Abstract
Ischemic stroke (IS) is a major cause of mortality and disability. However, no reliable prognostic or diagnostic biomarker has been utilized to date. Here, we have evaluated the serum S100B concentration and miR-602 expression as potential biomarkers for IS. Fifty-two IS patients and 52 age- and sex-matched healthy volunteers were enrolled. Blood samples were collected from all patients at the time of admission, 24 and 48 h later, at the time of discharge, and 3 months later. Real-time (RT) PCR was used to measure the serum level of miR602. We also measured the serum concentration of S100B using ELISA. As compared with healthy subjects, IS patients had a higher level of serum S100B and lower serum miR-602. ROC curve analyses revealed that miR-602 (AUC = 0.8168; P < 0.0001) and S100B (AUC = 0.8699; P < 0.0001) had acceptable ability to differentiate between IS patients from healthy subjects. Furthermore, serum S100B was a reliable predictor of the survival outcome at 3 months (P = 0.021). The expression of miR-602 was significantly higher in patients with bigger NIHSS scores. The lower levels of miR-602 and higher concentration of S100B in the sera of IS patients could be associated with clinically significant diagnostic utilities. S100B could be also introduced as a reliable prognostic marker for stroke and implemented in future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke (IS) is known as one of the main causes of mortality and disability in the world [1]. Despite significant progress having been made in the diagnosis of stroke, namely in imaging modalities, differentiating it from stroke mimic has remained a challenging issue. Some studies showed the prognostic and diagnostic roles of biomarkers, including interleukin-6 (IL-6), D-dimer, and C-reactive protein (CRP) in ischemic stroke [2,3,4].
S100 calcium-binding protein B (S100B) is a Ca2+-binding protein (MW 21 kD), expressed in schwann and astroglial cells [5]. Several reports have indicated an increased expression of S100B in IS patients [6]. Serum S100B could be a promising diagnostic biomarker [7] with prognostic value to predict the outcome of IS [8, 9].
MicroRNAs (miRNAs) are short (17–25 nucleotides) non-coding ribonucleic acids which are capable of modulating several cellular processes including protein expression by binding to complementary or partially complementary target sites in the 3′-untranslated regions (3′-UTRs) of messenger RNA (mRNA) [10, 11]. Circulating miRNAs in the serum have been reported to be stable, reproducible, and consistent among individuals and could be used in clinical settings [10, 12]. There is thriving evidence in favor of the role of miRNAs in modulating various IS-related biological mechanisms, such as inflammation, hypertension, atherosclerosis, and plaque rupture [13]. The expression of miRNAs in IS is also significant; the association of selected miRNAs have been demonstrated with the clinical subgroups of stroke that could be used as potential biomarkers [14]. The miRBase database (http://www.mirbase.org) has been used for the identification of miRNAs in humans and other species. Recent studies have shown that microRNAs target proteins. We have recently observed that the target protein of miR-602 was S100B (http://www.targetscan.org/vert_72/), and the expression of S100B is regulated by miR-602. Other studies have shown alterations in the expression of miR-602 [15] and S100B in stroke patients.
Although S100B has been introduced as an IS biomarker in limited studies, to the extent of our knowledge, no research has addressed the diagnostic and prognostic utility of miR-602 which controls the expression of S100B. Therefore, we aimed to evaluate serum concentrations of miR-602 and its target protein, S100B in stroke patients, and healthy subjects. We also assessed the diagnostic and prognostic values of miR-602 and S100B in IS.
Materials and methods
Study population and sample collection
We recruited fifty-two patients with IS from the Department of Neurology in Sayyad Shirazi Hospital, Gorgan, Iran. Stroke was diagnosed in accordance with the neurological deficit World Health Organization [16], CT scan, MRI, and related risk factors (history of hypertension, history of diabetes, smoking, etc.) [17]. We excluded all cases with a history of malignancy, acute or chronic infectious diseases, autoimmune disorders, renal or liver failure, and recurrent stroke. We also enrolled 52 age- and sex-matched healthy volunteers in the control group. Individuals with a medical family history of cardiovascular disorders, stroke, kidney or liver failure, and diabetes were also excluded. The study was approved by the Committee of Ethics, Golestan University of Medical Sciences (IR.GOUMS.REC.1395.23). All patients signed a written informed consent according to the declaration of Helsinki [6]. All clinical characteristics and laboratory data are shown in Table 1.
