Abstract
Purpose
To compare the peripapillary retinal nerve fiber layer (pRNFL), retinal nerve fiber layer (RNFL), and ganglion cell complex (GCC) thickness measurement in early-onset Alzheimer’s disease (EOAD) and controls using spectral domain optical coherence tomography (SD-OCT). We also assessed the relationship between SD-OCT measurements and cognitive measures, serum biomarkers for Alzheimer’s disease (AD), and cerebral microstructural volume.
Methods
pRNFL, RNFL, and GCC thicknesses were measured in 43 EOAD and 42 controls using SD-OCT. Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) were used to assess cognitive status, magnetic resonance imaging (MRI) tool was used to quantify cerebral microstructural volume, and serum biomarkers were quantified from peripheral blood.
Results
EOAD patients had thinner pRNFL (P < 0.001), RNFL (P = 0.008), and GCC (P = 0.018) thicknesses compared to controls after adjusting for multiple factors. pRNFL thickness correlated (P = 0.016) with serum t-tau level. Serum Aβ42 (P < 0.05) concentration correlated with RNFL thickness. Importantly, occipital lobe volume (P = 0.010) correlated with GCC thicknesses in EOAD patients.
Conclusion
Our findings suggest that retinal thickness may be useful markers for assessing neurodegenerative process in EOAD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early-onset Alzheimer’s disease (EOAD), defined as Alzheimer’s disease (AD) occurring before age 65, is significantly less well studied than the late-onset form (LOAD). However, EOAD often presents with high genetic predisposition, greater neuropathological burden, atypical clinical manifestations, and bad prognosis [1,2,3,4]. It may take a long time before EOAD is diagnosed, probably reflecting missed or delayed diagnosis or difficulties in diagnosis [5]. Currently, the diagnosis of AD primarily relies on the detection of amyloid β (Aβ) and tau levels in cerebrospinal fluid (CSF), as well as the identification of elevated depositions of Aβ and tau proteins in the cortex through positron emission tomography (PET). Although these clinical examinations have improved clinical diagnostic accuracy of AD, these modalities are invasive, time-consuming, and expensive. Therefore, it is urgent to develop a non-invasive, widely accessible, and cost-effective marker for the diagnosis of AD.
The cortex and the retina share many characteristics, including embryologic origin, precise neuronal cell layers, and microvasculature [6, 7]. Remarkably, the hallmark biomarkers of AD—amyloid beta (Aβ) plaques, and neurofibrillary tangles (NFTs) composed of the protein tau—have been identified in the retina of individuals with AD [8, 9]. Recently, optical coherence tomography (OCT) technology allows us to image and visualize retinal sub-layer structures in vivo with high resolution. Due to the benefits of retinal imaging, accumulating reports [10,11,12,13,14] focused on the identification, development, and validation of diagnostic and prognostic retinal biomarkers for Alzheimer’s disease. However, findings of retinal thickness in AD have been inconsistent [7, 15,16,17]. Moreover, the exploration of retinal thickness in EOAD has been notably scarce, with conflicting results [18] further complicating the current state of research. Additionally, although some reports have indicated a correlation between retinal thinning and cerebral cortical atrophy in EOAD evaluated using visual assessment scales [18], few studies have explored the relationship between retinal thickness and MRI-based quantification of cerebral microstructural volume in EOAD.
Recent studies [19, 20] suggest blood-based biomarkers of AD provide satisfactory performance in the diagnosis and prediction of disease progression and correlated well with cerebral pathological burden of AD. Studies [21, 22] suggested Aβ42/Aβ40 ratio in blood has shown satisfactory accuracies to detect AD from control. Phosphorylated tau (p-tau) 181 has also shown excellent diagnostic performances to detect and differentiate AD from other neurodegenerative diseases [23]. A previous study revealed retinal thickness correlated with tau and Aβ42 levels in CSF [24]. Nevertheless, the connection between retinal thickness and blood-based biomarkers even remains unknown.
Our study aims to address these gaps by examining retinal structural changes in EOAD patients and exploring the relationship between retinal structural thickness and cerebral microstructural volume and commonly used blood-based biomarkers in EOAD.
