Abstract
The purpose of this study was to evaluate the effectiveness of lactobacilli on vaginal health and proinflammatory cytokines. Sixty-seven patients with bacterial vaginosis (BV), 50 with intermediate flora and 42 with normal vaginal flora were enrolled in this double-blind study. The subjects were randomized to receive probiotic lactobacilli vaginal tablets (L. brevis CD2, L. salivarius subsp. salicinius, L. plantarum) or the vaginal pH tablet (active comparator). Cervico-vaginal lavage was collected to measure the concentrations of IL-1β, TNFα and IL-6 by ELISA. Neutral sphingomyelinase activity was also quantified in both arms before and after treatment. The probiotic vaginal tablet was well tolerated and no side effects were reported. The study demonstrated a cure rate of nearly 80 %; i.e., 32 % of the women could restore normal vaginal flora and 47 % had improved Nugent score, whereas 20 % of the subjects did not clear BV in the first follow-up (after 8 days treatment). The pH tablet containing pH lowering compounds induced resolution of BV and restoration of normal vaginal flora in 74 % and 26 %, respectively. The lactobacilli tablet was found to be better than the pH tablet in preventing BV in healthy subjects. A significant reduction in IL-1β and IL-6 vaginal cytokines was observed after treatment with lactobacilli, while the active comparator did not have any effect on local proinflammatory cytokines. Vaginal neutral sphingomyelinase activity was not modified in either group. Vaginal tablets containing lactobacilli can cure BV and reduce vaginal inflammatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vagina and cervix are the first line of physical and immunological defense against vaginal infections and sexually transmitted diseases [1, 2]. The presence of potentially pathogenic organisms including yeast, bacteria and viruses initiate an immune reaction resulting in increased vaginal secretion of immune stimulating molecules, irritation, vulvar pruritis, and foul odor, although it may be symptomatic or asymptomatic.
Bacterial vaginosis (BV) represents the most common vaginal syndrome afflicting premenopausal and pregnant women, with an incidence rate ranging from 10 % to 50 % [3–6]. BV is a polymicrobial disorder characterized by an overgrowth of strict or facultative anaerobic bacteria and a reduction of lactobacilli particularly those producing hydrogen peroxide. The microflora of the female genital tract has been shown to be an important determinant of both HIV acquisition and transmission [7, 8]. Studies have demonstrated a two-fold increase of HIV infection in women affected by BV and this effect has been attributed to lack of H2O2-producing lactobacilli, high vaginal pH and increased proinflammatory cytokines such as interleukin (IL)-1β and IL-6 [9, 10]. BV has also been associated with an increased risk for preterm delivery and chorioamnionitis, but antibiotic trials to treat BV before and during pregnancy have not resulted universally in a lowering of the risk for preterm delivery [11–13]. Therefore, it has been hypothesized that preterm delivery may not be only related to BV per se but rather to an inflammatory immune response, which may not diminish with treatment for infection [14]. BV is associated with higher concentrations of vaginal pro-inflammatory cytokines, especially IL-1β and IL-8 among both non-pregnant and pregnant women, which have been linked with preterm delivery [15–18].
Acidity of the vagina has long been understood to be a protective mechanism, not only eliminating the volatilization of amines, thus reducing fishy odor, but also making the vaginal environment unfavorable for colonization of bacterial pathogens and restoring lactobacilli as dominant members of healthy flora. Treatment of BV patients through normalization of vaginal pH by administration of probiotic lactic acid bacteria through production of lactic acid or by administration of lactic acid solutions [19] or gels, ascorbic acid tablets [20], etc., have been shown in many studies, but the role of these agents in vaginal immunity remain to be determined.
Women in India have higher concentration of cervicovaginal IL-1β, IL-6 and tumor necrosis factor (TNF)-α compared to reports from other parts of the world [21]. Moreover, a high proportion of women (73 %) have high vaginal pH associated with higher cervicovaginal IL-1β and TNF-α response irrespective of their vaginal flora status [21]. Thus, a large proportion of women might remain susceptible to increased HIV infection owing to decreased acidity, increased proinflammatory cytokines and increased lymphocyte activation due to high pH and therefore increased target cells for HIV.
