-56, Ca, USA) primary antibodies and Sekonder Antikor ABC staining system (Santa Cruz, sc-2023, Ca, USA) were used. A semi-quantitative grading system was used to compare the immunohistochemical staining. Intense of immunoreactivities were determined as mild (+), moderate (++), or strong (+++).
Treatment groups were compared to controls according to the number of osteoclasts, number of osteoblasts, number of capillaries, number of inflammatory cells, new bone formation, and immunohistochemical stainings.
Statistical analysis
Data are expressed as mean±standard error of the mean (SEM). Differences among four groups for amount of tooth movement, number of osteoclasts, number of osteoblasts, and number of capillaries were evaluated with SPSS (ver:14.0) using the Kruskal–Wallis test and pair-wise comparisons were made by the Mann–Whitney U test . A p value <0.05 was accepted as statistically significant. The number of inflammatory cells, new bone formation, and immunoreactivities were compared according to intensity.
Results
Metrical findings
Amount of tooth movement
According to metrical findings, no statistical difference was found for tooth movement rate between the control and study groups, although the amount of tooth movement was more in group II than in the other groups (Fig. 4).
Histological findings
Photomicrographs stained with H&E and those immunostained with anti-RANKL, anti-OPG, and anti-PCNA primary antibodies are represented in Fig. 5.
Photomicrographs of the groups (1: control group; 2: group 1-A (tooth movement only); 3: group 2-A (54 J laser); 4: group 3-A (15 J laser); 5: group 1-B; 6: group 2-B; 7: group 3-B). a Sections stained with H&E. b Sections immunostained with anti-RANKL primary antibody. c Sections immunostained with anti-OPG primary antibody. d Sections immunostained with anti-PCNA primary antibody. OB osteoblast, OC osteoclast, BV blood vessel, P periodontal ligament, AB alveolar bone, NB new bone
Number of osteoclasts
When the groups were compared for this aspect, the parameters of the groups II-A and II-B were statistically significantly higher than the others (Tables 1, 2). When we look at pair-wise comparisons, all of the paired comparisons except the one between group II-A and III-A were statistically significant (Table 3).
Number of osteoblasts
According to the comparison of A groups, there was no difference in the number of osteoblasts between groups (Table 1). However, B groups represented different results (Table 2). The difference between group II and III was insignificant, while the difference between group I and II and also the difference between group I and III were found to be statistically significant (Table 3).
Number of capillaries
Number of capillaries in both A and B groups were found significantly different from each other (Tables 1, 2). The difference between group I and II, group I and III and group II and III were found statistically significant (Table 3).
Number of inflammatory cells
Increase in the number of inflammatory cells was significantly more in group II than in the other groups. This parameter was found higher in group III than in group I (Table 4).
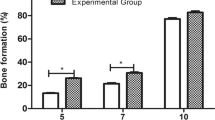
Bone formation
When new bone formation was compared between the groups, it was found to be greater in group II than in the other groups. Besides, group III represented more bone formation than group 1 (Table 4).
Immunohistochemical findings
RANKL immunoreactivity
RANKL-positive fibroblasts in PDL and osteoblasts and osteoclasts in the bone surface were observed in all of the force-applied groups both on the third-day and the eighth-day micrographs. The number and intensity of RANKL-positive cells in laser groups (groups II–III) were greater than in group I. Micrographs of group II represented stronger immunoreactivity than group III (Table 5).
OPG Immunoreactivity
OPG immunoreactivity of fibroblasts, osteoblasts, and osteoclasts were increased slightly in groups compared to the control group. However, there was no difference in the number and intensity of the OPG-positive cells between the groups (Table 5).
PCNA immunoreactivity
The number of PCNA-positive cells (fibroblasts, osteoblasts, and osteoclasts) was significantly higher in laser groups. When group II and III were compared, it was observed that micrographs of group II represented stronger immunoreactivity (Table 5).
Discussion
Several studies have represented the effects of LLLT on orthodontic tooth movement [10–12, 15–23]. In the present study, the effects of LLLT on tooth movement was investigated metrically, histologically, and immunohistochemically.
