Abstract
Purpose
In the vagina of healthy reproductive-aged women, several microbial species maintain a finely tuned mutualistic relationship with the host providing the first-line of defense against the colonization by opportunistic pathogens, which are the leading cause of dysbiosis or vaginal infections (bacterial vaginosis, vulvovaginal candidiasis, and aerobic vaginitis). The use of probiotic lactobacilli to prevent vaginal infections has a good rationale, and an excellent safety record, but so far only a few strains have been clinically proven to be effective, particularly to prevent BV. The aim of the clinical trial was to evaluate the changes in Nugent score in women with intermediate vaginal microbiota treated with oral Lactobacillus acidophilus GLA-14 and Lactobacillus rhamnosus HN001 mixture, in combination with bovine lactoferrin RCX™ (Respecta®) or placebo, for 15 days.
Methods
Vaginal swabs were collected from each woman at baseline and at the end of probiotic treatment and analyzed by RT-PCR. Both symptoms of abnormal vaginal micorbiota and adverse effects were assessed throughout the study.
Results
The results showed that oral intake of lactobacilli/lactoferrin mixture led to significant vaginal colonization by L. acidophilus GLA-14 and L. rhamnosus HN001 showing that both strains can colonize vagina following oral ingestion. The effect of such colonization is correlated with the restoration of normal Nugent score (values 0–3) and an improvement of symptoms of abnormal vaginal micorbiota including itching and discharge.
Conclusions
Oral consumption of lactobacilli/lactoferrin complex corroborates the effectiveness of using lactobacilli for supporting vaginal health and provides a rational basis for future studies on vaginal infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vaginal genital tract is populated by several microbial species, mainly lactobacilli spp., living in equilibrium as a balanced microbiota responsible for the healthy status of vagina and the maintenance of acidic pH (pH < 4.5) [1, 2]. Due to their capability to produce lactic acid and other soluble molecules such as H2O2 and bacteriocins, lactobacilli provide also a defense against opportunistic and pathogenic microorganisms, such as Gardnerella vaginalis and Atopobium vaginae. When number of vaginal lactobacilli is reduced (usually below 106 CFU/ml), the risk of vaginal infections increases significantly. Bacterial vaginosis (BV) is the most common cause of vaginal infection among childbearing women and its prevalence ranges between 4.9 and 36.0% according to European and American studies [3, 4]. BV is caused by bacteria (mainly anaerobic ones) able to adhere to vaginal epithelium, forming biofilm and producing enzymes such as cytolisin (vaginolisin) and sialidase [5]. G. vaginalis is virtually always present at high concentrations in women who have BV but it is also detected frequently in healthy women, so the ratio of logarithm concentration between Lactobacillus spp. and G. vaginalis should be considered as a reliable marker [6]. The most reliable methods to diagnose BV are Amsel criteria and Nugent score [7, 8]. In particular, Nugent score is a scoring system assessing the Gram stained preparations from vaginal smear by wet mount microscopy; it ranges from 0 to 10. Vaginal micorbiota is considered as normal if Nugent score (NS) is between 0 and 3; intermediate vaginal micorbiotia (IF) with NS between 4 and 6 and, finally, BV if NS is between 7 and 10. It is important to note that abnormal vaginal micorbiota or dysbiosis (Nugent score 4–6) shows almost the same characteristics of BV [9] in term of association with upper genital tract infections, sexually transmitted infections (STI) and unfavorable pregnancy outcomes [10, 11]. This condition is found in approximately 20% of clinically healthy women and could be considered a transitional step between normal micorbiota and bacterial vaginosis, which is related to several gynecologic or obstetric outcomes. Therefore, the early detection of vaginal disturbance and the proper management of the intermediate microbiota represents a pivotal matter especially for pregnant women [12].
The current study was performed to assess the degree and persistence of the vaginal colonization by probiotic strains from an orally administered mixture of lactobacilli containing Lactobacillus acidophilus GLA-14 and Lactobacillus rhamnosus HN001, in combination with bovine lactoferrin RCX™ (Respecta®). Its effect on parameters of vaginal health (e.g. total lactobacilli counts, vaginal pH and Nugent score) and subjective symptoms such as itching and vaginal discharge were also monitored.
Patients and methods
The study was performed from October 2016 to January 2017 as a double blind, randomized, placebo controlled clinical trial with two parallel groups receiving either verum or placebo (Fig. 1).