Therefore, a change of bacterial composition in the vagina should be accompanied with diminished inflammatory cytokine response and lower pH. Given the fact that lactobacilli demonstrate immunomodulatory activities in gut, it is plausible to believe that lactobacilli will have immunomodulatory effects at local sites such as the vagina [22]. Moreover, neutral sphingomyelinase (NSmase), a key regulatory enzyme of sphingolipid metabolism, plays a significant role in cell membrane organization and has been implicated in HIV infection [23]. It would be interesting to demonstrate if probiotic preparations would have any effect on local proinflammatory cytokines, vaginal health including vaginal flora and vaginal pH. In the present, randomized, double-blind study, we evaluated the effect of a probiotic lactobacilli tablet containing a blend of Lactobacillus brevis CD2, Lactobacillus salivarius subsp. salicinius and Lactobacillus plantarum vs pH lowering tablet on vaginal health and cervicovaginal proinflammatory cytokines (IL-1β, IL-6, and TNF-α).
Materials and methods
Study design
This study was a randomized, double-blind study conducted in a single center, NIN (National Institute of Nutrition, Indian Council of Medical Research), Hyderabad from June 2008 to December 2009.
Participants
Non-HIV, non-pregnant, sexually active women, living with husband, aged 20–40 years were recruited from urban slums after signed informed consent. The criteria for including subjects in the study were 8th to 10th day of menstrual cycle, absence of bleeding during examination, non-utilization of oral antibiotics or contraceptives or vaginal medication in the last 10 days and no sexual intercourse for the last 2 days before sample collection. All enrolled women denied using douches or tampons and were non-smokers. General and gynecological examinations were done to evaluate general health. The study was conducted in accordance with the Declaration of Helsinki and current Good Clinical Practice and was approved by Institute Ethics Committee, NIN.
Intervention
The test preparation consisted of probiotic vaginal tablet “Florisia™” (lactobacilli tablet) or a pH lowering vaginal tablet (pH tablet). The lactobacilli tablet contained at least 109CFU of viable lactobacilli (a blend of L. brevis CD2, L. salivarius subsp. salicinius and L. plantarum) with excipients, namely, starch, lactose, ascorbic acid, sodium bicarbonate, adipic acid, stearic acid and magnesium stearate). The pH tablet contained all excipients except the blend of probiotic strains. Both the vaginal tablets were similar in color and appearance and were packed in identical strips. Treatment consisted of one lactobacilli tablet or pH tablet inserted into the vagina daily at bedtime for 8 days. Florisia and pH tablets were supplied in cold conditions (4–8 °C) by CD Pharma India (Pvt. Ltd), which were maintained at 4–8 °C in a refrigerator before dispensing to subjects. Compliance to treatment was ensured by project staff by regular follow-up through phone as well as visiting each study subject at their place during the course of treatment. The subjects were asked to return empty packs to confirm compliance.
Adverse events
All observed or reported adverse events regardless of treatment group or suspected causal relationship to study drug were recorded on the adverse events page of the case report form. Abnormal findings and clinically significant changes such as abdomen pain, bleeding or discharge per vagina or local irritation or itching were evaluated on clinical and physical examination as part of adverse events.
Sample size calculation
The primary outcome measure was cure of BV as measured by Nugent score and sample size was calculated based on the primary outcome. The secondary outcome measure was effect of probiotic on proinflammatory cytokines for which sample size was not calculated due to lack of data in this area.
Expecting 50 % reduction in BV with treatment, a total of 28 subjects would be required in each group to study the effect of treatment at 5 % significance level and 80 % power. Expecting 10 % lost to follow-up, 30 patients with BV was arrived at for each group.
Randomization
Sequence generation
The random numbers were generated by computerized random number. The randomization list and numbered packing of the intervention was prepared by a person not involved in the study. Randomization was performed using permuted blocks of 10.
