Abstract
Previous studies failed to detect reduced value of the amplitude of low frequency fluctuation (ALFF) derived from resting state functional magnetic resonance imaging in the primary motor cortex in amyotrophic lateral sclerosis (ALS) though primary motor cortex was mainly affected with ALS. We aimed to investigate the cause of masking the abnormality in the primary motor cortex in ALS and usefulness of ALFF for differential diagnosis among diseases showing muscle weakness. We enrolled ten patients with ALS and eleven disease controls showing muscle weakness. Voxel-wise analysis revealed that significant reduction of ALFF value was present in the right sensorimotor cortex in ALS. There was a significant negative correlation between ALFF value in the right sensorimotor cortex and fractional anisotropy (FA) value in the posterior limbs of the internal capsule (PLIC). For a diagnostic tool, the area under receiver operating characteristic curve improved if the ALS patients with disease duration >1 year were excluded. The present findings raised the possibility of usefulness of ALFF value in the sensorimotor cortex for differential diagnosis of ALS, and supported the notion that adjustment for FA value in the PLIC could improve accuracy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blood oxygen level dependent (BOLD) signal is acquired using functional magnetic resonance imaging (fMRI). Resting state fMRI (RSfMRI) is an emerging technique to assess neuronal activity in brain, and many algorithms have been developed to analyze BOLD signal. The amplitude of low frequency fluctuation (ALFF) in BOLD signal was found to be more increased in gray matter than white matter [1, 2], and is thought to reflect the spontaneous neural function of the brain. Therefore, ALFF is accepted as an useful approach to detect the abnormalities in gray matter in neurodegenerative diseases. Indeed, this approach revealed the decreased value of ALFF in the posterior cingulate cortex, precuneus, inferior parietal and temporal lobe in Alzheimer’s disease [3], and decreased value of ALFF in the striatum [4], the supplementary motor cortex, the mesial prefrontal cortex, the middle frontal gyrus and the cerebellum in Parkinson’s disease (PD) [5]. In addition, several studies investigated brain activity in the PD patients under various conditions using ALFF [6–8]. However, in terms of amyotrophic lateral sclerosis (ALS), previous studies failed to detect reduced value of ALFF in the primary motor cortex [9, 10]. Primary motor cortex is mainly affected with ALS, which causes upper motor neuron signs [11]. The revised El Escorial criteria have been accepted as a tool for a clinical diagnosis, and required upper and lower motor neuron signs [12]. We aimed to investigate the cause of masking the abnormality of ALFF in the primary motor cortex in ALS and usefulness for differential diagnosis among diseases showing muscle weakness.
Materials and methods
Subjects
All subjects were recruited from the Department of Neurology of the Tokushima University Hospital over the period September 2014–September 2015. Ten patients with ALS [8 men and 2 women, age 70 ± 2.6 (mean ± SE)] were diagnosed according to the revised El Escorial criteria for clinically possible, probable-laboratory-supported, probable or definite ALS, and enrolled in this study. We evaluated age at scan, sex, age at onset, disease duration, onset form, prominently involved side and amyotrophic lateral sclerosis functional rating scale revised (ALSFRS-R). The definition of disease duration was from the onset of motor symptom to the time of magnetic resonance imaging (MRI) scan. Eleven disease controls (DC) were enrolled from the viewpoint of a differential diagnosis of muscle weakness [6 men and 5 women, age 69 ± 4.2 (mean ± SE)]. The control disorders were myasthenia gravis (MG), multifocal motor neuropathy (MMN), cervical spondylosis (CS) and lumbar spondylosis (LS). The diagnosis of MG was made based on clinical features and positivity for one or more of the following tests: edrophonium test, electrophysiological tests (i.e., repetitive nerve stimulation test and/or single-fiber electromyography), and presence of antibodies against the acetylcholine receptor or muscle-specific tyrosine kinase. Patients with MMN were diagnosed according to internationally accepted criteria [13]. Patients with CS or LS showed spondylosis in cervical or lumbar spine without any abnormalities in other regions. MRI did not reveal clear abnormality in control brains, such as stroke or brain tumor. Clinical characteristics of these subjects are summarized in Table 1. Informed consent was obtained from all participants under protocols approved by the local ethical committee.
MRI acquisition
Images were acquired in a 3.0 T Discovery 750 scanner (GE, Milwaukee, WI) with a standard head coil.
