Abstract
Objectives
To explore the association between uric acid (UA) levels and vascular dementia (VaD) and Parkinson’s disease dementia (PDD), a meta-analysis was conducted.
Methods
The relevant studies were identified by searching PubMed, Embase, Web of Science, and Cochrane Collaboration Database up to May 2022. Pooled analysis, sensitivity analysis, and publication bias examination were all conducted. All analyses were performed by using STATA 16.
Results
Twelve studies with a total of 2097 subjects were included. The pooled analysis showed that UA levels were not associated with VaD (WMD = - 10.99 μmol/L, 95% CI (- 48.05, 26.07), P = 0.561) but were associated with PDD (WMD = - 25.22 μmol/L, 95% CI (- 43.47, - 6.97), P = 0.007). The statistical stability and reliability were evaluated using sensitivity analysis and publication bias outcomes.
Conclusion
UA levels are associated with PDD but not with VaD. This study will help to strengthen our knowledge of the pathophysiologies of VaD and PDD, and promote the development of prevention and treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is characterized as a syndrome rather than a particular disease that can seriously affect an individual’s work and life [1]. Because of its high prevalence, dementia has become a serious public health issue around the world [2]. Vascular dementia (VaD) and Parkinson’s disease dementia (PDD) are both common types of dementia [3]. For a long time, factors related to VaD and PDD have attracted worldwide attention.
Previous studies have reported that dementia is influenced by many factors, including age, gender, educational level, obesity, and disease history [4,5,6]. Some researchers have demonstrated that neuronal injury can be caused by oxidative damage which might affect the pathophysiology of dementia [7,8,9]. Our previous study reported that low antioxidant capacity might be related to VaD, and treatment with antioxidants might mitigate cognitive impairment [10]. Similar results were also obtained in PDD [11].
As the most abundant endogenous antioxidant in blood, uric acid (UA) has been considered to exert neuroprotective effects by removing ROS and nitrite [12, 13]. However, studies have shown that high levels of UA can promote inflammation and oxidation in special environments and may cause neuronal injury [14, 15]. Many researchers have paid close attention to whether UA levels are associated with VaD and PDD, and several studies have been conducted. Nevertheless, no consistent conclusion has been reached on whether UA levels are related to VaD or PDD. Therefore, a meta-analysis was conducted in the study to investigate the association.
Methods
The study was performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search strategy
The PubMed, Embase, Web of Science, and Cochrane Collaboration Database were searched for all relevant citations up to May 2022. The following terms were used for searching: (uric acid OR UA OR urate OR hyperuricemia) AND (vascular dementia OR VaD OR Parkinson’s disease dementia OR PDD OR dementia). No language or other restrictions were applied to the search strategy. References of the retrieved studies were also screened to identify available articles.
Inclusion and exclusion criteria
Criteria for study inclusion were based on the following: (1) the association between UA levels and VaD and/or PDD was involved; (2) it should be a case-control study; (3) subjects in the case group were VaD or PDD patients, while the control group was not; and (4) the study should provide mean and standard deviation (SD) values of UA for each group. When only the median and interquartile ranges were provided, the mean and SD were estimated according to the formula [16].
Studies were excluded according to the following criteria: (1) reviews, case reports or letters; (2) animal or cell studies; and (3) repeated publication of data from the same population.
Data extraction
All data were recorded for further evaluation separately by two reviewers, and any discrepancy was resolved by the third investigator. The following baseline data were collected: first author’s name, year of publication, country, mean age, male percentage, number of patients in the case and control groups, and mean and SD values of UA in each group. Corresponding author was contacted for studies with inadequate information.
Quality assessment
The Newcastle-Ottawa quality assessment scale (NOS) [17], a nine-star system, was used to assess the quality of the included studies. This scale evaluates studies according to selection, comparability and exposure. A study awarded a score ≥ 6 was considered high quality.
Statistical analysis
Weighted mean difference (WMD) and 95% confidence interval (CI) were utilized to evaluate correlation strength between UA levels and VaD/PDD. Statistical heterogeneity was identified by Q statistic and I2 statistic. A random-effect model was applied when heterogeneity was defined as substantial (Q statistic P < 0.05 or I2 ≥ 50%); otherwise, a fixed-effect model was applied [18]. Subgroup analyses were performed according to continent (Asian, European or North American), male percentage (≥ 50% or < 50%) and sample size (≥ 200 or < 200) to detect sources of heterogeneity. The sensitivity analysis was performed by sequentially removing studies. Begg’s test and Egger’s test were applied to identify publication bias [19, 20]. Stata 16 (Stata Corporation, College Station, TX, USA) was utilized to conduct the analyses.
