Abstract
The production of biofuel from biomass waste is of great interest to the scientific community regarding the discovery of solutions to global energy demand and global warming. The pyrolysis of biomass to produce bio-oil is an easy, cheap and promising technology. In the current investigation, the pyrolysis of two different biomasses (cornelian cherry stones and grape seeds) was achieved at temperatures ranging from 300 to 700 °C. The effect of pyrolysis temperatures on the yields of each product was significant. The bio-oil yields were maximized at 500 °C for cornelian cherry stones and 700 °C for grape seeds. The compositions of bio-oils for both cornelian cherry stones and grape seeds were similar and contained mainly oxygenated hydrocarbons. The compounds observed in this investigation were composed of phenols, alkyl benzenes, alkanes, alkenes, fatty acids, fatty acid esters and a few nitrogen-containing compounds. Bio-char properties were amended in association with both the pyrolysis temperature and biomass type. Bio-chars from cornelian cherry stones contained higher carbon and lower oxygen levels than those from grape seeds under identical conditions. Increases in pyrolysis temperatures produced bio-chars containing higher carbon levels and heating values for both carnelian cherry stones and grape seeds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass is one of the best sources to meet the energy demands of the future as it is not only renewable but also environmentally friendly. The energy obtained from biomass is used mainly in heat production, electricity, and as fuels for vehicles (Houshfar et al. 2014). Various advanced technologies have been developed to obtain biofuels and/or biochemicals from biomass, which include the hydrothermal process (Jin and Enomoto 2011), pyrolysis (Torri et al. 2009), and combustion (Vassilev et al. 2013).
Pyrolysis of biomass is one of the most efficient technologies used to produce biofuel. The process is carried out at elevated temperatures under an inert atmosphere which is maintained using either argon or nitrogen gases. The process yields bio-oil, solid residue, and gaseous products. Process conditions, as well as the raw materials used, have varying effects on the yields of each product.
Bio-oils and bio-chars are two significant pyrolysis products, and they have their own significant values which can be utilized in a sustainable way. Bio-oils can be used as transportation fuels after they are upgraded using a process such as deoxygenation and dearomatization (Huber et al. 2006; Amen-Chen et al. 2001). Biofuels can be blended with diesel fuels with the assistance of surfactants (Huber et al. 2006; Bridgwater 2004). They can also be evaluated as a source of value-added chemicals after isolation and purification (Amen-Chen et al. 2001).
Bio-chars from pyrolysis of biomass are also perspective products which can be used to improve soil functions (Marousek 2013a). Production and storage of bio-chars in soils have some beneficial aspects such as the mitigation of climate change by sequestering carbon (Marousek et al. 2014a; Marousek 2013b), leading to an increase in the capacity of soil to store water (Marousek 2014), an increase in soil microbial biomass (Marousek 2014), and an increase in yields of crops (Marousek et al. 2014a).
Fruit wastes and their by-products are important biomass resources for the scientific community to evaluate as potential energy sources. Many studies are available in the literature concerning the pyrolysis of fruit wastes for the production of biofuel via pyrolysis, such as sesame, mustard and neem de-oiled cakes (Volli and Singh 2012), tamarind seed (Kader et al. 2013), jatropha oil cakes (Raja et al. 2010; Jourabchi et al. 2014), palm kernel cake (Ngo et al. 2013), cottonseed cake (Putun et al. 2006), and pomegranate seeds (Ucar and Karagoz 2009).
Nayan et al. investigated the production of bio-oil from the pyrolysis of neem seed at the temperature zone from 400 to 500 °C and a heating rate of 20 °C min−1 (Nayan et al. 2013). The greatest bio-oil yield (38 wt%) was accomplished at 475 °C. Hydrocarbons identified in the bio-oils were numerous and varied. Prominent compounds identified in the bio-oils were octadecanenitrile, oleanitrile, 9-octadecenoic acid methyl ester, and stearic acid methyl ester.
Pyrolysis of karanja seed to produce liquid fuel was carried at temperatures ranging between 500 and 600 °C (Shadangi and Mohanty 2014). The optimal temperature used for the production of the greatest bio-oil yield (55.2 wt%) was 550 °C. The bio-oil contained a mixture of oxygenated hydrocarbons including phenols, acids, esters, hexane, levoglucosan, amide, nitrile, benzene, furan, and other compounds.