Methods
This observational, cross-sectional study was conducted as part of an ongoing prospective study that recruits AD patients from the Neurology Department of West China Hospital, Sichuan University. The study was approved by the Biomedical Research Ethics Committee and the Committee on Human Research of West China Hospital, Sichuan University (Ethics Number: 2020–104) and followed the Declaration of Helsinki. Informed consent was obtained from participants or their guardians.
Sixty-eight patients diagnosed with EOAD were enrolled according to a prospective screening protocol from the Memory and Aging Unit of the Neurology Department between May 2020 and June 2021. Diagnosis of EOAD fulfilled the NIA-AA criteria [25]. Inclusion criteria were as follows: (1) provide informed consent and (2) ability to cooperate and complete MR imaging, OCT imaging, blood draw, and neuropsychological assessment. Controls were individuals who had their annual health checkups at our hospital and consented to enroll in the study. These individuals were matched in age with the EOAD group and did not exhibit impairment in any of the cognitive domains during the cognitive assessment. Exclusion criteria for all participants are psychiatric or neurological disorders (stroke, Parkinson’s disease, multiple sclerosis, autoimmune encephalitis, drug addiction, depression, and schizophrenia) and severe systemic disease such as heart failure and renal insufficiency.
All participants received the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA), which are brief dementia screening examinations with a total score of 30 and a higher score indicating better cognition. Medical histories including cardiovascular risk factors such as smoking status, previous stroke, hypertension, and diabetes mellitus and currently prescribed medications were recorded.
Blood samples were procured from the peripheral blood of individuals and examined at the West China Hospital of Sichuan University [26]. Serum Aβ40, serum Aβ42, and serum total tau (t-tau) were analyzed using Quanterix Simoa Human Neurology 3-Plex E essays, and p-tau 181 was measured using Quanterix Simoa p-tau 181 of the human tau protein. Assays were done using Simoa-HD1 according to the manufacturer’s manual as previously reported [26]. All samples were analyzed at the same time.
All participants underwent magnetic resonance imaging (MRI) as previously reported [27]. Briefly, MRI was performed on a 3-T magnetic resonance (MR) system (Magnetom Trio, Siemens Medical Systems, Erlangen, Germany). The scanning protocol was as follows: (i) whole-brain 3 D-T1 BRAVO sequence (TR/TE 8.5/3.2 ms; prep time 450 ms, flip angle 12°, voxel size 1.0 × 1.0 × 1.0 mm); (ii) T2 FLAIR (TR/TE/TI 9,000/95/2,474 ms; voxel size 0.93 × 0.93 × 5.0 mm; gap 1 mm); (iii) T2 propeller (TR/TE 5,039/110 ms; voxel size 0.58 × 0.58 × 5.0 mm; gap 1 mm); and (iv) 3D-ASL (TR/TE 4,809/10.7 ms; slice thickness 4 mm; post-label delay 2,024 ms; arms 8; number of excitation 3); during resting state, subjects were told not to concentrate on any particular subject, but just to relax with their eyes closed. The complete scanning protocol took 20 min. Automated brain volumetry analyses were performed for all the included subjects with AccuBrain® on 3D T1-weighted (T1W) MRI scans. In detail, given the T1W MRI data, several brain structures and three major brain tissues are segmented automatically based on prior anatomical knowledge specified by experienced radiologists. The anatomical information is automatically transformed into the individual brain. Then, the volume of the frontal lobe, temporal lobe, parietal lobe, and occipital lobe were automatically generated by the AccuBrain™ brain structure tool.
All participants underwent complete ophthalmic evaluation: a slit-lamp examination of the anterior chamber of the eye and optic nerve head (ONH) and fundus imaging using fundus photography. Photographs of the ONH were evaluated by an ophthalmologist (Dr. Ruilin Wang), and patients with ophthalmic disorders such as exudates, retinal hemorrhages, microaneurysms, cotton wool spots, and blurred optic discs were excluded.