For RSfMRI, scan parameters were as follows: the field of view (FOV), 240 mm; matrix, 64 × 64; TR, 2000 ms; TE, 27.2 ms; flip angle, 77°; slice thickness, 3.0 mm. For T1-weighted images scan parameters were as follows: the field of view (FOV), 240 mm; matrix, 256 × 256; TR, 7.77 ms; TE, 2.98 ms; flip angle, 8°; slice thickness, 0.9 mm. DTI were acquired with 33 diffusion gradient directions. Scan parameters were as follows: b value, 800 s/mm2; FOV, 240 mm; matrix, 128 × 128; TR, 15,000 ms; TE, 84.9 ms; flip angle, 90°; slice thickness, 2.5 mm.
Functional MRI and DTI analyses
The detailed method for the analyses except for ALFF calculation was described elsewhere [14, 15]. For fMRI, all volumes underwent motion correction, slice-timing correction, spatial smoothing and registration to the Montreal Neurological Institute (MNI-152: 2 × 2 × 2 mm3) template through 12-parameter affine transformation [16]. The resulting images were smoothed using 8 × 8 × 8 mm3 kernel. After preprocessing, the time series for each voxel was temporally band-pass filtered (0.01–0.08 Hz) to remove the effects of very-low-frequency drift and high frequency respiratory and cardiac noise [1, 17]. Filtered time series was transformed to a frequency domain using a fast Fourier transform, and the power spectrum was obtained. The square root was calculated at each frequency of the power spectrum and the averaged square root was obtained across 0.01–0.08 Hz at each voxel. The averaged square root across 0.01–0.08 Hz at each voxel was divided by the global mean value, which was taken as the ALFF in the present study. ALFF analysis was performed using REST software (http://restfmri.net/forum/REST_V1.8) [18].
In terms of DTI, images were corrected for Eddy current-induced distortions and head motion using the Eddy current correction routine in FSL. After brain extraction, fractional anisotropy (FA) maps were calculated for each subject using the DTIFIT routine in FSL to fit the diffusion tensor model, and were registered to the MNI-152 template (2 × 2 × 2 mm3) using a 12-parameter affine transformation. The resulting images were smoothed using 8 × 8 × 8 mm3 kernel.
Statistics
SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used to identify the regions showing a significant difference between groups. The regions were considered significant for both a voxel-level threshold of p < 0.001 and a cluster extent threshold of ten voxels. The sphere-shaped volumes of interest (VOI) were generated with a radius of 5 mm and center at the highest t value voxel in each region detected by SPM analysis using WFU Pickatlas [19]. Similarly, VOIs at the posterior limbs of the internal capsule (PLIC) were generated with a radius of 5 mm to assess FA value of the corticospinal tract (center at: ±22, −18, 0).
Pearson product-moment correlation coefficients were assessed between ALFF values in the sensorimotor or premotor cortex and FA values in each corresponding side of PLIC, disease duration or ALSFRS-R. Further, receiver operating characteristic (ROC) curves were drawn, and each area under curve (AUC) was computed. The analyses of correlation and ROC curve were performed using IBM SPSS Statistics version 21(IBM Corp., Armonk, NY).
Results
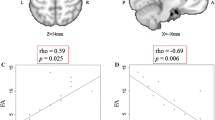
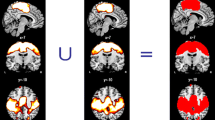
We first examined the difference between groups to assess the disease effect of ALS. In ALS, significant reduction of ALFF value was present in the right sensorimotor, anterior cingulate and left premotor cortices (Table 2; Fig. 1). We next evaluated correlation between ALFF value in the right sensorimotor or the left premotor cortex and FA value in the corresponding side of the PLIC to assess the relationship between cortical activity and the integrity of corticospinal tract in the ALS group. There was a significant negative correlation between ALFF value in the right sensorimotor cortex and FA value in the corresponding side of the PLIC (r = −0.70, p = 0.025; Fig. 2a). In contrast, no significant correlation was observed between ALFF value in the left premotor cortex and FA value in the corresponding side of the PLIC (r = −0.18, p = 0.620; Fig. 2b). ROC curve was depicted to assess the ability of ALFF to diagnose ALS. We assumed that longer disease duration contributed to lower value of FA. Indeed, AUC improved if the ALS patients with disease duration >1 year were excluded (all ALS patients: AUC = 0.87, sensitivity = 0.70, specificity = 0.91; ALS patients with disease duration <1 year: AUC = 0.96, sensitivity = 0.86, specificity = 0.91; Fig. 3a, b).