Shadangi and Singh explored the pyrolysis of polanga seed cake at temperatures ranging from 450 to 600 °C (Shadangi and Singh 2012). The maximal bio-oil yield was approximately 46 %, and it was produced at the temperature of 550 °C. GC–MS analysis showed that the bio-oil contained various compounds. The relative concentrations of the following compounds were high: oleic acid, hexadecanoic acid, octadecanonic acid, octadec-9-enoic acid, hexadecanenitrile, 9-octadecanamide, octadecanamide, phenol, oleanitrile, heptadecane, and pentadecane.
Figueiredo et al. produced bio-oil from the pyrolysis of castor seeds at the temperature of 380 °C (Figueiredo and Romeiro 2009). The product distribution was as follows: 50 wt% bio-oil, 29 wt% bio-char, and 8 wt% gaseous products. The fractions of the bio-oils contained long-chain hydrocarbons and polar compounds.
In the current paper, two types of fruit wastes (cornelian cherry stones and grape seeds) were exposed to pyrolysis under identical conditions. Pyrolysis temperatures from 300 to 800 °C were studied. Product distributions from the pyrolysis of cornelian cherry stones were compared with the pyrolysis of grape seeds under identical conditions. The bio-oils were analyzed via GC–MS and an elemental analyzer. The bio-chars were studied and classified by means of elemental analysis and scanning electron microscopy techniques.
Materials and methods
Feedstock
Cornelian cherry stones were purchased from a local market in Safranbolu, Turkey. Samples were separated from the skin of the fruits, washed, and air-dried. The air-dried samples were grinded in a blender. The samples were milled to a particle size: <0.5 mm. Grape seeds were obtained from a local market in Ankara, Turkey. Grape seed samples were used in the same condition of their purchase. Samples were milled to a particle size: <0.45 mm. The properties of waste biomasses are shown in Table 1.
Pyrolysis procedure
Pyrolysis experiments were completed at different pyrolysis temperatures (from 300 to 800 °C) for 1 h. An inert atmosphere was provided by a continuous nitrogen gas flow of 30 mL min−1. The nitrogen gas flow was introduced before the experiment and continued for 30 min to remove the air inside. A nitrogen purge was injected after the reaction was complete, including the cooling down of the reactor to room temperature. The pyrolysis reactor was made of stainless steel with 6 cm diameter and 21 cm height. The pyrolysis setup is shown in Fig. 1. The pyrolysis reactor was charged with biomass samples of 25 g (dry basis). The system was heated at a rate of 7 °C/min to the target temperature. The volatile products were swept by nitrogen gas from the reactor to the collection flasks. The first two collections flasks were cooled with a water and ice mixture. The third flask was cooled using water. The pyrolysis experiments were repeated three times with an average standard deviation of 2.5 wt% for bio-oil and bio-char.
Analysis of pyrolysis products
The liquid portion from the pyrolysis of biomass was exposed to liquid–liquid extraction using diethyl ether (300 mL) to recover bio-oils for analysis. The bio-oils were analyzed by gas chromatography–mass spectrometry (GC–MS) and elemental analysis. The GC–MS instrument was a 6890 Gas Chromatograph Agilent (30 m × 0.25 mm i.d. phenyl methyl siloxane capillary column HP-5MS). The injector temperature was held at 250 °C. The GC oven temperature was programmed in the following sequence: started at 40 °C; held for 10 min; raised at a rate of 2–170 °C; held for 5 min; raised to 250 °C at a rate of 8 °C; held for 15 min; raised to 300 °C at a rate 15 °C; and held at this final temperature for 10 min.
The functional groups in the raw materials, liquids (consisting of organic and aqueous phase), and bio-chars were determined with Fourier transform infrared spectrometry for attenuated total reflectance (FTIR-ATR, Perkin Elmer FTIR 100 spectrometer).
The elemental content of the raw samples and bio-char products was established using a LECO CHNS TRuSpec. The surface morphologies of the raw samples and bio-chars were studied with the assistance of a scanning electron microscope (FEI Quanta 450 FEG).