The modified Rankin Scale (mRS), which is a reliable measure for evaluating the level of dependence or disability in the activities of individuals incurred a stroke, was evaluated in all patients and used for further analyses. To examine the degree of IS, the National Institutes of Health Stroke Scale (NIHSS) system was used [18]. Accordingly, stroke patients with NIHSS ≤ 5 were categorized in NIHSS 1 group. Moderate stroke patients with scores between 6 and 13 were put in NIHSS 2 group and severe stroke patients with scores ≥ 13 were allocated in NIHSS 3 group.
We collected blood samples (serum) at five time points: (A) 0–12 h after stroke (at the time of admission), (B) 24 h after stroke, (C) 48 h after stroke, (D) at the time of discharge, and (E) 3 months after stroke. The blood samples were moved to the research laboratory in the Department of Biochemistry. We separated sera according to Alatas’s method [19] and transferred into two separate microtubes, kept at − 70 °C until analysis. The rest of the separated sera were immediately processed for the total RNA extraction.
RNA extraction and quantitative real-time PCR
The isolation of total RNA was performed using TRIzol LS reagent (Invitrogen, Carlsbad, USA) from separated sera of all individuals following established protocols [20]. The quality and quantity of extracted RNA were assessed using spectrophotometry (Picodrop, UK). A specific miR-602 and an endogenous U6 stem-loop control primer were used for the reverse transcription of 1 μg total RNA to cDNA based on the manufacturer’s guidelines of the thermo scientific kit (K1622, San Jose, CA, USA). Afterwards, cDNA was stored at − 20 °C.
Quantitative real-time PCR was performed using a fast real-time PCR system (lightcycler96, Roche, USA) using a RealQ Plus Master Mix Green (Ampliqon, Denmark). The final reaction volume was 20 μL containing miR-602 or U6 RT reaction product (3 μl), specific forward primer [miR-602 (5′-AGGACACGGGAGACAGC-3′) and U6 (5′-AAGGATGACACGCAAATTC-3′)] (1 μl), reverse primer (5′-GAGCAGGGTCCGAGGT-3′) (1 μl), RealQ Plus Master Mix Green (10 μl), and DEPC water (5 μl). A 96-well plate format was used with the following protocol: 95 °C for 15 min, followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. Eventually, the relative miR-602 level was normalized to the expression of the endogenous control U6 for each single sample in triplicates and was calculated using the 2−Δct method.
S100B ELISA assay
A commercially available ELISA kit (Elabscience Houston, Texas, USA) was used to measure serum S100B according to the protocols. Chromate 4300 (Biotek, USA) was applied to examine the optical densities (ODs) of all samples at the wavelength of 450 nm versus the reference wavelength of 630 nm. All of the experiments for each single sample were conducted in duplicates and the results were reported as picograms of S100B per ml.
Statistical analysis
Receiver operator characteristic (ROC) curve analysis was performed to determine the diagnostic utility of miR-602 and S100B. In order to model the relationship between a scalar response and one or more explanatory variables (or independent variables), linear regression was used. Two-way ANOVA or Kruskal–Wallis and relevant post hoc tests were conducted to compare the means between more than two groups. Kaplan–Meier survival analyses were performed after following the outcome of the disease (death or survival) to inspect the prognostic values of the selected variables in IS patients, and relevant plots were drawn. We used means and standard deviation to illustrate quantitative statistics, numbers, and percentages to represent qualitative data. In order to compare the demographic variables including age, blood pressure, and sex, T test and chi-square were used. The P values beneath 0.05 were granted as statistically significant. We evaluated the time course for the expression of the putative biomarkers, miR-602 and S100B, at five time points.
Results
The expression of miR-602 and S100B at time of admission
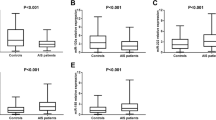
We found that serum miR-602 was lower in the IS patients compared with the normal controls (P < 0.0001) (Fig. 1a), and that serum S100B was significantly higher in patients in comparison with the healthy controls (P < 0.0001) (Fig. 1b). Serum levels of both S100B and miR-602 did not alter significantly at different time points until the end of the follow-up schedule (Fig. 2).