Allocation concealment
All the randomization numbers were concealed in separate envelopes and marked by patient number on the outer envelope.
Implementation
The randomization was performed by staff not involved with the study. The intervention was provided to the center. Patients were assigned the same serial number (corresponding to the randomization code on the sealed envelope) of the intervention.
Blinding
The individual sealed envelope method was used to maintain blinding of the investigators and study participants.
Study assessments
On visit 1, day 1, screening, randomization and beginning of the 8-day treatment took place. This involved informed consent and enrolment of patients as per the inclusion and exclusion criteria. Demographics and medical history concerning previous history of reproductive tract infections and any chronic disease were assessed at baseline. After pelvic and speculum examination, swabs were collected for gram stain and cervicovaginal wash for cytokine estimation.
On visit 2, day 9, was the end of treatment. Tolerability of the vaginal tablets was evaluated subjectively by the investigator. After pelvic and speculum examination swabs and cervicovaginal wash were collected for gram stain and cytokine estimation and sphingomyelinase assay.
Bacterial vaginosis by Nugent's score
Vaginal swabs for gram staining were obtained by a gentle rotation of a Dacron swab within the posterior fornix. Ayre’s spatula was used to collect samples for pH determination and amine odor. BV and intermediate flora were diagnosed based on Nugent’s score [24]. Three different bacterial morphotypes-lactobacilli, Gardnerella-like species (including G. vaginalis, Bacteroides species, Prevotella species, and Porphyromonas species), and Mobiluncus species were quantitatively evaluated according to the Nugent’s score method [24]. Women with Nugent scores of 0–3 were categorized as normal flora. Women with scores of 4–6 were classified as intermediate flora and women with Nugent score ≥7 were enrolled as BV.
Bacterial vaginosis by Amsel's criteria
The clinical criteria reported by Amsel [25] for diagnosing bacterial vaginosis were also evaluated: thin homogeneous discharge, vaginal pH greater than 4.5, positive “whiff” test or release of amine odor after addition of 10 % KOH, and clue cells on microscopic evaluation. The presence of any three of the four Amsel criteria confirms BV.
Cytokines assay
Quantitative Milliplex™ human cytokine kits (Millipore International, MA, USA) were used to measure the concentrations of TNF-α, IL-1β and IL-6 in the cervico-vaginal lavage (CVL). After testing the assay kit according to the manufacturer’s instructions, a dilution factor was necessary to measure the cytokines. Lower limits of detection for IL-1β, IL-6 and TNF- α were 0.4, 0.3 and 0.1 pg/ml, respectively. All samples and controls were assayed in duplicate. The intra-assay and inter-assay coefficients of variation of IL-1β were 6.1 % and 7.0 %, respectively; for IL-6 they were 8.1 % and 11.6 %, respectively; and for TNF-α, they were 10.5 % and 15.9 %, respectively. All the three cytokines analyzed were above the lower limit of detection range.
Sphingomyelinase assay
Neutral sphingomyelinase concentration in the vaginal wash samples was assessed by fluorimetry method [26] using Amplex Red kit (Invitrogen) and the activity was expressed as milliunits/mg protein/hr.
Statistical analysis
Since the data were skewed, log transformation was performed to analyze continuous variables. Two way repeat measure ANOVA F-test was performed to assess interactions over time. ANOVA was used for descriptive data. To evaluate the associations of different variables on the cytokine concentration of lavage samples we used multiple linear regression models. A p-value of 0.05 or less was considered statistically significant and a p-value of 0.001 or less was considered highly statistically significant. Statistical analysis was performed using SPSS statistical software version 19.0 (SPSS Inc, Chicago, IL, USA).