Retinal structural imaging with the OCT tool
The imaging was performed using an Avanti RTVue-XR OCT device (Optovue, Fremont, California, USA; software version 2017.1.0.151). The OCT device had an axial speed of 100 kHz and an 840-nm wavelength with a tuning range of 100 nm. The image resolution was 5.3 mm axially and 18 mm laterally with a scan speed of 70,000 A-scans per second. The peripapillary retinal nerve fiber layer (pRNFL) was obtained using the optic nerve head (ONH) map protocol, which covered 3.45 mm around the optic disc (Fig. 1). RNFL thickness was recorded from the ONH report, and the protocol involved obtaining a disc cube scan consisting of 128 B-scans of a 6 × 6 mm area centered on the optic nerve head. The built-in software was used to obtain a topographical map of the ONH and measure the nerve fiber layer thickness. The ganglion cell complex (GCC) thickness was obtained using the GCC scanning protocol, generated through scans centered 1 mm temporal to the fovea and covering a square grid on the central macula (7 × 7 mm) as shown in Fig. 1. The mean pRNFL, RNFL, and GCC thicknesses generated by the OCT device were used in the study, with the mean values of the OCT parameters representing the average values of the four quadrants. OCT data displayed in our study followed the OSCAR-IB quality criteria [28] and APOSTEL recommendation [29]. Exclusion criteria were as follows: retinal hemorrhage, retinal occlusion, a history of retinal surgery, age-macular degeneration, severe cataract, optic neuritis, diabetic retinopathy, and glaucoma. If a participant presented with any of these disorders in one eye, the other eye was used; if both eyes had the disorders, the participant was excluded from the study.
Statistical analysis
The normality of continuous variables was assessed using the Shapiro–Wilk test. For normally distributed variables, means ± standard deviation was reported, while skewed distributions were described using medians and interquartile ranges. Categorical variables were presented as frequencies and percentages. Demographic and clinical characteristic differences, as well as disparities in blood biomarkers and volume parameters between the EOAD and controls, were analyzed using the t-test and Mann–Whitney U test for continuous variables and the chi-square test for categorical variables. Linear regression with generalized estimating equation (GEE) was employed to investigate differences in retinal structure thicknesses (pRNFL, RNFL, and GCC) between EOAD and controls. This analysis was adjusted for age, gender, education, vascular risk factors (hypertension, diabetes mellitus), and inter-eye dependencies. GEE was also utilized to explore the associations between OCT parameters, blood serum biomarkers, and cognitive measures, adjusting for potential confounding factors such as age, gender, education, hypertension, diabetes mellitus, and inter-eye dependencies. Additionally, GEE was applied to assess the association between OCT parameters and cerebral microstructural volume while adjusting for potential confounders, including age, gender, TMV, hypertension, diabetes mellitus, and inter-eye dependencies. All statistical analyses were conducted using R version 4.2.1 and R Studio version 2022.06.0. Statistical significance was defined as P values less than 0.05 (P < 0.05).
Results
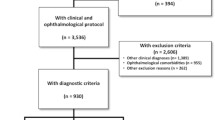
Figure 2 shows the flow chart of our study. Our final data analysis included 43 EOAD (59.77 ± 6.93 years; 48.84% males) and 42 controls (57.95 ± 6.86 years; 52.38% males).
Table 1 shows the demographics and clinical information of our study participants. EOAD patients showed reduced cerebral microstructural volume, increased serum biomarkers concentrations, and lower cognitive scores compared to controls. Moreover, EOAD patients had thinner pRNFL (P < 0.001), RNFL (P = 0.008), and GCC thicknesses (P = 0.018) compared to controls.
Table 2 shows the association of structural retinal parameters with cognitive scores and AD-related serum biomarkers in EOAD patients. pRNFL thicknesses correlated (P = 0.016) with serum t-tau level, while RNFL thickness correlated (P = 0.048) with serum Aβ42 level. After adjusting for multiple comparisons, the association remained significant. However, no significant correlation was seen between structural retinal parameters (pRNFL, RNFL, and GCC) and cognitive scores (MMSE and MoCA).
Table 3 shows the association of cerebral volumetric measures and OCT structural retinal parameters in EOAD patients. GCC thickness correlated with occipital lobe volume (P = 0.010) in EOAD patients after adjusting for potential confounding factors. No significant correlation (P > 0.05) was seen between pRNFL, RNFL thickness, and cerebral volumetric parameters.