Significant reduction of amplitude of low frequency fluctuation (ALFF) value. Significant reduction of ALFF value was found in the right sensorimotor, anterior cingulate cortices and the left premotor cortex (upper row). Arrow head indicated the right sensorimotor cortex (upper row), and t map was overlaid on standard structural brain (bottom row). Note that the region in the right sensorimotor cortex was confirmed. The black and white bar represents voxels thresholded at T = 3.58 (p < 0.001, uncorrected)
Correlation between amplitude of low frequency fluctuation (ALFF) and fractional anisotropy (FA) value. ALFF value in the right sensorimotor cortex negatively correlated with FA value in the corresponding side of the posterior limb of the internal capsule (PLIC) (a). In contrast, there was no significant correlation between ALFF value in the left premotor cortex and FA value in the corresponding side of the PLIC (b)
Discussion
This is the first study to demonstrate the reduction of ALFF value in the sensorimotor cortex in ALS, which supports the reliability of ALFF as an analytical tool for fMRI. Significant negative correlation between ALFF value in the sensorimotor cortex and FA value in the PLIC might indicate corticospinal tract integrity negatively regulated brain activity in the sensorimotor cortex. This finding raised the question of the mechanism underlying the negative correlation. The number of cortical interneurons in ALS was reduced [20], and MRS showed decreased concentration of GABA in the motor cortex in ALS [21]. In addition, interneurons were more vulnerable than motor neurons in the spinal cord of a zebrafish model of ALS [22]. Taken together, negative correlation between ALFF value in the sensorimotor cortex and FA value in the PLIC might indicate that reduced value of ALFF was attributed to the abnormality (e.g., hyperactivation) in GABAergic interneurons before neuronal death because corticospinal tract tended to be relatively intact and show almost normal FA value at the early phase of disease. In contrast, loss of GABAergic interneurons might cause increased value of ALFF at advanced stage of disease as corticospinal tract integrity was reduced. Considering these findings, we hypothesize that GABA concentration in the sensorimotor cortex depends on the phase of disease: that is, increased level of GABA at the early phase might induce cortical hypoactivity right before loss of interneurons and decreased level of GABA at the advanced phase might induce cortical hyperactivity after loss of interneurons. Finally, cortical activity could be distinctly reduced because all types of neurons were lost. To test the hypothesis, we need additional study which evaluates GABA concentration across disease stage. However, ALFF is thought to be a summation of local activity of neurons including excitatory and inhibitory activity derived from neurons in whole cortex layers. In this sense, ALFF-based findings could not be simply explained in terms of a single type of neuronal loss or the depletion of neurotransmitter.
ALS patients with upper motor neuron signs show decreased level of FA value in corticospinal tract including PLIC [23]. Previous studies using ALFF included patients with probable or definite ALS [9, 10]. In contrast, almost half of the enrolled patients were probable-laboratory-supported or possible in the present study, which indicated that they manifested less upper motor neuron signs than those of previous reports. Therefore, the patients in this study could show relatively normal FA value in the PLIC and decreased ALFF value in the sensorimotor cortex compared with previous studies. Another line of research supported the finding that ALFF value positively correlated with disease progression. One study reported positive correlation between ALFF value in the left middle frontal gyrus and disease duration [9]. The mean disease duration of our study was shorter than that of previous studies [9, 10]. However, we assumed that the patients at more advanced stage would show reduced value of ALFF because all types of neurons were degenerated. In aggregate, it was possible that ALFF value in the sensorimotor cortex was influenced by FA value, upper motor neuron signs and disease duration, and might be more useful at the early stage than that at the later stage of disease. Indeed, AUC improved if the only ALS patients with shorter disease duration were included. ALFF value could be adjusted for FA value to fit to diagnostic tool for ALS.
However, the present study had limitations. First, small number and heterogeneity of subjects contributed to the asymmetrical findings. Additional subjects could change the results.
In conclusion, we found the reduction of ALFF value in the sensorimotor cortex in ALS, and significant negative correlation between ALFF value in the sensorimotor cortex and FA value in the PLIC. These findings suggested that FA value, associated with corticospinal tract integrity and disease stage, contributed to ALFF value in the sensorimotor cortex in ALS. Further studies needs to be performed to confirm this notion.