Results and discussion
The TG profiles of the grape seeds and cornelian cherry stones are shown in Fig. 2. Moisture release for both grape seeds and cornelian cherry stones took place at approximately 80 °C. Decomposition of the biomasses started just below 200 °C. In the case of grape seeds, the main decomposition occurred at temperatures between 170 and 600 °C and resulted in 67.8 wt% weight losses. The main decomposition of cornelian cherry stones was observed at temperatures between 170 and 600 °C which resulted in 71.7 wt% weight losses.
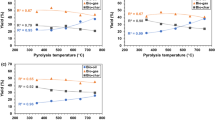
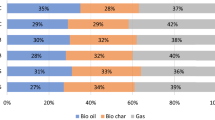
The distributions of products were influenced by both the pyrolysis temperature and biomass type in relation to temperature (as shown in Fig. 3). The trend for solid product yields was similar for both grape seeds and cherry stones. Solid product yields were gradually diminished with increasing the temperature for both grape seeds and cherry stones. The pyrolysis of cornelian cherry stones yielded less bio-char in comparison with grape seeds under identical conditions. The highest and lowest bio-char yields were 50.30 and 25.72 wt% for cornelian cherry stones and 53.88 and 29.82 wt% for grape seeds, respectively. Bio-oil yields from the pyrolysis of cornelian cherry stones were scaled up when the temperature was increased to 500 °C. After this temperature increase, further boosts in pyrolysis temperatures provided lower bio-oil yields from cornelian cherry stones. In the case of the pyrolysis of grape seeds, bio-oil yields increased as the pyrolysis temperature was raised (up to 700 °C). After further increases in the pyrolysis temperature from 700 to 800 °C, the bio-oil yields dropped for both biomass samples. Previous studies showed that bio-oil yields from the pyrolysis of biomass increased incrementally as the temperature was raised to a certain temperature. After this specific temperature that changed depending on the conditions (i.e., type of biomass, type of reactor, and type of pyrolysis), further increases in pyrolysis temperatures generated either lower yields of bio-oils (Ucar and Karagoz 2009) or the yields remained at almost the same levels (Shadangi and Singh 2012). It is believed that each biomass has the potential to provide a maximum bio-oil yield at a certain pyrolysis temperature. After this temperature, further increases in temperature helped to transform the bio-oil to gas products. Accordingly, gas product yields improved for both biomass samples when the pyrolysis temperatures were raised. The greatest gas yield (35 wt% ca.) was obtained at 800 °C for both the pyrolysis of grape seeds and cornelian cherry stones.
Figure 4 presents the FTIR spectra of bio-oil derived from the pyrolysis of grape seeds (Fig. 4a) and cornelian cherry stones (Fig. 4b) and their corresponding raw materials. Functional groups observed in bio-oils from the grape seeds were similar to those from the cornelian cherry stones. The broad band between 3,200 and 3,600 cm−1 represented the OH group. The peak at 2,924 cm−1 rose due to aliphatic CH2. The band at 2,855 cm−1 confirmed the stretching of C–H vibrations of the alkyl functional groups (CH2 and CH3). The peak at 1,743 cm−1 is assigned to the C=O functional group. The peaks between 1,100 and 1,200 cm−1 are assigned to C–O stretching bonds. The peak at 1,035 cm−1 represents C–H out-of-plane bending vibrations of the alkene groups. The peaks between 680 and 900 cm−1 are assigned to benzene rings (Lievens et al. 2008). Previous studies confirmed similar functional groups in bio-oils which were identified by FTIR from the pyrolysis of different biomass resources (Grierson et al. 2011; Huang et al. 2012; Demiral et al. 2012).