Discussion
In this study, EOAD patients displayed notable reductions in the pRNFL, RNFL, and GCC thickness, when compared to the control group. Notably, a negative association was identified between pRNFL thickness in EOAD patients and serum total tau levels, while RNFL thickness exhibited a positive correlation with serum Aβ42 levels. Furthermore, our study uncovered a correlation between occipital lobe volume and GCC thickness in EOAD patients.
While prior research has identified thinning retinal thickness in AD or LOAD [6, 8, 17], the retinal thickness changes associated with EOAD remain underexplored. Several studies with small sample sizes [18, 30, 31] have reported that retinal thickness did not exhibit significant differences between individuals with EOAD and controls. In contrast, our study has revealed that EOAD patients manifest thinner pRNFL, RNFL, and GCC thicknesses compared to controls. It is suggested that structural changes in the retinal structure may reflect neurodegenerative and microvascular changes within the brain, as observed in postmortem pathology of dementia and/or AD [8, 32]. These measurements of retinal structure thickness serve as markers for the integrity of retinal cells and axons, with thinner retinal thickness indicating the loss of neuroaxonal and neuronal components of the retina [33]. Consequently, we propose that thinner retinal thickness observed in EOAD patients may mirror the ongoing neurodegenerative processes within the brain during the progression of the EOAD.
We first discovered pRNFL thickness was negatively correlated with serum t-tau levels, while RNFL thickness was positively correlated with serum Aβ42 concentrations. A previous study from Santangelo et al. [24] reported RNFL thickness was correlated with CSF tau and Aβ42 concentrations providing robust support for our findings. Autopsy study [32, 34] revealed that AD patients with substantial brain amyloid plaque deposition also exhibited amyloid protein deposits in the retina. Previous study [32] further reinforced this observation by demonstrating a higher amyloid burden in the brain may be associated with a higher one in retina. Notably, lower concentrations of serum Aβ42 and elevated serum t-tau levels were identified as indicators of increased brain amyloid protein deposition and neurodegeneration resulting from amyloid protein accumulation [35], respectively. Our investigation revealed reduced Aβ42 concentrations and elevated serum t-tau levels were associated with the thinning of the retinal nerve fiber layer in the optic disc region. Consequently, we contend that RNFL thickness in the optic disc holds the potential to emerge as a non-invasive, cost-effective, and easily accessible biomarker for both diagnosing and monitoring disease progression of EOAD on a large scale in the future.
In our study, we observed EOAD patients exhibited reduced cerebral volumetric measures compared to controls, consistent with previous reports [36, 37]. Noteworthy findings from other studies have highlighted alterations in retinal nerve fiber layer (RNFL) and retinal ganglion cells (RGC) in occipital lobe stroke patients [38], significant loss of optic radiation fibers, and damage to the visual cortex in glaucoma and optic neuritis patients [39, 40]. These studies indicate a plausible link between the retina and the visual cortex, proposing trans-synaptic degeneration as the underlying mechanism driving this association within the visual pathway [39]. However, our current study revealed a negative correlation between GCC thickness and occipital lobe volume in EOAD patients. Similarly, other studies have demonstrated a positive association between degree of brain amyloid positivity and total retinal thickness in cognitively healthy people [41]. Given the known roles of the occipital lobe and GCC in visual processing and vision, our findings may offer insights into the mechanisms underlying visual symptoms in EOAD patients. The progression of Alzheimer’s disease is marked by increasing cerebral atrophy and brain amyloid burden. However, the longitudinal trends of GCC thickness throughout the course of AD remain unclear. Based on our results, we speculate that thicker GCC thickness may be related to more severe cerebral atrophy and advanced stages of AD. The GCC represents the three innermost retinal layers, encompassing the nerve fiber layer, the ganglion cell layer (GCL), and the inner plexiform layer (IPL). The IPL, being more susceptible to amyloid deposition compared to other retinal layers, is predominantly composed of capillaries [41]. This association may be attributed to retinal vascular abnormalities and neovascularization, previously shown to be associated with brain amyloid load [42]. Additionally, in response to injury, increased inflammation and activation of Müller cells in the retina were observed in mice [43]. Our findings emphasize the retinal thickness may be a useful biomarker to monitor the progression of EOAD.