Results
Studies and data included in the meta-analysis
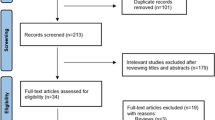
A flow chart of search strategy is displayed in Fig. 1. After screening the 2097 retrieved candidate articles, 12 studies were included [21,22,23,24,25,26,27,28,29,30,31,32]. Detailed information on the included studies is summarized in Table 1. The included studies were published from 1993 to 2020, with sample sizes ranging from 17 to 1135. Seven studies were from Europe, four were from Asia, and one was from North America. According to the quality evaluation, the research design is reasonable and the outcome is clear. No study was excluded from the meta-analysis for reasons of quality.
Pooled and subgroup analyses
There were 10 studies concerning the relationship between UA levels and VaD in the meta-analysis. Overall, UA levels were not associated with VaD (WMD = - 10.99 μmol/L, 95% CI (- 48.05, 26.07), P = 0.561) (Fig. 2(a)). Substantial heterogeneity was discovered among the included studies (I2 = 91.9%, P < 0.001).
Subgroup analysis was conducted for the correlation between UA levels and VaD. In the subgroup analysis, studies were classified by continent, male percentage and sample size. However, there was no significant discrepancy among the subgroups (Table 2).
There were 4 studies concerning the association between UA levels and PDD in the meta-analysis. Overall, UA levels were associated with PDD (WMD = - 25.22 μmol/L, 95% CI (- 43.47, - 6.97), P = 0.007). (Fig. 2(b)). No statistical heterogeneity was discovered among the included studies (I2 = 0.0%, P = 0.475).
Sensitivity analysis
The sensitivity analysis was performed by sequentially removing studies to assess the effect of a unitary study on the pooled WMD. Statistical analysis indicated that the results were stable and reliable (Fig. 3).
Publication bias
No evidence of obvious publication bias existed in the meta-analysis according to Begg’s correlation (VaD: P = 1.000; PDD: P = 0.089) and Egger’s regression (VaD: P = 0.697; PDD: P = 0.182). Additionally, no obvious asymmetry was shown in the shapes of funnel plots.
Discussion
Some studies have shown that oxidative damage might be related to dementia, including VaD and PDD. They have demonstrated that when neurons are exposed to oxidative damage factors (such as hypoxia, inflammatory factors, and aging), a large number of reactive oxygen species (ROS) will be produced. Excessive ROS can lead to oxidative damage, mainly manifested in protein denaturation, DNA breakage, and lipid peroxidation, resulting in neuronal death, which is closely related to the pathophysiologies of VaD and PDD [33, 34].
As a powerful antioxidant, UA has been supposed to alleviate neuronal damage via neuroprotective effects [35, 36]. However, evidence has also shown that high levels of UA are related to hypertension and metabolic syndrome, which might cause dementia by synergistically aggravating arteriosclerosis [37, 38]. Some evidence suggests that as an indicator of redox homeostasis, UA can relieve degenerative cascades in neurodegenerative diseases (such as dementia) [39, 40]. Furthermore, one study showed that exogenous UA might protect neurons from excitotoxic injury and play a neuroprotective role [41].
Ten independent studies were included in the current study, comprising 516 VaD patients and 1618 controls. The pooled analysis revealed that UA levels were not associated with the risk of VaD. We deduced that the antioxidant effect of UA might counteract its role in promoting arteriosclerosis. The pooled analysis showed that UA levels were associated with PDD, which was consistent with the findings of Huang et al. [42].
There are several limitations in this present study. First, the sample size was still relatively small in the meta-analysis, especially in exploring the association between UA and PDD. Second, although subgroup analysis and sensitivity analysis were conducted to explore the association between UA and VaD, the source of heterogeneity was not identified. Third, since only patients who visited the hospital were included in the study, some selection bias might affect the reliability of our findings. Despite the limitations, the statistical power was markedly improved based on substantial data from different studies. The statistical stability and reliability were further confirmed by sensitivity analysis and publication bias outcomes.