Increases in the pyrolysis temperatures did not change the functional groups in the bio-oils. The peak intensities were high in bio-oils from cornelian cherry stones in comparison with the raw materials. In the case of bio-oils produced from grape seeds, observed functional groups were similar to those of the raw material. The intensity of the peaks in bio-oils at between 2,800 and 3,200 cm−1 was high in comparison with the raw material. The total ion chromatograms of bio-oils obtained from the pyrolysis of grape seeds and cornelian cherry stones at 500 °C are presented in Figs. 5 and 6, respectively. The main peaks were demonstrated on the chromatograms. Small peaks on the chromatograms or peaks belonging to unidentified compounds are not presented in the figures. The main components in bio-oils for both grape seeds and cornelian cherry stones were oxygenated compounds such as phenol, 2-furanmethonol, 2-methoxyphenol, 4-ethyl-2-methoxyphenol, 2-methoxy-4-propylphenol, 2-ethylphenol, and n-hexadecanoic acid. The formation of phenolic compounds arose predominantly via cleavages of C–O and C–C bond networks in lignophenols (intermediate products of lignin). The following nitrogen-containing compounds were observed in bio-oils from grape seeds: (Z)-13-docosenamide and octadecanamide. The compound oleanitrile was observed in bio-oils derived from both grape seeds and cornelian cherry stones. Long-chain hydrocarbons (not including oxygen) such as decane, tridecane, tetradecane, pentadecane, and others were observed in bio-oils produced from grape seeds and cornelian cherry stones. The following compounds were also observed in bio-oils: o-xylene; p-xylene; butylbenzene; 1,2,3-trimethylbenzene; 1-ethyl; and 2-methylbenzene. Fabbri and co-workers produced bio-char from biomass using pyrolysis gas chromatography (py-GC–MS), and they reported that benzene derivatives mainly originated from the decomposition of char the fraction (Fabbri et al. 2012). The formation of benzene derivates can be explained by the decomposition of bio-chars from the pyrolysis of biomasses. Our results are in agreement with this previous report (Fabbri et al. 2012).
Total ion chromatograms for the bio-oil obtained from the pyrolysis of grape seeds at 500 °C. 1 2-Furanmethanol, 2 p-xylene, 3 1-nonene, 4 2-methyl-2-cyclopenten-1-one, 5 1,2,3-trimethylbenzene, 6 1-ethyl-2-methylbenzene, 7 phenol, 8 decane, 9 butylbenzene, 10 2-methylphenol, 11 3-methylphenol, 12 2-methoxyphenol, 13 undecane, 14 2-ethylphenol, 15 pentylbenzene, 16 naphthalene, 17 2-methoxy-4-methylphenol, 18 dodecane, 19 2,3,5-trimethylphenol, 20 4-ethyl-2-methoxyphenol, 21 tridecane, 22 (E)-2-tetradecene, 23 Tetradecane, 24 cyclopentadecane, 25 1-pentadecene, 26 1-hexadecene, 27 hexadecane, 28 cyclotetradecane, 29 1-heptadecene, 30 heptadecane, 31 5-octadecyne, 32 octadecane, 33 2-heptadecanone, 34 hexadecanoic acid, methyl ester, 35 n-hexadecanoic acid, 36 hexadecanoic acid, ethyl ester, 37 oleanitrile, 38 2-nonadecanone, 39 (Z,Z)-9,12-octadecadienoic acid, 40 leic acid, 41 (Z)-13-docosenamide, 42 octadecanamide, 43 stigmastan-3,5-diene
Total ion chromatograms for the bio-oil obtained from the pyrolysis of cornelian cherry stones at 500 °C. 1 Toluene, 2 furfural, 3 2-furanmethanol, 4 o-xylene, 5 2-methyl-2-cyclopenten-1-one, 6 5-methylfurfural, 7 phenol, 8 butylbenzene, 9 2-methylphenol, 10 2-methoxyphenol, 11 2-Methoxy-4-methylphenol, 12 1,2-benzenediol, 13 3-methoxy-1,2-benzenediol, 14 4-ethyl-2-methoxyphenol, 15 2-methoxy-4-vinylphenol, 16 2,6-dimethoxyphenol, 17 2-methoxy-4-propylphenol, 18 tetradecane, 19 1-pentadecene, 20 pentadecane, 21 1-hexadecene, 22 8-heptadecene, 23 heptadecane, 24 1-(4-hydroxy-3,5-dimethoxyphenyl)ethanone, 25 pentadecanenitrile, 26 hexadecanoic acid, methyl ester, 27 n-hexadecanoic acid, 28 oleanitrile, 29 8-octadecenoic acid, methyl ester, 30 octadecanoic acid, methyl ester, 31 (Z)-9,17-octadecadienal, 32 octadecanoic acid, 33 hexanedioic acid, bis(2-ethylhexyl) ester, 34 2-(3,4-himethoxyphenyl)-7-hydroxy-4H-1-benzopyran-4-one, 35 5-alpha-ergost-8(14)-ene
Fatty acids (FA) and fatty acid esters (FAE) were observed in bio-oil from both grape seeds and cornelian cherry stones. The observed FAs from grape seeds were n-hexadecanoic acid, (Z, Z)-9,12-octadecadienoic acid, and oleic acid. FAs from the cornelian cherry stones contained n-hexadecanoic acid and octadecanoic acid. FAEs from the grape seeds were hexadecanoic acid methyl ester and hexadecanoic acid ethyl ester. FAEs from the cornelian cherry stones were 8-octadecanoic acid methyl ester, octadecanoic acid methyl ester, and hexadecanoic acid bis (2-ethylhexyl) ester. FAs and FAEs in the bio-oils are responsible for the decomposition of extracts.