Our study did not reveal a significant correlation between retinal thickness (pRNFL, RNFL, and GCC) and MMSE and MoCA scores among EOAD patients. While prior studies have suggested a link between retinal thickness and cognitive performance in AD and older people [16, 24, 44,45,46], our findings align with the finding of Jurre den Haan et al. [15], who also reported no correlation between retinal thickness and MMSE score in EOAD. The potential factors contributing to conflicting results encompass participant heterogeneity, an overrepresentation of positive studies, variations in diagnostic criteria, and the diverse stages of the disease. To resolve these discrepancies, there is a pressing need for large-scale, standardized, and well-phenotyped EOAD cohorts with confirmed amyloid pathology to further elucidate the relationship between retinal structure and cognitive performance in EOAD.
Strength of study
This study focuses on EOAD, a distinctive form of AD with challenges in early diagnosis and a generally unfavorable prognosis. Notably, the mean age of EOAD is lower compared to those with LOAD dementia, diminishing the influence of aging—an important factor in AD. To ensure the accuracy of our findings, a certified ophthalmologist was engaged to systematically exclude participants with ophthalmic diseases, thereby mitigating potential confounding variables. Significantly, this research represents the inaugural exploration of the correlation between retinal thickness and blood biomarkers in EOAD. The outcomes may furnish additional evidence affirming the pivotal role of retinal biomarkers in enhancing the diagnostic capabilities for EOAD.
Limitation
First, the limitation of this study is its small number of patients and its cross-sectional design. To further assess the evolving characteristics of retinal structure and its relationship with Alzheimer’s disease pathological markers, a larger and longitudinal cohort is needed. Second, future studies ought to incorporate the results of amyloid β and tau protein PET. Finally, individuals with ophthalmic or neurological conditions were excluded from our study, potentially introducing selection bias and leading to an underestimation of the effect sizes.
Conclusion
In EOAD, we found peripapillary retinal nerve fiber layer thickness and retinal nerve fiber layer thickness correlated with serum t-tau and Aβ42 level respectively. Additionally, ganglion cell complex thickness associated with occipital lobe atrophy in EOAD. Taken together, these findings indicate that retinal imaging holds promise as a non-invasive, widely accessible, and cost-effective diagnostic tool for EOAD.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Ayodele T, Rogaeva E, Kurup JT, Beecham G, Reitz C (2021) Early-onset Alzheimer’s disease: what is missing in research? Curr Neurol Neurosci Rep 21:4. https://doi.org/10.1007/s11910-020-01090-y
Sirkis DW, Bonham LW, Johnson TP, La Joie R, Yokoyama JS (2022) Dissecting the clinical heterogeneity of early-onset Alzheimer’s disease. Mol Psychiatry 27:2674–2688. https://doi.org/10.1038/s41380-022-01531-9
Mendez MF (2019) Early-onset Alzheimer disease and its variants. Continuum (Minneap Minn) 25:34–51. https://doi.org/10.1212/CON.0000000000000687
Stanley K, Walker Z (2014) Do patients with young onset Alzheimer’s disease deteriorate faster than those with late onset Alzheimer’s disease? A review of the literature. Int Psychogeriatr 26:1945–1953. https://doi.org/10.1017/S1041610214001173
Eriksson H et al (2014) Differences in routine clinical practice between early and late onset Alzheimer’s disease: data from the Swedish Dementia Registry (SveDem). J Alzheimers Dis 41:411–419. https://doi.org/10.3233/JAD-132273
Alber J et al (2020) Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: what we know, what we don’t, and how to move forward. Alzheimers Dement 16:229–243. https://doi.org/10.1002/alz.12006
Snyder PJ et al (2021) Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimers Dement 17:103–111. https://doi.org/10.1002/alz.12179