The type of laser device is chosen according to the target tissue and desired effect. The best wavelength for biostimulation is between 550 and 950 nm. Because absorption of infrared light is low by hemoglobin and water, the beams at this wavelength penetrate deeper in the tissues. An infrared light emitting laser (820 nm) was chosen due to the aim of this study, which is to stimulate bone cells placed under soft tissues and deeper in the alveolus [24].
The most difficult issue about LLLT is to define the effective dose. There are several studies in the literature promoting low-level laser therapy as a useful treatment with doses used between 2 and 54 J [10, 11, 15–17]. On the other hand, some researchers used doses of 8.1–27 J and attained negative results [18, 19]. However, the doses which have been found as non-effective are in between the effective dose range. For this reason, it’s not sensible to decide whether the amount of energy’s being appropriate for biostimulation depending just on the dose (Joules). Laser spot area should be known to be able to have an opinion about the density of the energy given to the tissue. Because, when the spot area is doubled, the energy density decreases four times, or if the spot area is halved, the energy density quadruples. However, this point has not been emphasized in any of the studies. Even the diameter of the probe has not been indicated in some of the studies [11, 17, 19].
Another important point regarding the energy dose is scattering, which reduces the effectiveness of light. It is accepted that the ideal biostimulation range is 2–12 J/cm2 [1]. However, Luger et al.’s results conflicted with this. Although the researchers used light energy of 64 J/cm2, which was quite high for biostimulation, they promoted that the energy amount at the target area was 3–6% of the total energy due to scattering of light while transmitting through the tissue [25]. According to Kawasaki and Shimizu, Yamagishi et al. claimed that only 50% of the light of a diode laser could reach 1 mm depth in bovine mandibular cortical bone [10]. Therefore, this situation should be considered when defining the energy dose.
In five of nine animal studies about stimulation effects of LLLT on orthodontic tooth movement [10, 12, 16, 17, 19–23], experiments were performed on albino Wistar rats using the same laser device and the same parameters [10, 12, 20–22]. Even though the energy density used in these studies was considerably higher (54 J, 19.108 J/cm2) than it is thought to be appropriate for biostimulation (2–12 J/cm2), it was concluded in all of the five studies that laser radiation had stimulated tooth movement. When the other animal studies were examined, it was noticed that there were differences about subject type, the energy dose given, and about the results. It is known that the efficiency of low-level laser therapy depends on the dose and the nature of the irradiated tissue. Because the aim of the present study was to highlight the mechanisms of biostimulation process, it was considered that it would be better to base this study on studies performed on Wistar rats, and the dose was defined as 54 J. Additionally, another group was composed to assess the changes when the dose was lower (15 J).
In the present study, orthodontic tooth movement was carried out on maxillary incisors. However, the distance between distal proximal sides of maxillary incisors is approximately 3–4 mm. Therefore, it was considered that the laser radiation applied to distal side of one of the teeth might distribute in tissue and affect the opposite tooth. Then it would be impossible to define the energy density affecting one tooth distinctly and perhaps the dose given would be more than the desired. For this reason, only the right maxillary incisors were irradiated.
According to metrical findings, tooth movement rate did not show any difference between groups. While the most tooth movement was observed in group II (54 J) both on the third and eighth day of the experiment, the difference between groups was not statistically significant. This finding is in contrast with the findings of the researchers who acclaimed that orthodontic tooth movement could be stimulated with low-level laser therapy [10, 12, 20–22]. It is thought that this conflicting result might be due to the limited number of test subjects. Moreover, the teeth were moved for a close distance in the present study model and only one of the teeth was irradiated. However, tooth movement was measured as the distance between two incisors, one of them being non-irradiated. This condition might prevent the rise of a significant increase in the amount of tooth movement between groups.