Elemental contents and heating values of bio-chars obtained from the pyrolysis of cornelian grape seeds and cherry stones are shown in Tables 2 and 3, respectively. Bio-chars included higher carbon contents and lower oxygen contents than those of their corresponding raw materials. Both carbon contents and heating values of bio-chars from cornelian cherry stones were higher than those from grape seeds under identical conditions. H/C atomic ratios of bio-chars for both of the biomasses were diminished when the pyrolysis temperature increased. The lowest H/C ratio was attained at the highest pyrolysis temperature which indicates that the aromatic contents of bio-chars were the highest. When the aromatic contents of bio-chars were compared, the contents from cornelian cherry stones were greater than those from grape seeds under identical conditions.
The heating value is an important factor which affects the quality of bio-chars. Biomass type (McHenry 2009), retention time (Marousek et al. 2014b), and heating rates (Angin 2013) are some significant pyrolysis parameters that change the heating values. In addition, new techniques in pyrolysis systems can allow for the production of profitable bio-chars. In a previous study reported by Marousek and colleagues, it was shown that the production of high-quality bio-chars is possible from anaerobically fermented residues in designated continuous pyrolysis systems (Marousek et al. 2014b).
The highest heating values were 32.44 for bio-char from cornelian cherry stones and 27.32 MJ kg−1 for bio-char from grape seeds. The pyrolysis process that was applied to both cornelian cherry stones and grape seeds led to deoxygenation in bio-chars as O/C ratios were lower than their corresponding biomasses.
The functional groups in bio-chars from the pyrolysis of grape seeds and cornelian cherry stones (shown in Fig. 7) revealed that the functional groups in bio-chars were similar and that they started to disappear with the increase in pyrolysis temperatures. Some of the functional groups observed in bio-chars at 300 and 400 °C for both grape seeds and cornelian cherry stones are as follows: the peaks at 2,918 and 2,853 cm−1 are assigned to C–H vibrations, saturated C–C, and C–H; the peaks at 1,407 and 1,555 cm−1 confirmed the aromatic ring C=C stretching vibration. The peak at 1,035 cm−1 represents C–H out-of-plane bending vibrations of the alkene groups. This peak was also observed at 500 °C. After 500 °C, a baseline sloping down to the right was observed and peaks were distorted at the temperatures of 600, 700, and 800 °C. These results demonstrate that the diamond ATR technique is not suitable for the analysis of char samples obtained at high temperatures.
SEM images of bio-chars obtained from grape seeds and cornelian cherry stones are shown in Figs. 8 and 9, respectively. The surfaces of bio-chars were altered with the increase in temperatures for both biomass samples. In case of bio-chars from grape seeds, it is clearly seen that the formation of pores started at 300 °C which is due to the first release of volatiles, mainly cellulose and hemicellulose in the biomass. At 400 °C, the pores were distinct. After further increases in temperature to 500 and 600 °C, it is believed that the pores were closed by the volatile contents of lignin factions. After 600 °C, the cavities were reformed which can be explained by the complete removal of lignin-derived volatiles in the biomass.