Results
Evaluation of the effectiveness of lactobacilli on the vaginal health and proinflammtory cytokines
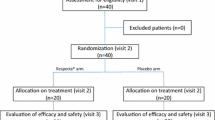
One hundred eighty-nine subjects were screened, of whom 30 were excluded as they did not fulfill inclusion criteria (Fig. 1). Of the 159 women, 67 with bacterial vaginosis (BV) (Nugents’ score ≥ 7), 50 with intermediate flora (IF) (Nugents’ score 4 –6) and 42 with normal vaginal flora (NVF) (Nugents’ score 1–3) participated in the trial. Of the 67 patients with BV, 37 were randomly assigned to receive the lactobacilli tablet and 30 to the pH tablet. There were six dropouts from the BV group (did not return for next visit) leaving 61 evaluable patients (lactobacilli tablet, n = 34; pH tablet, n = 27). Of the 50 IF cases, 23 were randomized to receive the lactobacilli tablet and 27 were randomized to receive the pH tablet. With two dropouts we had 48 evaluable cases of IF (lactobacilli tablet, n = 21; pH tablet, n = 27). Of the 42 women with NVF, 22 were randomly assigned to receive the lactobacilli tablet and 20 to receive the pH tablet. There were three dropouts from this group leaving 39 evaluable subjects (lactobacilli tablet, n = 20; pH tablet, n = 19). Abnormal findings and clinically significant changes such as pain abdomen, bleeding or discharge per vagina or local irritation or itching were evaluated on clinical and physical examination. The vaginal tablet containing three strains of lactobacilli or the pH tablet used in the present study were well tolerated and no side effects were reported. Baseline characteristics of women, randomly assigned to lactobacilli tablet or pH tablet, were demographically and clinically similar (Table 1). The mean body mass index (BMI) (21.0) was similar in women with or without BV and one third of the women in the study had less than 18.5 kg/m2 BMI.
The data in Table 1 depict the vaginal health of women treated locally with lactobacilli tablet/ pH tablet, irrespective of their baseline BV status (includes women with BV, intermediate flora and normal vaginal flora). At the end of treatment (9th day), the proportion of subjects with vaginal pH > 4.5 reduced in the lactobacilli treated group, while there was an increase in the pH tablet group; however these differences were not significant. Mean Nugent’s score and proportion of women with Amsel’s criteria (positive for BV) decreased significantly at the end of treatment in both the lactobacilli tablet group and the pH tablet group; however, more significant change was observed in the lactobacilli treated group. Of the total 82 subjects treated with lactobacilli tablet, 37 patients had BV at day 0 (37/82). After 8 days treatment with lactobacilli tablet, the proportion of patients with BV (8/75 and 7 lost to follow-up) reduced significantly (p < 0.0001). In the pH tablet-treated group, there were 30 patients with BV (30/77) at day 0, and after 8 days treatment 12 (12/73) patients continued with BV (p = 0.004) (4 subjects were lost to follow-up). Moreover, significantly (p = 0.007) higher proportion of subjects achieved normal vaginal flora in the lactobacilli treated group as compared to the pH tablet group (Table 1). Subjects with Clue cells, known to be associated with bacterial vaginosis reduced significantly (p = 0.01) after treatment in the lactobacilli tablet group, while in the pH tablet group there was no significant change. No improvement was observed in the subjects with vaginal discharge, vaginal candida infection or leucorrhea with either of the interventions; though, the proportion of women with vaginal WBC (leucorrhea) reduced non-significantly in the lactobacilli group.
Figure 2 depicts the effect of local lactobacilli/ pH lowering tablet on BV (intergroup analysis) at follow-up visit (after 8 days treatment) in women with BV at baseline. Out of 37 BV patients in the lactobacilli tablet-treated group, three patients were lost to follow-up and therefore data was available for 34 patients on both day 0 and day 9. Nearly 80 % had improved vaginal flora after treatment. However, 20 % of the women with BV at baseline continued with BV after treatment. In the pH tablet group, of 30 patients with BV, three patients were lost to follow-up, and therefore data for 27 patients was analyzed on both day 0 and day 9. A total of 26 % continued to have BV after treatment with the pH tablet. Moreover, 32 % and 26 % attained NVF in the lactobacilli treated group and pH tablet group, respectively, after treatment.