Study product (verum) was a lactobacilli mixture (Respecta® class IIa medical device in Europe, studied and developed by Giellepi S.p.A. Health Science, Lissone, MB, Italy), formulated in capsules including 5 × 109 CFU probiotic blend (Lactobacillus acidophilus GLA-14, LMG S-29159 and Lactobacillus rhamnosus HN001, AGAL NM07/09514), in combination with bovine lactoferrin RCX™ (50 mg). Placebo consisted of an identical capsule containing maltodextrin (100 mg); the excipients were the same in both verum and placebo.
The study was evaluated and approved by the National Committee of Bioethics for Medicines and Medical Devices, Bucharest (no. 3DM/16.02.2016). Clinical procedures have been completed in accordance with Good Clinical Practices and the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments regarding the ethical principles for medical research involving human subjects adopted during the 64th WMA General Assembly at Fortaleza in Brazil on October 2013.
Forty (40) women of reproductive age (18–50 years old) were enrolled with signs or symptoms of vaginitis/vaginosis. The symptoms (itching; vaginal discharge; burning or dryness; fishy odor) were reported from the patients during gynecologic visits using self-completion questionnaire for comprehensive assessment of the severity according to the following four level scale: absent; mild; moderate and severe.
Written informed consent was obtained from all individual participants included in the study. Patients were included if they: (1) need supplementation and/or restoration of the vaginal microbiota (Nugent score 4–6); (2) want to cooperate with investigators during the study and comply with the study procedures; and (3) abstain from sexual relations and vaginal douche during the study. The exclusion criteria were: (1) age < 18 and > 50 years old; (2) Nugent score different from 4 to 6 or other vaginal infections as candidiasis, BV or trichomoniasis; (3) hypersensitivity to any ingredient in the study product; (4) pregnancy, breastfeeding or menopause state; (5) the use of intrauterine device; (6) participation to other clinical trial which could influence urogenital tract micorbiota; (7) the use of any other oral or vaginal probiotics; (8) antibiotic therapy; (9) hormonal therapy.
The full study cycle consisted of three visits at gynecology centers. The first visit was devoted to initial screening to ascertain the inclusion/exclusion criteria; vaginal swabs were collected to define Nugent score and vaginal culture was included to exclude yeast, bacterial or protozoal vaginitis. After informed consent during second visit, all women with intermediate Nugent score were randomized into two groups (20 women per group) according to treatments (verum or placebo). For treatment allocation, a random number table list was created using SPSS program (version 20). The randomization codes were generated as a serial number of four digits indicating the treatment patient should receive (A or B). The randomization scheme ratio was of 1:1; each time a patient met the eligibility criteria, a randomization code was requested from the headquarter by the investigator based on randomization codes distributed at that site. The randomization list was kept in a secure place with limited access and unknown to the clinical investigators and laboratory researchers. The randomization list was opened at the end of the study for study results presentation.
During the third visit (end of the treatment), data regarding efficacy and safety of the intervention were collected. Nugent score at the end of treatment in comparison to baseline values was evaluated and Real-Time PCR (RT-PCR) was carried out for detecting L. acidophilus and L. rhamnosus from vaginal swabs to assess whether the strains transferred from the gut to the vaginal site. The microbiologists able to read Gram staining were blinded.
All enrolled women ingested one capsule of verum/placebo per day for 15 days. Patients were not allowed to take any medicine, intravaginal products (e.g. spermicides, lubricants, etc.), or food that could interact with the study treatments during the entire study period. Participants utilizing/consuming the above-mentioned products were excluded from the evaluation and statistical analysis; women were only allowed to use a neutral detergent for intimate hygiene.
Nugent score is a Gram stain scoring system useful as screening method to differentiate normal vaginal micorobiota from bacterial vaginosis. Vaginal specimens were collected according to Good Clinical Practice from each woman by careful rotation of sterile cotton swab against sidewalls in the upper third area of the vagina. Each vaginal swab was used for doing a smear on a microscope slide and stained for Nugent score estimation. The Nugent score was calculated from the microscopic evaluation according to Nugent et al. [8]. Regarding the criteria for interpreting the results, Nugent score ranging from 0 to 3 indicates normal vaginal micorbiota; Nugent score from 4 to 6 indicates intermediate vaginal microbiota or dysbiosis; Nugent score from 7 to 10 indicates bacterial vaginosis. Collectively, Nugent score greater than three have been referred to as “abnormal” vaginal micorbiota.