Multinucleated osteoclasts, Howship lacunes, and bone resorption were found increased in all of the study groups. The increase was significantly more in group II-A and II-B than the other groups. It has been already known that low-level laser therapy stimulates differentiation and activation of osteoclasts [7, 12, 20, 26]. According to Karu et al., the mitochondrial cytochromes absorb the photon energy and this absorption improves the potential activity of the cells via increasing ATP synthesis [27]. Because osteoclasts are multinuclear cells with mitochondria of high activity, they are affected from low-level laser radiation readily. Furthermore, Hentunen et al. reported that the bone matrix liberates a protein that stimulates osteoclast formation, which is light-dose dependent [28]. This also explains the higher resorption levels in the irradiated animals.
RANKL and OPG are two important regulators of bone remodeling. In other words, RANK/RANKL/OPG system reflects osteoclasts’ differentiation and function. Not surprisingly, RANKL immunoreactivity was increased more in the laser groups than in group I. As for OPG levels, there was no difference between groups. Ogasawara et al. reported that no OPG-positive osteoclasts were observed in cases of experimental tooth movement [14]. Although there was no difference in OPG levels, RANKL/OPG ratio, which defines osteoclastic activity, remained high because of the increased RANKL levels. It is possible that the OPG levels stayed unchanged in order to prevent excessive increase in RANKL/OPG ratio in order to get under control the resorption process due to increased osteoclastic activity.
It is known that osteoclastic activity may influence posterior osteoblastic activity and vice versa [29]. Zaidi et al. reported that both osteoblasts and osteoclasts have hormonal interaction [30]. In the present study, it was found that the number of osteoblasts was increased more in group II than in the others at the end of the experiment, while there was no difference between groups on the third day. Because osteoclastic activity begins before the formation process in orthodontic tooth movement, laser radiation did not alter osteoblastic activity on the third day. However, the bone formation started afterwards was stimulated by low-level laser beams in harmony with the results of the following studies [9, 26, 31]. Laser radiation has the potential of stimulating maturation of osteoblastic cells and it accelerates organic matrix synthesis and mineralization via increasing osteoblastic activity [31].
As was expected on the basis of the above findings, the amount of new bone formation was greater in group II than in the other groups. Hamajima et al. reported that the increased expression of the osteoglycin gene by LLLT in the early proliferation stage of cultured osteoblastic cells might play an important role in stimulation of bone formation [32].
Orthodontic tooth movement occurs as a result of inflammatory response of the tissue. Laser irradiation multiplied inflammation since it accelerated tooth movement. Although Shimizu et al. claimed that PGE2 synthesis was inhibited by laser radiation [33], Fujita et al. found that cytokine depression decelerated tooth movement while they accelerated tooth movement with low-level laser application [20]. Moreover, Glinkowski and Pokora indicated that biostimulating laser application to bone increased phagocytosis and cytokine (IL-1, TGF-β) synthesis via accelerating macrophage migration [1].
The increases in almost all of the parameters belonging to group III were higher than group I but lower than group II. This indicated that the stimulation effect of low-level laser radiation was dose-dependent. It was thought that energy of the applied dose remaining incapable caused indifference of some parameters.
It has been thought that excessive doses cause inhibition rather than stimulation according to Arndt-Schultz law [1]. Although the energy density was too high in this study, tooth movement was stimulated. Furthermore, group II presented more positive results than group III, indicating that the density of group III was inadequate for stimulation though being higher than it was accepted in literature. Histological and immunohistochemical findings support this idea. It was thought that the differences in dose applications were due to scattering of the light in the tissue.
Any significant differences being found for the amount of orthodontic tooth movement between groups was attributed to the small sample size of this study. Sample size should be increased and various doses should be applied to be able to define the effective dose for biostimulation of tooth movement.
In the present study, it was determined that low-level laser therapy increased the osteoclastic activity by exacerbating the inflammatory response to orthodontic forces. It is known that inflammatory cytokines trigger RANKL release which regulates osteoclastic cell activity. Additionally, under inflammatory conditions, RANKL is released not only from osteoblasts but also from immune system cells. In this way, RANKL release was found to be increased in our study. Low-level laser therapy is known to be a stimulator of the current biological process in tissue. This is why osteoclastic activity varied while osteoblastic activity was similar between groups on the third day of the experiment. At the end of the experiment, because bone formation had already started, the number of osteoblasts was found to be increased in the laser groups.