Figure 3 depicts the effect of 8-day administration of local lactobacilli/ pH tablet on subjects with NVF at baseline. A significantly higher proportion (p = 0.014) of subjects retained NVF following treatment with lactobacilli tablet in comparison to the pH tablet group. In the lactobacilli treated group, none of the subjects with NVF at baseline developed BV during treatment; however one subject had intermediate flora after treatment. In the pH tablet-treated group, 32 % of women with NVF at enrollment shifted to intermediate vaginal flora and one subject was BV positive at follow-up visit.
Distribution of subjects with normal vaginal flora (NVF) at baseline. Between group differences (Lactobacilli tablet vs pH tablet) after treatment were compared by Pearson's chi-squared test. Within group differences (subjects retaining NVF vs subjects not able to retain NVF) after treatment were analysed using binomial test
Figure 4 depicts the proinflammatory cytokines IL-1β, IL-6 and TNF-α before and after the treatment in subjects with NVF at recruitment. The mean and SD of IL-1β at baseline was 88.69 ± 125.71 and 69.48 ± 114.06 pg/ml in lactobacilli and pH tablet groups, respectively. IL-6 was 268.70 ± 345.50 and 321.13 ± 658.32 pg/ml; and TNF-α was 21.66 ± 44.96 and 12.79 ± 17.26 in the lactobacilli and pH tablet groups, respectively. There was a significant reduction in IL-1β (p < 0.001) and IL-6 (p = 0.015) at the end of treatment in the lactobacilli group, but no significant change was observed in TNF-α levels. However, in the pH tablet group, there were no significant changes in levels of any of the proinflammatory cytokines.
Neutral sphingomyelinase (NSmase) activity ranged from 1.0 to 1030 milliunits/mg protein/hr and was similar in women with or without BV. NSmase activity was not altered with lactobacilli or pH tablet treatment.
Discussion
This study investigated the effect of vaginal administration of pH adjustment and lactobacilli tablets on BV and other parameters indicative of vaginal health. Since pH and cervicovaginal inflammatory cytokines are increased in BV, cure of BV should be accompanied with normalization of pH and reduction in inflammatory response. With this assumption we ascertained the effect of lactobacilli on BV and local proinflammatory cytokines.
The present study demonstrated a BV cure rate of nearly 80 %; i.e., 32 % of Lactobacillus-treated women could restore normal vaginal flora, 47 % had intermediate flora, and 20 % of the subjects did not clear BV in the follow-up visit. This is in contrast to what had been reported earlier with similar preparation. In the earlier study with Florisia (lactobacilli tablet), all the patients were free of BV at the end of treatment and 83 % had normal vaginal flora [27]. The reason for lower efficacy in the present study is not clear, but women in this study were more frequently undernourished and had very poor hygiene practices that could have affected the outcome. Since the pH tablet used in the present study contained pH lowering compounds, there was substantial effect on the vaginal flora with pH tablet. Although treatment of BV with pH-lowering compounds has been reported to be successful in some studies, other trials have shown vaginal acidification to be ineffective treatment for BV [28, 29]. The pH tablet used in the present study had substantial effect on BV cure, though to a lesser extent when compared to the lactobacilli tablet.
Earlier studies have shown increased vaginal IL-1β, impaired innate mucosal immunity and increased production of sialidase and prolidase in BV-affected women [30, 31]. Given the fact that increased levels of sialidase, prolidase and IL-1β alter mucosal protective factors, successful treatment of bacterial vaginosis should also aim at lowering inflammation in the lower reproductive tract. It was interesting to note significant reduction in IL-1β and IL-6 proinflammatory cytokines with lactobacilli treatment in the present study. To our knowledge this is the first study demonstrating the anti-inflammatory effect of local lactobacilli treatment.
The mean vaginal pH was similar to that reported in women of other ethnic groups [32]. A large proportion of women had high vaginal pH, which was associated with inflammatory cytokines in our earlier study [21]. Though the probiotic tablet showed considerable effect on proinflammatory cytokines, it had no significant effect on the mean vaginal pH.