Blanks JC, Hinton DR, Sadun AA, Miller CA (1989) Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res 501:364–372. https://doi.org/10.1016/0006-8993(89)90653-7
Hinton DR, Sadun AA, Blanks JC, Miller CA (1986) Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med 315:485–487. https://doi.org/10.1056/NEJM198608213150804
Bayhan HA, Aslan Bayhan S, Celikbilek A, Tanık N, Gürdal C (2015) Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin Exp Ophthalmol 43:145–151. https://doi.org/10.1111/ceo.12386
Chan VTT et al (2019) Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology 126:497–510. https://doi.org/10.1016/j.ophtha.2018.08.009
Cheung CY et al (2015) Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 45:45–56. https://doi.org/10.3233/JAD-141659
Ferrari L et al (2017) Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J Alzheimers Dis 56:1101–1107. https://doi.org/10.3233/jad-160886
Gao L, Liu Y, Li X, Bai Q, Liu P (2015) Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr 60:162–167. https://doi.org/10.1016/j.archger.2014.10.011
den Haan J et al (2019) Retinal thickness as potential biomarker in posterior cortical atrophy and typical Alzheimer’s disease. Alzheimers Res Ther 11:62. https://doi.org/10.1186/s13195-019-0516-x
Oktem EO et al (2015) The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci 36:1141–1146. https://doi.org/10.1007/s10072-014-2055-3
den Haan J, Verbraak FD, Visser PJ, Bouwman FH (2017) Retinal thickness in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement (Amst) 6:162–170. https://doi.org/10.1016/j.dadm.2016.12.014
den Haan J et al (2018) Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer’s disease and controls. Alzheimers Dement (Amst) 10:49–55. https://doi.org/10.1016/j.dadm.2017.10.005
Teunissen CE et al (2022) Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol 21:66–77. https://doi.org/10.1016/S1474-4422(21)00361-6
Olsson B et al (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 15:673–684. https://doi.org/10.1016/S1474-4422(16)00070-3
Brickman AM et al (2021) Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement 17:1353–1364. https://doi.org/10.1002/alz.12301
Xu C, Zhao L, Dong C (2022) A review of application of Abeta42/40 ratio in diagnosis and prognosis of Alzheimer’s disease. J Alzheimers Dis 90:495–512. https://doi.org/10.3233/JAD-220673
Karikari TK et al (2021) Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry 26:429–442. https://doi.org/10.1038/s41380-020-00923-z
Santangelo R et al (2020) Neuro-retina might reflect Alzheimer’s disease stage. J Alzheimers Dis 77:1455–1468. https://doi.org/10.3233/JAD-200043
McKhann GM et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Kac PR et al (2022) Diagnostic value of serum versus plasma phospho-tau for Alzheimer’s disease. Alzheimers Res Ther 14. https://doi.org/10.1186/s13195-022-01011-w
Zhang S et al (2021) Choriocapillaris changes are correlated with disease duration and MoCA score in early-onset dementia. Front Aging Neurosci 13:656750. https://doi.org/10.3389/fnagi.2021.656750
Tewarie P et al (2012) The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE 7:e34823. https://doi.org/10.1371/journal.pone.0034823
Aytulun A et al (2021) APOSTEL 2.0 Recommendations for reporting quantitative optical coherence tomography studies. Neurology 97:68–79. https://doi.org/10.1212/WNL.0000000000012125
den Haan J et al (2019) Retinal thickness as a potential biomarker in patients with amyloid-proven early- and late-onset Alzheimer’s disease. Alzh Dement-Dadm 11:463–471. https://doi.org/10.1016/j.dadm.2019.05.002
Jorge L et al (2020) The retinal inner plexiform synaptic layer mirrors grey matter thickness of primary visual cortex with increased amyloid beta load in early Alzheimer’s disease. Neural Plast 2020:8826087. https://doi.org/10.1155/2020/8826087
Koronyo Y et al (2017) Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2. https://doi.org/10.1172/jci.insight.93621
Ong YT et al (2015) Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neurosci Lett 584:12–16. https://doi.org/10.1016/j.neulet.2014.10.010