The pH of secretions collected from the lateral vaginal wall was measured using paper strips, whose color indicator ranged from 3.5 to 5.2.
A second swab was collected for assessing lactobacilli colonization by RT-PCR analysis. Total DNA was extracted from vaginal swabs using the genomic DNA extraction PureLink Mini Kit (Thermo Fisher Scientific) and DNA Cleanup Micro kit according to the manufacturer protocols. Extracts purity was determined using a biophotometer (Eppendorf). In the following stage, the concentrations of total DNA were calculated in all samples and a quantitative PCR was performed using the highest concentration of DNA common to all samples (100 ng). In all PCR reactions, positive controls (DNA extracted from lactobacilli cultures) and negative controls were used for internal control amplification. RT-PCR results were reported as genome containing particles (GCP)/100 ng total DNA (number of copies of DNA lactobacilli in 100 ng of total DNA).
DNA amount of the two studied microorganisms was assessed by RT-PCR according to methods described by De Alberti et al. [13]. Two sets of primers were used; the first set of primers was LacidoF (50-TGCAAAGTGGTAGCGTAAGC-30) and LacidoR (50-CCTTTCCCTCACGGTACTG-30), which was designed to amplify a 200 bp on the region between 16S rDNA and 32S rDNA of L. acidophilus. The second set of primers was LrhamF (50-TGCTTGCATCTTGATTTAATTTTG-30) and LrhamR (50-GGTTCTTGGATYTATGCGGTATTAG-30), which was designed to amplify a 120 bp on the region of the 16S rDNA of L. rhamnosus.
DNA amplification conditions were as follows: 3 min initial denaturation at 95 °C, 35 denaturation cycles at 95 °C for 30 s, 35 denaturation-annealing and annealing-elongation cycles at 60 °C for 30 s. A Quant Studio 3 Real Time-PCR System was used for RT-PCR analyses. RT-PCR was performed with TaqMan Multiplex Master Mix (Applied Biosystems, Thermo Fisher Scientific). After TaqMan amplification, a dissociation curve analysis was performed to confirm the absence of nonspecific amplification products.
Adverse events were classified as light, moderate or severe (an adverse event was considered severe if it was fatal, life threatening, requiring hospitalization, leading to permanent handicaps or congenital abnormalities).
Causality of any adverse events was considered as: correlated if the investigator had sufficient information and considered the symptom to be correlated to the study treatment. Uncorrelated if the investigator had sufficient information and considered the symptom not to be correlated to the study treatment. Unable to determine correlation if the investigator had insufficient information or was not able to determine the correlation or non-correlation of the symptom to the study treatment.
For normality testing, Shapiro–Wilk test was performed. Descriptive statistics was used in this study to present quantitative descriptions of different variables. Unpaired Student’s t test was used for assessing differences between treatment groups. Chi-Square test was used for the analysis to tests the association between tested variables. The null hypothesis to be tested was no difference between the group treated with the lactobacilli/lactoferrin mixture compared to placebo group.
Results
Demographic characteristics of the recruited women are presented in Table 1. No difference was observed between the two treated groups (Respecta® and placebo). Compliance was 100% for all participants in both study groups during the treatment. During clinical examinations, symptoms such as itching, vaginal discharge, fishy odor, burning and dryness were monitored in both groups. As expected, no woman showed burning sensation or dryness. Women taking lactobacilli/lactoferrin mixture experienced a general improvement of symptoms; in particular, itching and vaginal discharge decreased significantly at the end of treatment with Respecta® compared to baseline and placebo group (P < 0.001). Results are shown in Fig. 2.
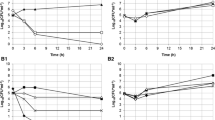
Nugent score results are shown in Table 2. The intra-group analysis for the evaluation of Nugent score evolution from baseline to the end of the study showed that Nugent score was significantly improved (from intermediate to normal score) in the group treated with lactobacilli/lactoferrin mixture after 15 days treatment (P = 0.0004). On the other hand, the administration of placebo did not induce any modifications in Nugent score. Intergroup comparison at the end of treatment revealed that Nugent score was significantly improved after lactobacilli/lactoferrin mixture oral administration compared to placebo (P = 0.0110).
The mean vaginal pH varied between 4.42 and 4.09 in verum group and between 4.03 and 4.47 in the placebo group (Table 3). No statistical difference was observed neither between baseline and the end of the study in each arm, nor between the two arms.