Some researchers have reported the effects of LLLT on cellular responses [31, 34]. Conlan et al. showed that the stimulation of photoreceptors in mitochondrial respiratory chain changed the cellular adenosine triphosphate levels and cell membrane stabilization through bioelectrical effects [34]. However, it should be considered that LLLT’s effects on cells are wavelength- and dose-dependent, and molecular absorption of laser light is a prerequisite for any cellular effect [31]. Due to the biological differences between animals and human beings, we were not able to characterize the exact mechanism of the biostimulatory effect of laser irradiation on human physiologic state and it is not suitable to suggest that these doses and wavelength are appropriate for human beings.
Conclusions
On the basis of the findings of the present study, it is obvious that LLLT accelerates the bone remodeling process by stimulating osteoblast and osteoclast cell proliferation and function during orthodontic tooth movement. Since the bio-stimulant effects of laser therapy have been shown in many studies, further studies with different doses should be performed to determine the appropriate dose to provide clinical advantage.
References
Glinkowski W, Pokora L (2001) Lasery w therapii (in Polish) (Lasers in theraphy). Laser instruments-Centrum techniki Laserowej, Warsaw
Van Breugel HH, Bar PR (1992) Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med 12:528–537
Schultz RJ (1985) Effects of varying intensities of laser energy on articular cartilage. Lasers Surg Med 5:577–588
Poon VK, Huang L, Burd A (2005) Biostimulation of dermal fibroblast by sublethal Q-switched Nd:YAG 532-nm laser: collagen remodeling and pigmentation. J Photochem Photobiol B 81:1–8
Mohammed IF, Al-Mustawfi N, Kaka LN (2007) Promotion of regenerative processes in injured peripheral nerve induced by low-level laser therapy. Photomed Laser Surg 25:107–111
Maiya GA, Kumar P, Rao L (2005) Effect of low intensity helium-neon (He-Ne) laser irradiation on diabetic wound healing dynamics. Photomed Laser Surg 23:187–190
Nicolau R, Jorgetti V, Rigau J, Pacheco M, Reis LM, Zangaro R (2003) Effect of low-power GaAlAs laser (660 nm) on bone structure and cell activity: an experimental animal study. Lasers Med Sci 18:89–94
Turhani D, Scheriau M, Kapral D, Benesch T, Jonke E, Bantleon HP (2006) Pain relief by single low-level laser irradiation in orthodontic patients undergoing fixed appliance therapy. Am J Orthod Dentofacial Orthop 130(3):371–377
Saito S, Shimizu N (1997) Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop 111(5):525–532
Kawasaki K, Shimizu NV (2000) Effects of low-energy irradiation on bone remodelling during experimental tooth movement in rats. Lasers Surg Med 26:282–291
Youssef M, Ashkar S, Hamade E, Gutknecht N, Lampert F, Mir M (2008) The effect of low-level laser therapy during orthodontic movement: a preliminary study. Lasers Med Sci 23(1):27–33
Yamaguchi M, Fujita S, Yoshida T, Oikawa K, Utsunomiya T, Yamamoto H, Kasai K (2007) Low-energy laser irradiation stimulates the tooth movement velocity via expression of M-CSF and c-fms. Orthod Waves 66:139–148
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin—a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Ogasawara T, Yoshimine Y, Kiyoshima T, Kobayashi I, Matsuo K, Akamine A, Sakai H (2004) In situ expression of RANKL, RANK, Osteoprotegerin and cytokines in osteoclasts of rat periodontal tissue. J Periodontal Res 39:42–49
Cruz D, Kohara E, Ribeiro M, Wetter N (2004) Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: a preliminary study. Lasers Surg Med 35:117–120