While acid Smase has been shown to induce virulence and inflammation, neutral Smase has been demonstrated to be anti-inflammatory [26–33]. In our earlier study acid Smase activity was higher in women with BV; however, in the current study the neutral Smase activity was similar in women with or without BV and was not affected by lactobacilli treatment [21]. The vaginal tablet containing three strains of lactobacilli used in the present study was well tolerated and no side effects have been reported, which was similar to the earlier findings. These strains have been chosen for their ability to adhere and colonize at high levels; but this is primarily because the preparation when used in Italy achieved a cure rate in the lower range of typical pharmacological therapies [27]. However, as suggested earlier, longer and repeated treatments could be indicated for better outcome.
Major drawbacks include not attempting to isolate lactobacilli strains used for the treatment, which could have actually monitored the treatment effect. The strengths of the study are its large sample size, restricted use of agents (oral or external contraceptives, douches, etc.) or means (coitus) that can alter the vaginal environment and the homogenous hormonal status (sample collection was restricted to 9th and 10th day of the cycle) of the enrolled women that could have influenced the results. Moreover, to our knowledge this is the first study demonstrating the beneficial effect of lactobacilli treatment on local proinflammatory cytokines. A more complete understanding of the effect of lactobacilli on local and systemic inflammation would be useful in designing strategies to control BV thus reducing the pathological conditions associated with the alteration of the vaginal microbiota.
References
Gage JR, Sandhu AK, Nihira M, da Bonecini-Almeida MG, Cristoforoni P, Kishimoto T, Montz FJ, Martínez-Maza O (2000) Effects of human papillomavirus-associated cells on human immunodeficiency virus gene expression. Obstet Gynecol 96(6):879–885
Fan SR, Liu XP, Liao QP (2008) Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int J Gynaecol Obstet 103(1):50–54
Bhalla P, Chawla R, Garg S, Singh MM, Raina U, Bhalla R, Sodhanit P (2007) Prevalence of bacterial vaginosis among women in Delhi, India. Indian J Med Res 125(2):167–172
Madhivanan P, Krupp K, Chandrasekaran V, Karat C, Arun A, Cohen CR, Reingold AL, Klausner JD (2008) Prevalence and correlates of bacterial vaginosis among young women of reproductive age in Mysore, India. Indian J Med Microbiol 26(2):132–137
Bang RA, Bang AT, Baitule M, Choudhary Y, Sarmukaddam S, Tale O (1989) High prevalence of gynaecological diseases in rural Indian women. Lancet 1(8629):85–88
Sumati AH, Saritha NK (2009) Bacterial vaginosis with special reference to anaerobes. Indian J Pathol Microbiol 52(1):56–58
Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG (1998) Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12(13):1699–1706
Sha BE, Zariffard MR, Wang QJ, Chen HY, Bremer J, Cohen MH, Spear GT (2005) Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis 191(1):25–32
Sturm-Ramirez K, Gaye-Diallo A, Eisen G, Mboup S, Kanki PJ (2000) High levels of tumor necrosis factor-alpha and interleukin-1beta in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J Infect Dis 182(2):467–473
Osborn L, Kunkel S, Nabel GJ (1989) Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA 86(7):2336–2340
Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P (2003) Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 189(1):139–147
Leitich H, Brunbauer M, Bodner-Adler B, Kaider A, Egarter C, Husslein P (2003) Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol 188(3):752–758
Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M (2006) Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. Am J Obstet Gynecol 194(3):617–623
Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S (2007) The role of inflammation and infection in preterm birth. Semin Reprod Med 25(1):21–39
Basso B, Giménez F, López C (2005) IL-1beta, IL-6 and IL-8 levels in gyneco-obstetric infections. Infect Dis Obstet Gynecol 13(4):207–211