In summary, the meta-analysis shows that UA levels are associated with PDD but not with VaD. The study will help to strengthen the understanding of the pathophysiologies of VaD and PDD, and promote the development of prevention and treatment strategies. To obtain a clearer conclusion, more large sample size studies are needed.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Long JM, Day GS (2018) Autoimmune dementia. Semin Neurol 38(3):303–315. https://doi.org/10.1055/s-0038-1660480
Achterberg W, Lautenbacher S, Husebo B et al (2019) Pain in dementia. Pain Rep 5(1):e803. https://doi.org/10.1097/PR9.0000000000000803
Gale SA, Acar D, Daffner KR (2018) Dementia. Am J Med 131(10):1161–1169. https://doi.org/10.1016/j.amjmed.2018.01.022
Flores-Cordero JA, Pérez-Pérez A, Jiménez-Cortegana C et al (2022) Obesity as a risk factor for dementia and Alzheimer’s disease: the role of leptin. Int J Mol Sci 23(9):5202. https://doi.org/10.3390/ijms23095202
Gorelick PB, Scuteri A, Black SE et al (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 42(9):2672–2713. https://doi.org/10.1161/STR.0b013e3182299496
Tariq S, Barber PA (2018) Dementia risk and prevention by targeting modifiable vascular risk factors. J Neurochem 144(5):565–581. https://doi.org/10.1111/jnc.14132
Ilari S, Russo P, Proietti S et al (2022) DNA damage in dementia: evidence from patients affected by severe Chronic Obstructive Pulmonary Disease (COPD) and meta-analysis of most recent literature. Mutat Res Genet Toxicol Environ Mutagen 878:503499. https://doi.org/10.1016/j.mrgentox.2022.503499
Loh KP, Huang SH, De Silva R et al (2006) Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res 3(4):327–337. https://doi.org/10.2174/156720506778249515
Castagne V, Gautschi M, Lefevre K et al (1999) Relationships between neuronal death and the cellular redox status. Focus on the developing nervous system. Prog Neurobio 59(4):397–423. https://doi.org/10.1016/s0301-0082(99)00012-x
Hou X, Xu H, Chen W et al (2020) Neuroprotective effect of dimethyl fumarate on cognitive impairment induced by ischemic stroke. Ann Transl Med 8(6):375. https://doi.org/10.21037/atm.2020.02.10
Annanmaki T, Pohja M, Parviainen T et al (2011) Uric acid and cognition in Parkinson’s disease: a follow-up study. Parkinsonism Relat Disord 17:333–337. https://doi.org/10.1016/j.parkreldis.2011.01.013
Becker BF (1993) Towards the physiological function of uric acid. Free Radic Biol Med 14(6):615–631. https://doi.org/10.1016/0891-5849(93)90143-i
Squadrito GL, Cueto R, Splenser AE et al (2000) Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys 376(2):333–337. https://doi.org/10.1006/abbi.2000.1721
Santos CX, Anjos EI, Augusto O (1999) Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys 372(2):285–294. https://doi.org/10.1006/abbi.1999.1491
Seet RC, Kasiman K, Gruber J et al (2010) Is uric acid protective or deleterious in acute ischemic stroke? A prospective cohort study. Atherosclerosis 209(1):215–219. https://doi.org/10.1016/j.atherosclerosis.2009.08.012
Wan X, Wang W, Liu J (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Serdarevic N, Stanciu AE, Begic L et al (2020) Serum uric acid concentration in patients with cerebrovascular disease (ischemic stroke and vascular dementia). Med Arch 74(2):95–99. https://doi.org/10.5455/medarh.2020.74.95-99
Liu H, Reynolds GP, Wei X (2020) Uric acid and high-density lipoprotein cholesterol are differently associated with Alzheimer’s disease and vascular dementia. J Alzheimers Dis 73(3):1125–1131. https://doi.org/10.3233/JAD-191111
Tuven B, Soysal P, Unutmaz G et al (2017) Uric acid may be protective against cognitive impairment in older adults, but only in those without cardiovascular risk factors. Exp Gerontol 89:15–19. https://doi.org/10.1016/j.exger.2017.01.002
Xu Y, Wang Q, Cui R et al (2016) Uric acid is associated with vascular dementia in Chinese population. Brain Behav 7(2):e00617. https://doi.org/10.1002/brb3.617