RT-PCR was performed to assess vaginal colonization by both probiotic strains (L. acidophilus GLA-14 and L. rhamnosus HN001) after lactobacilli/lactoferrin mixture oral intake. The results are showed in Figs. 3 and 4 for L. acidophilus GLA-14 and L. rhamnosus HN001, respectively. Both strains increased significantly in the vagina of women in the active arm at the end of the study in comparison to baseline. On the contrary, vaginal colonization did not change significantly in the placebo group anytime. Intergroup comparison at the end of treatment revealed that lactobacilli vaginal colonization was significantly improved after lactobacilli/lactoferrin mixture oral administration compared to placebo (P < 0.001).
During the study period no adverse event occurred, neither correlated nor unrelated. Both products (verum and placebo) were well tolerated by all subjects participating in the study.
Discussion
The manipulation of vaginal microbiota by supplementation of probiotics represents a strong rationale for restoring the homeostasis of unbalanced vaginal micorbiota, which causes dysbiosis and vaginal infections [14, 15]. Nevertheless, not all probiotic strains are suitable for vaginal indications. To be effective, a strain must reach and colonize the human vagina. Substantial of experimental evidence exists about vaginal colonization by lactobacilli, also following oral intake [13, 16,17,18]. Unfortunately, also pathogenic bacteria (e.g. Streptococcus agalactiae, Escherichia coli, Gardnerella vaginalis) can move from the gastrointestinal tract to the lower uro-genital tract [19] thus causing diseases such as urinary tract infections (UTI), bacterial vaginosis (BV), aerobic vaginitis (AV) and vulvovaginal candidiasis (VVC).
Some studies have suggested that even a transient colonization through a short probiotic intervention may be important and sufficient to correct a non-balanced microbiota and this may be relevant in case of intermediate microbiota/dysbiosis [18] and BV during pregnancy [20].
Oral supplementation with probiotics, which may colonize the vagina, can be the first step in the prevention of further development of vaginal dysbiosis [21]. Jang et al. [22] found that orally administered L. acidophilus GLA-14 and L. rhamnosus HN001, in association with bovine lactoferrin, were able to reach and colonize the vagina in mice and attenuate bacterial vaginosis experimentally induced in treated animals. In particular, it has been shown that oral administration of the lactobacilli mixture was more effective against G. vaginalis-induced BV than intravaginal administration in mice. A previous clinical study showed that oral administration of lactobacilli mixture with bovine lactoferrin (Respecta®) led to a significant increase in vaginal colonization by L. acidophilus GLA-14 and L. rhamnosus HN001 in healthy women after 1 week of treatment; on the contrary, the placebo did not increased lactobacilli in the vagina of treated women. Interestingly, the levels of both strains increased 1 week after the end of oral probiotic intake, suggesting a prolonged persistence of lactobacilli in the vagina [13]. In addition, Strus and co-workers [21] showed also a significant vaginal colonization after oral administration of a lactobacilli mixture containing L. fermentum 57A, L. plantarum 57B, and L. gasseri 57C.
Following vaginal colonization, lactobacilli can counteract the proliferation of opportunistic or pathogenic microorganisms by different ways. Lactobacilli compete with other bacteria for both nutrients and adhesion sites onto the vaginal epithelium. In addition, they exert antimicrobial activities by producing lactic acid, which decreases vaginal pH and produce soluble molecules including H2O2 and bacteriocins able to cause either microbicidal effects by disrupting bacteria membrane or inhibiting the activity of enzymes (i.e. sialidases and prolidases) needed for bacterial virulence [23, 24]. These characteristics must be considered as key parameters to select strains for probiotic vaginal therapy.
A recent in vitro study showed that L. acidophilus GLA-14 and L. rhamnosus HN001, exert antimicrobial effects against some pathogenic bacteria responsible for lower genital tract infections by inhibiting the proliferation of Gardnerella vaginalis, Atopobium vaginae, Staphylococcus aureus and Escherichia coli [25]. The hypothesized mechanism of action by which lactobacilli exert such an effect against pathogenic bacteria could be linked to the production of soluble antimicrobial compounds such as bacteriocins and lactic acid. Most likely, an additional mechanism by which lactobacilli reduce pathogen colonization is by competition and displacement on the vaginal tissue. Jang et al. [22] showed that L. acidophilus GLA-14 and L. rhamnosus HN001 significantly inhibited the adherence of G. vaginalis to HeLa cells (a human cervical cancer cell line) and pathogen growth in vitro. In addition, the authors demonstrated that either oral or intravaginal administration of a selected lactobacilli mixture, in combination with bovine lactoferrin, significantly inhibited G. vaginalis-induced epithelial cell disruption.