References
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541
Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF et al (2010) The oscillating brain: complex and reliable. Neuroimage 49(2):1432–1445
Liu X, Wang S, Zhang X, Wang Z, Tian X, He Y (2014) Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer’s disease. J Alzheimers Dis 40(2):387–397
Hou Y, Wu X, Hallett M, Chan P, Wu T (2014) Frequency-dependent neural activity in Parkinson’s disease. Hum Brain Mapp 35(12):5815–5833
Skidmore FM, Yang M, Baxter L, von Deneen KM, Collingwood J, He G et al (2013) Reliability analysis of the resting state can sensitively and specifically identify the presence of Parkinson disease. Neuroimage 75:249–261
Yao N, Pang S, Cheung C, Chang RS, Lau KK, Suckling J et al (2015) Resting activity in visual and corticostriatal pathways in Parkinson’s disease with hallucinations. Parkinsonism Relat Disord 21(2):131–137
Luo C, Guo X, Song W, Chen Q, Yang J, Gong Q et al (2015) The trajectory of disturbed resting-state cerebral function in Parkinson’s disease at different Hoehn and Yahr stages. Hum Brain Mapp 36(8):3104–3116
Skidmore FM, Yang M, Baxter L, von Deneen K, Collingwood J, He G et al (2013) Apathy, depression, and motor symptoms have distinct and separable resting activity patterns in idiopathic Parkinson disease. Neuroimage 81:484–495
Luo C, Chen Q, Huang R, Chen X, Chen K, Huang X et al (2012) Patterns of spontaneous brain activity in amyotrophic lateral sclerosis: a resting-state FMRI study. PLoS One 7(9):e45470
Zhu W, Fu X, Cui F, Yang F, Ren Y, Zhang X et al (2015) ALFF value in right parahippocampal gyrus acts as a potential marker monitoring amyotrophic lateral sclerosis progression: a neuropsychological, voxel-based morphometry, and resting-state functional MRI study. J Mol Neurosci 57(1):106–113
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O et al (2011) Amyotrophic lateral sclerosis. Lancet 377(9769):942–955
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299
Joint Task Force of the EFNS and the PNS (2010) European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst 15(4):295–301
Sako W, Fujita K, Vo A, Rucker JC, Rizzo JR, Niethammer M et al (2015) The visual perception of natural motion: abnormal task-related neural activity in DYT1 dystonia. Brain 138(Pt 12):3598–3609
Sako W, Abe T, Izumi Y, Harada M, Kaji R (2016) Fractional anisotropy in the supplementary motor area correlates with disease duration and severity of amyotrophic lateral sclerosis. Neurol Sci 37(4):573–577
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2):825–841
Lowe MJ, Mock BJ, Sorenson JA (1998) Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 7(2):119–132
Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ et al (2011) REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6(9):e25031
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19(3):1233–1239
Maekawa S, Al-Sarraj S, Kibble M, Landau S, Parnavelas J, Cotter D et al (2004) Cortical selective vulnerability in motor neuron disease: a morphometric study. Brain 127(Pt 6):1237–1251
Foerster BR, Callaghan BC, Petrou M, Edden RA, Chenevert TL, Feldman EL (2012) Decreased motor cortex gamma-aminobutyric acid in amyotrophic lateral sclerosis. Neurology 78(20):1596–1600
McGown A, McDearmid JR, Panagiotaki N, Tong H, Al Mashhadi S, Redhead N et al (2013) Early interneuron dysfunction in ALS: insights from a mutant sod1 zebrafish model. Ann Neurol 73(2):246–258
Sage CA, Peeters RR, Gorner A, Robberecht W, Sunaert S (2007) Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 34(2):486–499
Acknowledgements
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 15K19487, Grants-in-Aid from the Research Committee of CNS Degenerative Diseases, the Ministry of Health, Labor and Welfare of Japan and the Research Committee on Establishment of Novel Treatments for Amyotrophic lateral Sclerosis, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sako, W., Abe, T., Izumi, Y. et al. Spontaneous brain activity in the sensorimotor cortex in amyotrophic lateral sclerosis can be negatively regulated by corticospinal fiber integrity. Neurol Sci 38, 755–760 (2017). https://doi.org/10.1007/s10072-017-2831-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-2831-y