The serum concentration of miR-602 and S100B levels at the time of admission. miR-602 is downregulated in IS patients (a), while S100B is expressed in higher quantities (b). The calculations of the expression levels for miR-602 were conducted by 2−ΔCt method. To compare the means between two groups, Students’ T test was used (patients 52, healthy subjects 52). Statistics on each scattered plot demonstrates mean ± SD. Level of significant P values were 0.05
The serum concentration of miR-602 and S100B in 5 different time points. miR-602 serum levels did not alter (a), while S100B serum levels did not change significantly in all 5 time points among IS patients (b). The calculations of the expression levels for miR-602 were conducted by 2−ΔCt method. One-way ANOVA and Tukey’s post-test or Kruskal–Wallis and Dunn–Bonferroni post-test were used to evaluate the differences between the means of various groups. (patients 52, healthy subjects 52). Bar charts show mean ± SD for each value. Level of significant P values were 0.05
S100B and miR-602 as suitable diagnostic markers
The area under the curve (AUC) for the expression of miR-602 was 0.8168 (95% CI 0.7281–0.9054; P < 0.0001). The cut-off point was set at the fold change level of 0.1290 with the sensitivity of 78.85% (95% CI 65.30–88.94), the specificity of 84.62% (95% CI 71.92–93.12), and likelihood ratio (LR) of 5.125 (Fig. 3a). Similarly, the calculated AUC for S100B was 0.8699 (95% CI 0.7981–0.9417; P < 0.0001). The cut-off value was set at the optimum level of 39.99 ng/ml with the sensitivity of 76.47% (95% CI 62.51–87.21), the specificity of 82.69% (95% CI 69.67–91.77), and likelihood ratio (LR) of 4.192 (Fig. 3b).
ROC curve analyses. Area under the curve (AUC) for miR-602 was 0.8168 (P < 0.0001). Setting the cut-off value (blue arrows) at 0.1290 gave a sensitivity of 78.85%, specificity of 84.62%, and likelihood ratio (LR) of 5.125 (a). AUC for S100B was 0.8699 (P < 0.0001). The optimum cut-off value at the level of 39.99 gave a sensitivity of 76.47%, specificity of 82.69%, and likelihood ratio (LR) of 4.192 (b). All values are the expression levels at the time of zero (initial referring to the hospital)
S100B and miR-602 were reliable predictors of IS prognosis
The levels of both biomarkers were subdivided into two categories of low and high levels based on the optimum cut-off points derived from ROC curve analyses. Log-rank test results revealed that miR-602 was not capable of predicting the outcome (death; N, 26) of IS (P = 0.6863) (Fig. 4a), while the low levels of S100B were associated with good prognosis in IS patients (P = 0.0212) (Fig. 4b). We also found a negative but weak correlation between the miR-602 and S100B upon admission (rs = − 0.264, P = 0.007).
Survival analyses. IS patients were subdivided into two categories of high and low expression on the basis of the optimum cut-off points of suggested biomarkers. Log-rank test results showed that miR-602 was not able to predict the outcome (death) of the disease after 90 days of follow-up (a), while the lower expression of S100B was associated with a good prognosis of the disease (b)
miR-602 was reduced in IS patients with the maximum modified Rankin Scale
In order to measure the expression of selected variables and determine the association of S100B and miR-602 with the disability status of IS patients, a modified Rankin Scale (mRS scoring ( was utilized. Although not statistically significant, the expression of miR-602 was decreased among IS patients with the maximum disability (mRS = 5). However, S100B was not varied between different groups of mRS (Fig. 5).
Alterations of S100B and miR-602 after 3 months of follow-up in different categories of mRS. miR-602 was downregulated in IS patients of the MRS 5 category (a), while no remarkable change was observed in the expression of S100B between 5 groups of MRS (b). IS patients falling in the 6th category of mRS had passed away and no record of S100B or miR-602 was available. One-way ANOVA and Tukey’s post-test or Kruskal–Wallis and Dunn–Bonferroni post-test were used to evaluate the differences between the means of various groups. Bar charts show mean ± SD for each value. Level of significant P values were 0.05
The overexpression of miR-602 in IS patients with higher NIHSS scores
Patients were categorized into three different groups according to their NIHSS scores, as abovementioned. The expression of miR-602 was significantly higher in patients in NIHSS 2 (P < 0.05) and NIHSS 3 (P < 0.01) subgroups (Fig. 6a). However, no significant difference was shown between various subgroups of NIHSS regarding the serum concentration of S100B (Fig. 6b).