Abnormal vaginal microbiota (dysbiosis or intermediate Nugent score) appears less resilient to disturbance/symptom and more susceptible to disease. Ideally, ‘intermediate microbiota’ condition represents a turning point from a healthy status into BV, or conversely from BV to normal condition. Interestingly, women having a disturbed microbiota (intermediate Nugent score 4–6) are in most cases not treated with any antibiotic regimen because both the lack of symptoms and clear guideline in regards of the management of borderline situation. Considering that this category represents a ‘pivot point’ and can eventually lead to a serious range of complications including mid-trimester pregnancy loss, the ‘classic’ full-blown BV, a strategic probiotic supplementation may represent a safe method to re-establish a healthy vaginal environment.
The results from the current study indicate that the oral intake of selected probiotics for a short time (i.e. 15 days) increase significantly the vaginal levels of both lactobacilli species, L. acidophilus GLA-14 and L. rhamnosus HN001 and confirm the results from the study of De Alberti et al. [13]. This effect determines a significant regression of Nugent score from intermediate towards a normal value (0–3) and a resolution of symptoms such as itching and vaginal discharge reported in women with symptomatic vaginal dysbiosis.
No significant reduction on vaginal pH was observed after neither verum nor placebo treatment. Nevertheless, lactobacilli/lactoferrin mixture oral intake induced a positive trend towards lower pH values. This is not surprising considering the baseline value of pH were found < 4.5 in both groups (i.e. 4.42 and 4.03 in verum arm and placebo arm, respectively) and the short period of probiotic administration.
The results of this study suggest that oral supplementation with probiotics proven to colonize vagina can be the first step in the treatment of dysbiosis or intermediate Nugent score. The resolution of all these forms of abnormal microbiota will be a major challenge for future treatment studies. The new insights in the differential diagnosis of all types of abnormal vaginal microbiota have created renewed interest in screening and treating pregnant and non-pregnant women to prevent acquisition of STI or pregnancy complications as preterm labour [26].
Our results may suggest a potential risk reduction for women with elevated Nugent score. Furthermore, the lack of evidence-based clinical guidelines for the management of these subtypes of abnormal vaginal microbiota, such as intermediate Nugent score, needs to be addressed urgently.
The results found in this double-blind, placebo-controlled randomized study suggest that the tested lactobacilli mixture in combination with bovine lactoferrin may be a new candidate for vaginal dysbiosis and further studies are needed to analyze similar protocols in women with vaginal infections such as bacterial vaginosis or aerobic vaginitis.
References
Larsen B, Monif GR (2001) Understanding the bacterial flora of the female genital tract. Clin Infect Dis 32(4):69–77
Oakley BB, Fiedler TL, Marrazzo JM, Fredricks DN (2008) Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl Environ Microbiol 74:4898–4909
Cristiano L, Rampello S, Noris C, Valota V (1996) Bacterial vaginosis: prevalence in an Italian population of asymptomatic pregnant women and diagnostic aspects. Eur J Epidemiol 12(4):383–390
Kasaro MP, Husnik MJ, Chi BH, Reid C, Magure T, Makanani B, Tembo T, Ramjee G, Maslankowski L, Rabe L, Brad Guffey M (2017) Impact of targeted counseling on reported vaginal hygiene practices and bacterial vaginosis: the HIV Prevention Trials Network 035 study. Int J STD AIDS 28(5):467–475
Cauci S, Hitti J, Noonan C (2002) Vaginal hydrolytic enzymes immunoglobulin A against Gardenerella vaginalis toxin and risk of early preterm birth among women in preterm labor with bacterial vaginosis or intermediate flora. Am J Obstet Gynecol 187(4):877–881
Donders GG (2007) Definition and classification of abnormal vaginal flora. Best Pract Res Clin Obstet Gynaecol 21:355–373
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74(1):14–22
Nugent RP, Krohn MA, Hillier SL (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29(2):297–301