In the case of bio-chars from cornelian cherry stones, crevices and small pores were observed at 300 °C. Likewise, cracks and slits were observed at 400 °C. When the temperature rose to 500 °C, pores were observed. A number of pores with several dimensions were observed at 600, 700, and 800 °C. It is believed that volatiles were completely removed at temperatures between 600 and 800 °C, and this occurrence caused the formation of cavities.
Conclusion
Pyrolysis of two different biomass samples was carried out at different temperatures from 300 to 800 °C. The results show that product distributions under identical conditions were strongly affected by the types of biomass samples. The bio-oil yields reached their peaks at 500 for cornelian cherry stones and 700 °C for grape seeds. The highest bio-yields were 47.50 wt% for cornelian cherry stones and 41.04 wt% for grape seeds. The contents of bio-oils for both cornelian cherry stones and grape seeds were similar and mainly composed of oxygenated hydrocarbons. Hydrocarbons that did not contain oxygen were also observed. Elemental contents and heating values varied for different raw materials. Both carbon contents and heating values of cornelian cherry stones were higher than those of grape seeds at the same pyrolysis temperatures. Bio-chars contained less oxygen than their corresponding raw materials. The surface topography was varied at different pyrolysis temperatures. The formation of many large and small cavities on the surface of the bio-chars from grape seeds was apparent at pyrolysis temperatures of 700 and 800 °C.
In the current paper, it was shown that waste biomasses can be converted into profitable bio-products with high economic value including bio-oils and bio-chars. The production of valuable bio-products from waste biomasses is important in terms of environmental concerns and economical factors.
References
Amen-Chen C, Pakdel H, Roy C (2001) Production of monomeric phenols by thermochemical conversion of biomass: a review. Bioresour Technol 79:277–299. doi:10.1016/S0960-8524(00)00180-2
Angin D (2013) Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour Technol 128:593–597. doi:10.1016/j.biortech.2012.10.150
Bridgwater AV (2004) Biomass fast pyrolysis. Therm. Sci. 8(2):21–49. doi:10.2298/TSCI0402021B
Demiral I, Eryazıcı A, Sensoz S (2012) Bio-oil production from pyrolysis of corncob (Zea mays L.). Biomass Bioenerg 36:43–49. doi:10.1016/j.biombioe.2011.10.045
Fabbri D, Torri C, Spokas KA (2012) Analytical pyrolysis of synthetic chars derived from biomass with potential agronomic application (biochar). Relationships with impacts on microbial carbon dioxide production. J Anal Appl Pyrol 93:77–84. doi:10.1016/j.jaap.2011.09.012
Figueiredo MK-K, Romeiro GA (2009) Low temperature conversion (LTC) of castor seeds—a study of the oil fraction (pyrolysis oil). J Anal Appl Pyrol 86:53–57. doi:10.1016/j.jaap.2009.04.006
Grierson S, Strezov V, Shah P (2011) Properties of oil and char derived from slow pyrolysis of Tetraselmis chui. Bioresour Technol 102:8232–8240. doi:10.1016/j.biortech.2011.06.010
Houshfar E, Wang L, Vaha- Savo N, Brink A, Lovas T (2014) Characterisation of CO/NO/SO2 emission and ash-forming elements from the combustion and pyrolysis process. Clean Technol Environ Policy. doi:10.1007/s10098-014-0762-3
Huang Y, Wei Z, Qiu Z, Yin X, Wu C (2012) Study on structure and pyrolysis behavior of lignin derived from corncob acid hydrolysis residue. J Anal Appl Pyrol 93:153–159. doi:10.1016/j.jaap.2011.10.011
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev 106:4044–4098. doi:10.1021/cr068360d
Jin F, Enomoto H (2011) Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: chemistry of acid/basecatalysed and oxidation reaction. Energy Environ Sci 4:382–397. doi:10.1039/C004268D