Shi H et al (2023) Retinal arterial Abeta(40) deposition is linked with tight junction loss and cerebral amyloid angiopathy in MCI and AD patients. Alzheimers Dement 19:5185–5197. https://doi.org/10.1002/alz.13086
Jack CR Jr et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Leung KK et al (2013) Cerebral atrophy in mild cognitive impairment and Alzheimer disease: rates and acceleration. Neurology 80:648–654. https://doi.org/10.1212/WNL.0b013e318281ccd3
Frenzel S et al (2019) A biomarker for Alzheimer’s disease based on patterns of regional brain atrophy. Front Psychiatry 10:953. https://doi.org/10.3389/fpsyt.2019.00953
Donaldson L, Chen M, Margolin E (2023) Transsynaptic ganglion cell degeneration in adult patients after occipital lobe stroke. J Neuroophthalmol 43:243–247. https://doi.org/10.1097/WNO.0000000000001657
Balk LJ et al (2015) Bidirectional trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. J Neurol Neurosurg Psychiatry 86:419–424. https://doi.org/10.1136/jnnp-2014-308189
Martucci A, Cesareo M, Nucci C, Mancino R (2018) Macular ganglion cells alteration in a patient with left homonymous hemianopia subsequent to surgical excision of an arteriovenous malformation. Am J Ophthalmol Case Rep 12:9–14. https://doi.org/10.1016/j.ajoc.2018.07.009
Rotenstreich Y et al (2022) Thicker macula in asymptomatic APOE E4 middle-aged adults at high AD risk. Alzheimers Dement (Amst) 14:e12275. https://doi.org/10.1002/dad2.12275
O’Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP (2018) Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol 136:1242–1248. https://doi.org/10.1001/jamaophthalmol.2018.3556
Antes R et al (2015) VEGF Mediates ApoE4-induced neovascularization and synaptic pathology in the choroid and retina. Curr Alzheimer Res 12:323–334. https://doi.org/10.2174/1567205012666150325182504
Iseri PK, Altinaş O, Tokay T, Yüksel N (2006) Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol 26:18–24. https://doi.org/10.1097/01.wno.0000204645.56873.26
Kim HM et al (2022) Association between retinal layer thickness and cognitive decline in older adults. JAMA Ophthalmol 140:683–690. https://doi.org/10.1001/jamaophthalmol.2022.1563
Shi Z et al (2016) The utilization of retinal nerve fiber layer thickness to predict cognitive deterioration. J Alzheimers Dis 49:399–405. https://doi.org/10.3233/JAD-150438
Funding
The study was funded by the National Natural Science Foundation of China (92049115), by the Science and Technology Program of Sichuan Province (2023YFS0266), and by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYG D18009).
Author information
Authors and Affiliations
Contributions
Conceptualization: ZZ, SZ, WRK. Methodology: ZZ, WRK, LC, RW. Formal analysis and investigation: ZZ, WRK, LC, ZF, PL, RW. Writing—original draft preparation: ZZ, WRK. Writing—review and editing: ZZ, WRK, SZ. Funding acquisition: BW, SZ. Resources: BW, SZ. Supervision: BW, SZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Biomedical Research Ethics Committee and the Committee on Human Research of West China Hospital, Sichuan University (Ethics Number: 2020–104). Informed consent was obtained from participants or their guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Why carry out this study?
1. The association of between retinal sublayer thickness and AD-related typical blood-based biomarkers and quantitative brain volume measures is underexplored in EOAD patients.
2. Could retinal thickness reflect established AD-related markers changes in blood and neuroimaging?
What was learned from the study?
1. Thickness of pRNFL correlated with serum t-tau level, serum Aβ42 concentration correlated with RNFL thickness, and occipital lobe volume correlated with GCC thicknesses in EOAD patients.
2. Thickness of pRNFL may be a useful marker for assessing serum t-tau level and GCC thickness may be a reflection of occipital lobe atrophy in EOAD.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Kwapong, W.R., Cao, L. et al. Correlation between serum biomarkers, brain volume, and retinal neuronal loss in early-onset Alzheimer’s disease. Neurol Sci 45, 2615–2623 (2024). https://doi.org/10.1007/s10072-023-07256-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07256-z