Guédou FA, Van Damme L, Deese J, Crucitti T, Mirembe F, Solomon S, Becker M, Alary M (2014) Intermediate vaginal flora and bacterial vaginosis are associated with the same factors: findings from an exploratory analysis among female sex workers in Africa and India. Sex Transm Infect 90(2):161–164
Schwebke JR (2003) Gynecologic consequences of bacterial vaginosis. Obstet Gynecol Clin North Am 30:685–694
Hauth JC, Macpherson C, Carey JC, Klebanoff MA, Hillier SL, Ernest JM, Leveno KJ, Wapner R, Varner M, Trout W, Moawad A, Sibai B (2003) Early pregnancy threshold vaginal pH and Gram stain scores predictive of subsequent preterm birth in asymptomatic women. Am J Obstet Gynecol 188(3):831–835
Ugwumadu A (2007) Role of antibiotic therapy for bacterial vaginosis and intermediate flora in pregnancy. Best Pract Res Clin Obstet Gynaecol 21(3):391–402
De Alberti D, Russo R, Terruzzi F, Nobile V, Ouwehand AC (2015) Lactobacilli vaginal colonisation after oral consumption of Respecta® complex: a randomised controlled pilot study. Arch Gynecol Obstet 292(4):861–867
Reid G (2017) The development of probiotics for women’s health. Can J Microbiol 63(4):269–277
Reid G, Bruce AW (2001) Selection of Lactobacillus strains for urogenital probiotic applications. J Infect Dis 183(Suppl 1):S77–S80
Reid G, Bruce AW, Fraser N (2001) Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbio 30:49–52
Antonio MA, Rabe LK, Hillier SL (2005) Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J Infect Dis 192(3):394–398
Mezzasalma V, Manfrini E, Ferri E, Boccarusso M, Di Gennaro P, Schiano I, Michelotti A, Labra M (2017) Orally administered multispecies probiotic formulations to prevent uro-genital infections: a randomized placebo-controlled pilot study. Arch Gynecol Obstet 295(1):163–172
El Aila NA, Tency I, Claeys G, Verstraelen H, Saerens B, Santiago GL, De Backer E, Cools P, Temmerman M, Verhelst R, Vaneechoutte M (2009) Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC Infect Dis 9:167
Reid G, Bocking A (2003) The potential for probiotics to prevent bacterial vaginosis and preterm labor. Am J Obstet Gynecol 189(4):1202–1208
Strus M, Chmielarczyk A, Kochan P, Adamski P, Chełmicki Z, Chełmicki A, Pałucha A, Heczko PB (2012) Studies on the effects of probiotic Lactobacillus mixture given orally on vaginal and rectal colonization and on parameters of vaginal health in women with intermediate vaginal flora. Eur J Obstet Gynecol Reprod Biol 163(2):210–215
Jang SE, Jeong JJ, Choi SY, Kim H, Han MJ, Kim DH (2017) Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus GLA-14 attenuate Gardnerella vaginalis-infected bacterial vaginosis in mice. Nutrients 9(6):23
Todorov SD, Furtado DN, Saad SM, de Melo Gombossy, Franco BD (2011) Bacteriocin production and resistance to drugs are advantageous features for Lactobacillus acidophilus La-14, a potential probiotic strain. New Microbiol 34(4):357–370
Stoyancheva G, Marzotto M, Dellaglio F, Torriani S (2014) Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol 196(9):645–653
Bertuccini L, Russo R, Iosi F, Superti F (2017) Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int J Immunopathol Pharmacol 30(2):163–167
Krauss-Silva L, Moreira ME, Alves MB, Rezende MR, Braga A, Camacho KG, Batista MR, Savastano C, Almada-Horta A, Guerra F (2010) Randomized controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with intrauterine infection: study protocol. Reprod Health 30:7–14
Acknowledgements
We are grateful to all the staff of Cebis International, Lugano (Switzerland) for their assistance in data management and clinical procedures and to all patients that participated to the study.
Author information
Authors and Affiliations
Contributions
RR: Project development; data analysis; manuscript writing. AE: Data management; clinical operations. FDS: Project development; data analysis; manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
RR is employed by Giellepi. He had no influence on the interpretation of results. AE and FDS have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Russo, R., Edu, A. & De Seta, F. Study on the effects of an oral lactobacilli and lactoferrin complex in women with intermediate vaginal microbiota. Arch Gynecol Obstet 298, 139–145 (2018). https://doi.org/10.1007/s00404-018-4771-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4771-z