The expression of S100B and miR-602 in different groups of NIHSS at the time of admission. miR-602 was significantly overexpressed in NIHSS subgroups with higher scores (a), while S100B was not altered in different subgroups of NIHSS (b). Kruskal–Wallis and Dunn–Bonferroni post-test were used to evaluate the differences between the means of various groups. Bar charts show mean ± SD for each value. Level of significant P values were 0.05
Discussion
A reliable diagnosis of IS at early stages could reduce the complications of the disease and increase the survival rate [20]. However, there remains a lack of a reliable diagnostic and prognostic marker for IS, with just the current reliance on clinical examinations and imaging. There are two main types of biomarkers: exposure biomarkers which are utilized in risk prediction, and disease biomarkers in screening, diagnosis, and monitoring of disease progression [21, 22]. Some studies have previously indicated that biomarkers of IS include neuron-specific enolase (NSE), myelin basic protein (MBP), matrix metallopeptidase 9 (MMP-9), glial fibrillary acidic protein (GFAP), interleukin 6 (IL-6), and D-Dimer [23]. However, none of these biomarkers have gained sufficient support to be included in the routine laboratory repertoire.
Recently, there has been rising evidence that serum S100B may be a unique biomarker for the diagnosis of IS [24]. Serum protein S100B has been also introduced a diagnostic biomarker for distinguishing posterior circulation stroke from Vertigo of nonvascular causes [25]. The mechanisms leading to the increased levels of serum S100B are unknown [26]. Moreover, numerous researches have diversely shown alterations of microRNAs in the central nervous system following injury and their involvement in apoptosis, inflammatory neuronal death, and oxidative stress. Recently, microRNAs especially circulating microRNAs have been introduced as sensitive biomarkers for brain damage [5, 6, 27]. Therefore, circulating microRNAs could be applicable biomarkers for the diagnosis of IS and monitoring the outcome of the disease. Here, we evaluated the expression of circulating miR-602 and its potential target (S100B) as appropriate diagnostic and prognostic markers for ischemic stroke.
We found that serum miR-602 levels in IS patients were less than their counterparts in healthy controls. However, no indicative difference in serum miR-602 expression was observed between different time points of follow-up. To the extent of our knowledge, this is the first report of the serum miR-602 after 3 months of follow-up following IS. We also found that the serum level of S100B was increased in IS patients compared with the normal controls. S100B levels were not essentially changed at the five time intervals after stroke. Other studies have also reported that serum S100B is significantly higher in IS and intracranial hemorrhage patients [5, 22, 23]. The results by ROC curve analyses implied that miR-602 and S100B had admissible sensitivities for the diagnosis of ischemic stroke in patients. They could be favorable serum biomarkers for the diagnosis of ischemic stroke in association with the previous reports [28, 29].
Kaplan–Meier plots (log-rank) analysis of survival rates revealed that miR-602 was not able to predict the outcome (death) of IS, while serum S100B was a powerful prognostic marker for IS in our study. Although miR-602 is a prognostic biomarker in other diseases such as glioblastoma and pancreatic ductal adenocarcinoma [22, 30], other studies have demonstrated that S100B is of prognostic value in IS [31, 32]. Several studies have demonstrated that elevator S100B serum levels could be negatively correlated with IS, suggesting that higher serum levels of S100B could lead to more serious condition and worse prognoses for IS patients [33]. We also demonstrated a negative but weak correlation between the serum expression of miR-602 and S100B upon admission, which may indicate the regulatory effects of miR-602 on the expression of S100B. Here, we have evaluated the alterations of miR-602 and S100B at different time points of mRS after 3 months of following up and found that miR-602 was expressed in lower levels among IS patients with the maximum disability.