Hatanaka H, Hanyu H, Fukasawa R et al (2015) Differences in peripheral oxidative stress markers in Alzheimer’s disease, vascular dementia and mixed dementia patients. Geriatr Gerontol Int 15(1):53–58. https://doi.org/10.1111/ggi.12659
Cervellati C, Romani A, Seripa D et al (2014) Oxidative balance, homocysteine, and uric acid levels in older patients with late onset Alzheimer’s disease or vascular dementia. J Neurol Sci 337(1–2):156–161. https://doi.org/10.1016/j.jns.2013.11.041
González-Aramburu I, Sánchez-Juan P, Sierra M et al (2014) Serum uric acid and risk of dementia in Parkinson’s disease. Parkinsonism Relat Disord 20(6):637–639. https://doi.org/10.1016/j.parkreldis.2014.02.023
Maetzler W, Stapf AK, Schulte C et al (2011) Serum and cerebrospinal fluid uric acid levels in lewy body disorders: associations with disease occurrence and amyloid-β pathway. J Alzheimers Dis 27(1):119–126. https://doi.org/10.3233/JAD-2011-110587
Polidori MC, Mattioli P, Aldred S et al (2004) Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dement Geriatr Cogn Disord 18(3–4):265–270. https://doi.org/10.1159/000080027
Foy CJ, Passmore AP, Vahidassr MD et al (1999) Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. QJM 92(1):39–45. https://doi.org/10.1093/qjmed/92.1.39
Tohgi H, Abe T, Takahashi S et al (1993) The urate and xanthine concentrations in the cerebrospinal fluid in patients with vascular dementia of the Binswanger type, Alzheimer type dementia, and Parkinson’s disease. J Neural Transm Park Dis Dement Sect 6(2):119–126. https://doi.org/10.1007/BF02261005
Maesaka JK, Wolf-Klein G, Piccione JM et al (1993) Hypouricemia, abnormal renal tubular urate transport, and plasma natriuretic factor(s) in patients with Alzheimer’s disease. J Am Geriatr Soc 41(5):501–506. https://doi.org/10.1111/j.1532-5415.1993.tb01885.x
Davies MJ (2005) The oxidative environment and protein damage. Biochim Biophys Acta 1703(2):93–109. https://doi.org/10.1016/j.bbapap.2004.08.007
Shao A, Lin D, Wang L et al (2020) Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis 11(6):1537–1566. https://doi.org/10.14336/AD.2020.0225
Yu ZF, Bruce-Keller AJ, Goodman Y et al (1998) Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res 53(5):613–625. https://doi.org/10.1002/(SICI)1097-4547(19980901)53:5%3c613::AID-JNR11%3e3.0.CO;2-1
Chamorro A, Obach V, Cervera A et al (2002) Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke 33(4):1048–1052. https://doi.org/10.1161/hs0402.105927
Borghi C, Rosei EA, Bardin T et al (2015) Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 33(9):1729–1741. https://doi.org/10.1097/HJH.0000000000000701
Raffaitin C, Gin H, Empana JP et al (2009) Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City Study. Diabetes Care 32(1):169–174. https://doi.org/10.2337/dc08-0272
Vannorsdall TD, Jinnah HA, Gordon B et al (2008) Cerebral ischemia mediates the effect of serum uric acid on cognitive function. Stroke 39(12):3418–3420. https://doi.org/10.1161/STROKEAHA.108.521591
Paganoni S, Schwarzschild MA (2017) Urate as a marker of risk and progression of neurodegenerative disease. Neurotherapeutics 14(1):148–153. https://doi.org/10.1007/s13311-016-0497-4
Beydoun MA, Beydoun HA, Gamaldo AA et al (2014) Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 14:643. https://doi.org/10.1186/1471-2458-14-643
Moccia M, Picillo M, Erro R et al (2015) Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson’s disease. Eur J Neurol 22(1):93–98. https://doi.org/10.1111/ene.12533
Acknowledgements
We are appreciative of all the participants in this study.
Author information
Authors and Affiliations
Contributions
Qian Li wrote the first draft, searched the literature, and extracted the data. Kaiwen Cen and Ying Cui searched the literature and extracted the data. Xu Feng conducted the data analysis. Xiaowen Hou designed the study and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Cen, K., Cui, Y. et al. Uric acid levels and their association with vascular dementia and Parkinson’s disease dementia: a meta-analysis. Neurol Sci 44, 2017–2024 (2023). https://doi.org/10.1007/s10072-023-06620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06620-3