Hedges SR, Barrientes F, Desmond RA, Schwebke JR (2006) Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis 193(4):556–562
Genc MR, Witkin SS, Delaney ML, Paraskevas LR, Tuomala RE, Norwitz ER, Onderdonk AB (2004) A disproportionate increase in IL-1beta over IL-1ra in the cervicovaginal secretions of pregnant women with altered vaginal microflora correlates with preterm birth. Am J Obstet Gynecol 190(5):1191–1197
González Bosquet E, Ferrer I, Valls C, Borrás M, Lailla JM (2005) The value of interleukin-8, interleukin-6 and interleukin-1beta in vaginal wash as predictors of preterm delivery. Gynecol Obstet Invest 59(3):175
Bahamondes MV, Portugal PM, Brolazo EM, Simões JA, Bahamondes L (2011) Use of a lactic acid plus lactoserum intimate liquid soap for external hygiene in the prevention of bacterial vaginosis recurrence after metronidazole oral treatment. Rev Assoc Med Bras 57(4):415–420
Petersen EE, Genet M, Caserini M, Palmieri R (2011) Efficacy of vitamin C vaginal tablets in the treatment of bacterial vaginosis: a randomised, double blind, placebo controlled clinical trial. Arzneimittelforschung 61(4):260–265
Hemalatha R, Ramalaxmi BA, Krishnaswetha G, Kumar PU, Rao DM, Balakrishna N, Annapurna V. Cervicovaginal inflammatory cytokines and sphingomyelinase in women with and without bacterial vaginosis. Am J Med Sci. 2011 Nov 16. [Epub ahead of print]
Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D (2008) Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol 10(1–2):37–54
Finnegan CM, Rawat SS, Puri A, Wang JM, Ruscetti FW, Blumenthal R (2004) Ceramide, a target for antiretroviral therapy. Proc Natl Acad Sci USA 101(43):15452–15457
Nugent RP, Krohn MA, Hillier SL (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29(2):297–301
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74(1):14–22
Rybakina EG, Nalivaeva NN, Pivanovich YU, Shanin SN, Kozinets A, Korneva EA (2001) The role of neutral sphingomyelinase in interleukin-1beta signal transduction in mouse cerebral cortex cells. Neurosci Behav Physiol 31(4):439–444
Mastromarino P, Macchia S, Meggiorini L, Trinchieri V, Mosca L, Perluigi M, Midulla C (2009) Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect 15(1):67–74
Brzezinski A, Stern T, Arbel R, Rahav G, Benita S (2004) Efficacy of a novel pH-buffering tampon in preserving the acidic vaginal pH during menstruation. Int J Gynaecol Obstet 85(3):298–300
Holley RL, Richter HE, Varner RE, Pair L, Schwebke JR (2004) A randomized, double-blind clinical trial of vaginal acidification versus placebo for the treatment of symptomatic bacterial vaginosis. Sex Transm Dis 31(4):236–238
Yasodhara P, Raghunath M, Sreeramulu D, Venu L, Hemalatha R, Krishna TP (2006) Local immunity in Indian women with bacterial vaginosis. J Reprod Immunol 70(1–2):133–141
Cauci S, Guaschino S, Driussi S, De Santo D, Lanzafame P, Quadrifoglio F (2002) Correlation of local interleukin-8 with immunoglobulin A against Gardnerella vaginalis hemolysin and with prolidase and sialidase levels in women with bacterial vaginosis. J Infect Dis 185(11):1614–1620
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ (2011) Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108(Suppl 1):4680–4687
Becker KA, Riethmüller J, Lüth A, Döring G, Kleuser B, Gulbins E (2010) Acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. Am J Respir Cell Mol Biol 42(6):716–724
Acknowledgments
The study was funded by CD Pharma India Pvt. Ltd., New Delhi.
The authors thank Mr. S. Ananda Rao, Mr. D. Madusudhan Rao and Ms. Bhavani for technical help.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Registration status of trial: Clinical Trial Registry India (CTRI), CTRI/2007/091/000022; Registered on 26/12/2007.
Rights and permissions
About this article
Cite this article
Hemalatha, R., Mastromarino, P., Ramalaxmi, B.A. et al. Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: a randomized, double-blind study. Eur J Clin Microbiol Infect Dis 31, 3097–3105 (2012). https://doi.org/10.1007/s10096-012-1671-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-012-1671-1