The NIHSS is a 15-item scale that measures the standards and criteria for basic neurologic examination and focuses on aspects most related to stroke [34]. The NIHSS provides an ordinal, nonlinear measure of acute stroke-related impairments by assigning values to various aspects of the function of the brain [6]. Most stroke patients with NIHSS ≤ 5 (NIHSS 1) were discharged. Moderate stroke patients with scores between 6 and 13 (NIHSS 2) went to acute rehabilitation, and severe stroke patients with scores ≥ 13 (NIHSS 3) required nursing facility (NF) placement [34]. In contrast to the previous findings which have demonstrated a correlation between National Institutes of Health Stroke Scale (NIHSS) at admission time and plasma S100B levels [6], we showed no significant correlation, which could be due to the limited samples size of our study. Although we expected trending changes in the expression of S100B among different groups of NIHSS, limited sample size could confine the conclusion and more compelling data is needed to confirm or reject our findings. Moreover, we found a significant correlation between miR-602 and NIHSS; miR-602 was overexpressed in higher NIHSS scores. However, the overexpression of miR-602 in patient groups with moderate and higher NIHSS could not thoroughly reflect the severity of the disease due to the lack of sample size in the NIHSS 2 (6 IS patients) and 3 (11 IS patients) groups and further investigations are needed in this regard.
In conclusion, we aimed to investigate the diagnostic and prognostic accuracy of miR-602 and S100B as novel biomarkers for IS. We found that serum miR-602 was lower in patients with IS, and serum S100B was higher in these patients, which may be associated with clinically significant diagnostic utilities. Although miR-602 was not capable of predicting the outcome of IS, the level of S100B in the sera could be introduced as a powerful prognostic marker for stroke. It will be useful in future studies to establish whether these findings are specific to IS, or whether there are differences for hemorrhagic stroke.
References
Béjot Y, Daubail B, Giroud M (2016) Epidemiology of stroke and transient ischemic attacks: current knowledge and perspectives. Rev Neurol 172(1):59–68
Di Napoli M, Schwaninger M, Cappelli R et al (2005) Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 6(6):1316–1329
Schafer BW, Heizmann CW (1996) The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci 21(4):134–140
Wunderlich MT, Ebert AD, Kratz T et al (1999) Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke. 30(6):1190–1195
Park SY, Kim MH, Kim OJ, Ahn HJ, Song JY, Jeong JY, Oh SH (2013) Plasma heart-type fatty acid binding protein level in acute ischemic stroke: comparative analysis with plasma S100B level for diagnosis of stroke and prediction of long-term clinical outcome. Clin Neurol Neurosurg 115(4):405–410
Alatas OD, Gurger M, Atescelik M et al (2015) Neuron-specific enolase, S100 calcium-binding protein B, and heat shock protein 70 levels in patients with intracranial hemorrhage. Medicine. 94(45):e2007
Gonzalez-Garcia S, Gonzalez-Quevedo A, Fernandez-Concepcion O et al (2012) Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem 45(16–17):1302–1307
Lagos-Quintana M, Rauhut R, Lendeckel W et al (2001) Identification of novel genes coding for small expressed RNAs. Science 294(5543):853–858
Kouhkan F, Mobarra N, Soufi-Zomorrod M, Keramati F, Hosseini Rad SM, Fathi-Roudsari M, Tavakoli R, Hajarizadeh A, Ziaei S, Lahmi R, Hanif H, Soleimani M (2016) MicroRNA-129-1 acts as tumour suppressor and induces cell cycle arrest of GBM cancer cells through targeting IGF2BP3 and MAPK1. J Med Genet 53(1):24–33
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18(10):997–1006
Koutsis G, Siasos G, Spengos K (2013) The emerging role of microRNA in stroke. Curr Top Med Chem 13(13):1573–1588
Hogg DR, Harries LW (2014) Human genetic variation and its effect on miRNA biogenesis, activity and function. Biochem Soc Trans 42(4):1184–1189
Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K (2009) Expression profile of microRNAs in young stroke patients. PLoS One 4(11):e7689
Hatano S (1976) Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 54(5):541–553
Azarpazhooh MR, Etemadi MM, Donnan GA, Mokhber N, Majdi MR, Ghayour-Mobarhan M, Ghandehary K, Farzadfard MT, Kiani R, Panahandeh M, Thrift AG (2010) Excessive incidence of stroke in Iran: evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke. 41(1):e3–e10