Jourabchi SA, Gan S, Ng HK (2014) Pyrolysis of Jatropha curcas pressed cake for bio-oil production in a fixed-bed system. Energy Convers Manage 78:518–526. doi:10.1016/j.enconman.2013.11.005
Kader MA, Islam MR, Parveen M, Haniu H, Takai K (2013) Pyrolysis decomposition of tamarind seed for alternative fuel. Bioresour Technol 149:1–7. doi:10.1016/j.biortech.2013.09.032
Lievens C, Yperman J, Cornelissen T, Carleer R (2008) Study of the potential valorisation of heavy metal contaminated biomass via phytoremediation by fast pyrolysis: part II: characterisation of the liquid and gaseous fraction as a function of the temperature. Fuel 87:1906–1916. doi:10.1016/j.fuel.2007.10.023
Marousek J (2013a) Two-fraction anaerobic fermentation of grass waste. J Sci Food Agric 93:2410–2414. doi:10.1002/jsfa.6046
Marousek J (2013b) Removal of hardly fermentable ballast from the maize silage to accelerate biogas production. Ind Crop Prod 44:253–257. doi:10.1016/j.indcrop.2012.11.022
Marousek J (2014) Significant breakthrough in biochar cost reduction. Clean Technol Environ Policy. doi:10.1007/s10098-014-0730-y
Marousek J, Haskova S, Zeman R, Vanickova R (2014a) Managerial preferences in relation to financial indicators regarding the mitigation of global change. Sci Eng Ethics. doi:10.1007/s11948-014-9531-2
Marousek J, Zeman R, Vanickova R, Haskova S (2014b) New concept of urban green management. Clean Technol Environ Policy. doi:10.1007/s10098-014-0736-5
McHenry MP (2009) Agricultural bio-char production, renewable energy generation and farm carbon sequestration in Western Australia: certainty, uncertainty and risk. Agric Ecosyst Environ 129:1–7. doi:10.1016/j.agee.2008.08.006
Nayan NK, Kumar S, Singh RK (2013) Production of the liquid fuel by thermal pyrolysis of neem seed. Fuel 103:437–443. doi:10.1016/j.fuel.2012.08.058
Ngo T-A, Kim J, Kim S–S (2013) Fast pyrolysis of palm kernel cake using a fluidized bed reactor: design of experiment and characteristics of bio-oil. J Ind Eng Chem 19:137–143. doi:10.1016/j.jiec.2012.07.015
Putun E, Uzun BB, Putun AE (2006) Fixed-bed catalytic pyrolysis of cotton-seed cake: effects of pyrolysis temperature, natural zeolite content and sweeping gas flow rate. Bioresour Technol 97:701–710. doi:10.1016/j.biortech.2005.04.005
Raja SA, Kennedy ZR, Pillai BC, Lee C (2010) Flash pyrolysis of jatropha oil cake in electrically heated fluidized bed reactor. Energy 35:2819–2823. doi:10.1016/j.energy.2010.03.011
Shadangi KP, Mohanty K (2014) Thermal and catalytic pyrolysis of Karanja seed to produce liquid fuel. Fuel 115:434–442. doi:10.1016/j.fuel.2013.07.053
Shadangi KP, Singh RK (2012) Thermolysis of polanga seed cake to bio-oil using semi batch reactor. Fuel 97:450–456. doi:10.1016/j.fuel.2012.02.058
Torri C, Lesci IG, Fabbri D (2009) Analytical study on the production of a hydroxylactone from catalytic pyrolysis of carbohydrates with nanopowder aluminium titanate. J Anal Appl Pyrolysis 84:25–30. doi:10.1016/j.jaap.2008.10.002
Ucar S, Karagoz S (2009) The slow pyrolysis of pomegranate seeds: the effect of temperature on the product yields and bio-oil properties. J Anal Appl Pyrol 84:151–156. doi:10.1016/j.jaap.2009.01.005
Vassilev SV, Baxter D, Vassileva CG (2013) An overview of the behaviour of biomass during combustion: part I. Phase-mineral transformations of organic and inorganic matter. Fuel 112:391–449. doi:10.1016/j.fuel.2013.05.043
Volli V, Singh RK (2012) Production of bio-oil from de-oiled cakes by thermal pyrolysis. Fuel 96:579–585. doi:10.1016/j.fuel.2012.01.016
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Alper, K., Tekin, K. & Karagöz, S. Pyrolysis of agricultural residues for bio-oil production. Clean Techn Environ Policy 17, 211–223 (2015). https://doi.org/10.1007/s10098-014-0778-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-014-0778-8