Goulart CS, Nouer PRA, Mouramartins L, Garbin IU, Lizerelli RDZ (2006) Photoradiation and orthodontic movement: experimental study with canines. Photomed Laser Surg 242:192–196
Sun X, Zhu X, Xu C, Ye N, Zhu H (2001) Effects of low energy laser on tooth movement and remodeling of alveolar bone in rabbits. Hua Xi Kou Qiang Yi Xue Za Zhi 19(5):290–293
Limpanichkul W, Godfrey K, Srisuk N, Rattanayatikul C (2006) Effects of low-level laser therapy on the rate of orthodontic tooth movement. Orthod Craniofac Res 9:38–43
Seifi M, Shafeei HA, Daneshdoost S (2007) Effects of two types of low-level laser wave lengths (850 and 630 nm) on the orthodontic tooth movements in rabbits. Lasers Med Sci 22:261–264
Fujita S, Yamaguchi M, Utsunomiya T, Yamamoto H, Kasai K (2008) Low-energy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod Craniofac Res 11:143–155
Yoshida T, Yamaguchi M, Utsunomiya T, Kato M, Arai Y, Kaneda T, Yamamoto H, Kasai K (2009) Low-energy laser irradiation accelerates the velocity of tooth movement via stimulation of the alveolar bone remodeling. Orthod Craniofac Res 12(4):289–298
Yamaguchi M, Hayashi M, Fujita S, Yoshida T, Utsunomiya T, Yamamoto H, Kasai K (2010) Low-energy laser irradiation facilitates the velocity of tooth movement and the expressions of matrix metalloproteinase-9, cathepsin K, and alpha(v) beta(3) integrin in rats. Eur J Orthod 32:131–139
Kim YD, Kim SS, Kim SJ, Kwon DW, Jeon ES, Son WS (2010) Low-level laser irradiation facilitates fibronectin and collagen type I turnover during tooth movement in rats. Lasers Med Sci 25:25–31
Hillenkamp F (1990) Interaction between laser radiation and biological systems. In: Hillenkamp F, Pratesi R, Scassi CA (eds) Lasers in biology and medicine. Plenum Press, New York, pp 37–68
Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102
Aihara N, Yamaguchi M, Kasai K (2006) Low-energy laser stimulates formation of osteoclast-like cells via RANK expression in vitro. Lasers Med Sci 21:24–33
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol 49:1–17
Hentunen TA, Cunningham NS, Vuolteenaho O, Reddi AH, Vaananen HK (1994) Osteoclast recruiting activity in bone matrix. Bone Miner 25:183–198
MacDonald BR, Gowen M (1993) The cell biology of bone. Baillières Clin Rheumatol 7:421–443
Zaidi M, Pazianas M, Shankar VS, Bax BE, Bax CM, Bevis PJ, Stevens C, Huang CL, Blake DR, Moonga BS (1993) Osteoclast function and its control. Exp Physiol 78:721–739
Kim YD, Song WW, Kim SS, Kim GC, Hwang DS, Shin SH, Kim UK, Kim JR, Chung IK (2009) Expression of receptor of nuclear factor-kB ligand, receptor activator of nuclear factor-kB, and osteoprotegerin, following low-level laser treatment on deproteinized bovine bone graft in rats. Lasers Med Sci 24(4):577–584
Hamajima S, Hiratsuka K, Kiyama-Kishikawa M, Tagawa T, Kawahara M, Ohta H, Sasahara H, Abiko Y (2003) Effect of low-level laser irradiation on osteoglycin gene expression in osteoblasts. Lasers Med Sci 18:78–82
Shimizu N, Yamaguchi M, Goseki T, Shibata Y, Takiguchi H, Iwasawa T (1995) Inhibition of PGE2 and IL-1β production by low-power laser irradiation in stretched human periodontal ligament cells. J Dent Res 74:1382–1388
Conlan MJ, Rapley JW, Cob CM (1996) Biostimulation of wound healing by low-energy laser irradiation: a review. J Clin Periodontol 23:492–496
Acknowledgements
This study was supported by the Scientific Research Project Fund of Cumhuriyet University under the project number DIS-055.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altan, B.A., Sokucu, O., Ozkut, M.M. et al. Metrical and histological investigation of the effects of low-level laser therapy on orthodontic tooth movement. Lasers Med Sci 27, 131–140 (2012). https://doi.org/10.1007/s10103-010-0853-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0853-2