WM Association (2013) World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Bruno A, Saha C, Williams LS (2009) Percent change on the National Institutes of Health Stroke Scale: a useful acute stroke outcome measure. J Stroke Cerebrovasc Dis 18(1):56–59
Kroh EM, Parkin RK, Mitchell PS, Tewari M (2010) Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods. 50(4):298–301
Prugger C, Luc G, Haas B et al (2013) Multiple biomarkers for the prediction of ischemic stroke: the PRIME study. Arterioscler Thromb Vasc Biol 33(3):659–666
Mayeux R (2004) Biomarkers: potential uses and limitations. NeuroRx 1(2):182–188
Jickling GC, Sharp FR (2011) Blood biomarkers of ischemic stroke. Neurotherapeutics 8(3):349–360
Selcuk O, Yayla V, Cabalar M et al (2014) The relationship of serum S100B levels with infarction size and clinical outcome in acute ischemic stroke patients. Noro Psikiyatr Ars 51(4):395–400
Lu YL, Wang R, Huang HT et al (2018) Association of S100B polymorphisms and serum S100B with risk of ischemic stroke in a Chinese population. Sci Rep 8(1):971
Vemuganti R (2013) All’s well that transcribes well: non-coding RNAs and post-stroke brain damage. Neurochem Int 63(5):438–449
Purrucker JC, Herrmann O, Lutsch JK et al (2014) Serum protein S100β is a diagnostic biomarker for distinguishing posterior circulation stroke from vertigo of nonvascular causes. Eur Neurol 72(5–6):278–284
Vijayan M, Reddy PH (2016) Peripheral biomarkers of stroke: focus on circulatory microRNAs. Biochim Biophys Acta 1862(10):1984–1993
Kouhkan F, Soleimani M, Daliri M, Behmanesh M, Mobarra N, Mossahebi Mohammadi M, Mohammad S, Mokhtari M, Lahmy R (2013) miR-451 up-regulation, induce erythroid differentiation of CD133+cells independent of cytokine cocktails. Iran J Basic Med Sci 16(6):756–763
Su Q, Zhu EC, Qu YL et al (2018) Serum level of co-expressed hub miRNAs as diagnostic and prognostic biomarkers for pancreatic ductal adenocarcinoma. J Cancer 9(21):3991–3999
Li R, Gao K, Luo H et al (2014) Identification of intrinsic subtype-specific prognostic microRNAs in primary glioblastoma. J Exp Clin Cancer Res 33:9
Goyal A, Failla MD, Niyonkuru C, Amin K, Fabio A, Berger RP, Wagner AK (2013) S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J Neurotrauma 30(11):946–957
Harrison JK, McArthur KS, Quinn TJ (2013) Assessment scales in stroke: clinimetric and clinical considerations. Clin Interv Aging 8:201–211
Goldstein LB, Bertels C, Davis JN (1989) Interrater reliability of the NIH stroke scale. Arch Neurol 46(6):660–662
Ye H, Wang L, Yang X-K et al (2015) Serum S100B levels may be associated with cerebral infarction: a meta-analysis. J Neurol Sci 348(1–2):81–88
Schlegel D, Kolb SJ, Luciano JM, Tovar JM, Cucchiara BL, Liebeskind DS, Kasner SE (2003) Utility of the NIH stroke scale as a predictor of hospital disposition. Stroke. 34(1):134–137
Acknowledgments
Data for this article was derived from a M.Sc. thesis in the field of Clinical Biochemistry written by Mina Rahmati at Gorgan School of Medicine of Golestan University of Medical Sciences, Gorgan, Iran.
Funding
The current study was financially supported by the Deputy of Research and Technology, Golestan University of Medical Sciences (Grant number: 960427092).
Author information
Authors and Affiliations
Contributions
MR: acquisition of data, analyses and interpretations of data, manuscript drafting, revision of the manuscript. MRAP: participation in data acquisition. HE: participation in data analysis. GAF: participation in data acquisition and manuscript drafting. MGM: participation in data acquisition and manuscript drafting. HG: participation in data acquisition. NM: study design and concept, participation in literature bibliography, data acquisition and analysis, manuscript drafting, and critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the Committee of Ethics, Golestan University of Medical Sciences (IR.GOUMS.REC.1395.23). All patients signed a written informed consent according to the declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahmati, M., Azarpazhooh, M.R., Ehteram, H. et al. The elevation of S100B and downregulation of circulating miR-602 in the sera of ischemic stroke (IS) patients: the emergence of novel diagnostic and prognostic markers. Neurol Sci 41, 2185–2192 (2020). https://doi.org/10.1007/s10072-020-04323-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04323-7